Abstract
Main conclusion
We demonstrate here that the reduction of InsP 3 , the key component of the phosphoinositol pathway, results in changes in ROS-scavenging machinery and, subsequently, increases the tolerance of tomato plants to light stress.
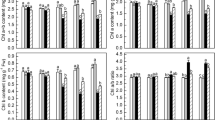
Different plant stress signaling pathways share similar elements and, therefore, ‘cross-talk’ between the various pathways can exist. Links between the phosphoinositol signaling pathway and light signaling were recently found. Tomato plants expressing InsP 5-ptase and exhibiting reduction in the level of inositol 1,4,5-triphosphate (InsP3) demonstrated enhanced tolerance to stress caused by continuous light exposure. To understand the molecular basis of observed stress tolerance in tomato lines with decreased amount of InsP3, we monitored the expression of enzymatic antioxidants as well as important factors in light signaling associated with non-enzymatic antioxidants (secondary metabolites). Here, we demonstrated that InsP 5-ptase transgenic plants accumulate less hydroxide peroxide and maintain higher chlorophyll content during stress caused by continuous light exposure. This observation can be explained by documented activation of multiple enzymatic antioxidants (LeAPX1, SICAT2, LeSOD) at levels of gene expression and enzymatic activities during continuous light exposure. In addition, we noticed the up-regulation of photoreceptors LePHYB and LeCHS1, key enzymes in flavonoid biosynthesis pathway, transcription factors LeHY5, SIMYB12, and early light-inducible protein (LeELIP) genes in transgenic tomato seedlings exposed to blue or red light. Our study confirmed the existence of a correlation between phosphoinositol signaling pathway modification, increased tolerance to stress caused by continuous light exposure, activation of ROS-scavenging enzymes, and up-regulation of molecular activators of non-enzymatic antioxidants in InsP 5-ptase expressing tomato lines.








Similar content being viewed by others
Abbreviations
- InsP3 :
-
Inositol 1,4,5-triphosphate
- InsP 5-ptase:
-
Inositol trisphosphate 5 phosphatase
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- LeCHS1:
-
Lycopersicon esculentum chalcone synthase
- LeHY5:
-
Lycopersicon esculentum long hypocotyl 5 transcription factor
- LePHYB:
-
Lycopersicon esculentum photochrome B
- SICRY1:
-
Solanum lycopersicum cryptochrome 1
- LeELIP:
-
Lycopersicon esculentum early light-inducible protein
References
Ahmad P, Sarwat M, Sharma S (2008) Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol 51(3):167–173
Alimohammadi M, de Silva K, Ballu C, Ali N, Khodakovskaya MV (2012) Reduction of inositol (1, 4, 5)-trisphosphate affects the overall phosphoinositol pathway and leads to modifications in light signalling and secondary metabolism in tomato plants. J Exp Bot 63(2):825–835
Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1(2):213–222
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Aravind P, Prasad MNV (2003) Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L.: a free floating freshwater macrophyte. Plant Physiol Biochem 41(4):391–397
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Bergey DR, Howe GA, Ryan CA (1996) Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci 93(22):12053–12058
Berridge MJ (2005) Unlocking the secrets of cell signaling. Annu Rev Physiol 67:1–21
Bruno AK, Wetzel CM (2004) The early light-inducible protein (ELIP) gene is expressed during the chloroplast-to-chromoplast transition in ripening tomato fruit. J Exp Bot 55(408):2541–2548
Cashmore AR, Jarillo JA, Wu YJ, Liu D (1999) Cryptochromes: blue light receptors for plants and animals. Science 284(5415):760–765
Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell Online 10(5):673–683
Chen M, Chory J, Fankhauser C (2004) Light signal transduction in higher plants. Annu Rev Genet 38:87–117
Chory J, Reinecke D, Sim S, Washburn T, Brenner M (1994) A role for cytokinins in de-etiolation in Arabidopsis (det mutants have an altered response to cytokinins). Plant Physiol 104(2):339–347
Clé C, Hill LM, Niggeweg R, Martin CR, Guisez Y, Prinsen E, Jansen MA (2008) Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum; consequences for phenolic accumulation and UV-tolerance. Phytochemistry 69(11):2149–2156
Dalkin K, Bowles DJ (1989) Local and systemic changes in gene expression induced in tomato plants by wounding and by elicitor treatment. Planta 179(3):367–375
Dhindsa RS, Plumb-Dhindsa PAMELA, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32(1):93–101
Elstner EF (1991) Mechanisms of oxygen activation in different compartments of plant cells. In: Pelland EJ, Steffen KL (eds) Active oxygen/oxidative stress in plant metabolism. American Society of Plant Physiologists, Rockville, pp 13–25
Fankhauser C, Staiger D (2002) Photoreceptors in Arabidopsis thaliana: light perception, signal transduction and entrainment of the endogenous clock. Planta 216(1):1–16
Fini A, Brunetti C, Di Ferdinando M, Ferrini F, Tattini M (2011) Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal Behav 6(5):709–711
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143(1):81–96
Heijde M, Ulm R (2012) UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci 17(4):230–237
Hutin C, Nussaume L, Moise N, Moya I, Kloppstech K, Havaux M (2003) Early light-induced proteins protect Arabidopsis from photooxidative stress. Proc Natl Acad Sci 100(8):4921–4926
Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. The Plant Cell Online 9(4):627–640
Khan NA, Singh S, Nazar R (2007) Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress. J Agron Crop Sci 193(6):435–444
Khodakovskaya M, Sword C, Wu Q, Perera IY, Boss WF, Brown CS, Winter Sederoff H (2010) Increasing inositol (1, 4, 5)-trisphosphate metabolism affects drought tolerance, carbohydrate metabolism and phosphate-sensitive biomass increases in tomato. Plant Biotechnol J 8(2):170–183
Kimura M, Yamamoto YY, Seki M, Sakurai T, Sato M, Abe T et al (2003) Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem Photobiol 77(2):226–233
Kleine T, Kindgren P, Benedict C, Hendrickson L, Strand Å (2007) Genome-wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol 144(3):1391–1406
Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y et al (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell Online 19(3):731–749
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Lightner J, Pearce G, Ryan CA (1993) Isolation of signaling mutants of tomato (Lycopersicon esculentum). Mol General Genet MGG 241(5–6):595–601
Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR (1998) Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc Natl Acad Sci 95(5):2686–2690
Liu YS, Gur A, Ronen G, Causse M, Damidaux R, Buret M et al (2003) There is more to tomato fruit colour than candidate carotenoid genes. Plant Biotechnol J 1(3):195–207
Løvdal T, Olsen KM, Slimestad R, Verheul M, Lillo C (2010) Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 71(5):605–613
Malan C, Greyling MM, Gressel J (1990) Correlation between CuZn superoxide dismutase and glutathione reductase, and environmental and xenobiotic stress tolerance in maize inbreds. Plant Sci 69(2):157–166
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164(5):601–610
Mosblech A, König S, Stenzel I, Grzeganek P, Feussner I, Heilmann I (2008) Phosphoinositide and inositolpolyphosphate signalling in defense responses of Arabidopsis thaliana challenged by mechanical wounding. Mol Plant 1(2):249–261
Munnik T (2001) Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci 6(5):227–233
Nazar R, Khan NA, Singh S, Anjum NA (2008) Photosynthetic traits and activities of antioxidant enzymes in blackgram (Vigna mungo L. Hepper) under cadmium stress. Am J Plant Physiol 3(1):25–32
O’Donnell PJ, Truesdale MR, Calvert CM, Dorans A, Roberts MR, Bowles DJ (1998) A novel tomato gene that rapidly responds to wound-and pathogen-related signals. Plant J 14(1):137–142
Olsen K, Hehn A, Jugdé H, Slimestad R, Larbat R, Bourgaud F, Lillo C (2010) Identification and characterization of CYP75A31, a new flavonoid 3′5′-hydroxylase, isolated from Solanum lycopersicum. BMC Plant Biol 10(1):21
Pandhair V, Sekhon BS (2006) Reactive oxygen species and antioxidants in plants: an overview. J Plant Biochem Biotechnol 15(2):71–78
Peña-Cortés H, Fisahn J, Willmitzer L (1995) Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc Natl Acad Sci 92(10):4106–4113
Pérez FJ, Villegas D, Mejia N (2002) Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O 2 in grapevine leaves. Phytochemistry 60(6):573–580
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell Online 6(1):65–74
Quail PH (2002) Photosensory perception and signalling in plant cells: new paradigms? Curr Opin Cell Biol 14(2):180–188
Sagar AD, Briggs WR (1990) Effects of high light stress on carotenoid-deficient chloroplasts in Pisum sativum. Plant Physiol 94(4):1663–1670
Salinas- Mondragon RE, Kajla JD, Perera IY, Brown CS, Sederoff HW (2010) Role of inositol 1, 4, 5-triphosphate signalling in gravitropic and phototropic gene expression. Plant, Cell Environ 33(12):2041–2055
Sellaro R, Hoecker U, Yanovsky M, Chory J, Casal JJ (2009) Synergism of red and blue light in the control of Arabidopsis gene expression and development. Curr Biol 19(14):1216–1220
Sharma P, Shanker Dubey R (2005) Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of osmolytes as enzyme protectant. J Plant Physiol 162(8):854–864
Srivastava AK, Bhargava P, Rai LC (2005) Salinity and copper-induced oxidative damage and changes in the antioxidative defence systems of Anabaena doliolum. World J Microbiol Biotechnol 21(6–7):1291–1298
Stracke R, Favory JJ, Gruber H, Bartelniewoehner L, Bartels S, Binkert M et al (2010) The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant, Cell Environ 33(1):88–103
Sun QP, Guo Y, Sun Y, Sun DY, Wang XJ (2006) Influx of extracellular Ca2+ involved in jasmonic-acid-induced elevation of [Ca2+] cyt and JR1 expression in Arabidopsis thaliana. J Plant Res 119(4):343–350
Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G (2004) Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol 163(3):547–561
Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell Online 11(7):1195–1206
Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK et al (2004) Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J 39(1):45–58
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66
Xiong L, Zhu JK (2001) Abiotic stress signal transduction in plants: molecular and genetic perspectives. Physiol Plant 112(2):152–166
Yang Y, Han C, Liu Q, Lin B, Wang J (2008) Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiologiae Plantarum 30(4):433–440
Zlatev ZS, Lidon FC, Ramalho JC, Yordanov IT (2006) Comparison of resistance to drought of three bean cultivars. Biol Plant 50(3):389–394
Acknowledgments
Funding from EPSCoR-NSF-P3 Center (Grant P3-202 to MVK) and NASA-EPSCoR (Grant to MVK) is highly appreciated. Authors thank EPSCoR-NSF-P3 Center, Graduate Institute of Technology and College of Science, UALR for providing graduate assistantship to Mohammad Alimohammadi. We are grateful to Dr. Julian Post for designing and building of LED light boxes used for light exposure experiment. The editorial assistance of Dr. Marinelle Ringer is also acknowledged.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alimohammadi, M., Lahiani, M.H. & Khodakovskaya, M.V. Genetic reduction of inositol triphosphate (InsP3) increases tolerance of tomato plants to oxidative stress. Planta 242, 123–135 (2015). https://doi.org/10.1007/s00425-015-2289-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2289-1