Abstract
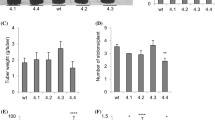
Potato (Solanum tuberosum L.) tuberization is regulated by many signals, such as abscisic acid (ABA), sucrose and gibberellic acid (GA). ABA and sucrose are positive modulators, while GA is an inhibitor of the process. ABF (ABRE-binding factor) proteins are transcription factors involved in ABA and stress signaling. Previously, we reported that S. tuberosum StABF1 could mediate the ABA effects on tuberization. The aim of the present study was to evaluate the potential use of ABF genes to enhance tuberization and to determine the molecular mechanism involved. For this purpose, transgenic potato plants expressing the Arabidopsis ABF4 or ABF2 genes were generated, and their tuberization capacity and response to tuberization-related signals were analyzed in vitro. The results indicate that both ABF4 and ABF2 proteins positively regulate potato tuber induction; however, only ABF4 expression significantly increases the number and weight of the tubers obtained, without stunting growth. ABF4 and ABF2 transgenic plants exhibit ABA hypersensitivity during tuberization, accompanied by a GA-deficient phenotype. ABF4 expression triggers a significant rise in ABA levels in stolons under tuber-inducing conditions as compared with wild-type plants and a transcriptional deregulation of GA metabolism genes. Our results demonstrate that Arabidopsis ABF4 functions in potato ABA-GA signaling crosstalk during tuberization by regulating the expression of ABA- and GA-metabolism genes. ABF4 gene might be a potential tool to increase tuber production, since its heterologous expression in potato enhances tuber induction without affecting plant growth.










Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- GA:
-
Gibberellic acid
- ABF:
-
ABRE-binding factor
- AREB:
-
ABA-response element binding factor
References
Appleford NEJ, Wilkinson MD, Ma Q, Evans DJ, Stone MC, Pearce SP, Powers SJ, Thomas SG, Jones HD, Phillips AL, Hedden P, Lenton JR (2007) Decreased shoot stature and grain α-amylase activity following ectopic expression of a gibberellin 2-oxidase gene in transgenic wheat. J Exp Bot 58:3213–3226
Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14:2085–2096
Bastías A, López-Climent M, Valcárcel M, Rosello S, Gómez-Cadenas A, Casaretto JA (2011) Modulation of organic acids and sugar content in tomato fruits by an abscisic acid-regulated transcription factor. Physiol Plant 141:215–226
Bravo-Almonacid F, Rudoy V, Welin B, Segretin ME, Bedogni MC, Stolowicz F, Criscuolo M, Foti M, Gomez M, López M, Serino G, Cabral S, Dos Santos C, Huarte M, Mentaberry A (2012) Field testing, gene flow assessment and pre-commercial studies on transgenic Solanum tuberosum spp. tuberosum (cv. Spunta) selected for PVY resistance in Argentina. Transgenic Res 21:967–982
Brown PTH, Lange FD, Kranz E, Lorz H (1993) Analysis of single protoplasts and regenerated plants by PCR and RAPD technology. Mol Gen Genet 237:311–317
Casaretto JA, Ho TH (2005) Transcriptional regulation by abscisic acid in barley (Hordeum vulgare L.) seeds involves autoregulation of the transcription factor HvABI5. Plant Mol Biol 57:21–34
Choi H, Hong J, Ha J, Kang J, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275:1723–1730
Choi HI, Park HJ, Park JH, Kim S, Im MY, Seo HH, Kim YW, Hwang I, Kim SY (2005) Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiol 139:1750–1761
Destefano-Beltrán L, Knauber D, Huckle L, Suttle JC (2006) Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol Biol 61:687–697
Dijkstra C, Adams E, Bhattacharya A, Anthony P, Kourmpetli S, Power JB, Lowe KC, Thomas SG, Hedden P, Phillips AL, Davey MR (2008) Over-expression of a gibberellin 2-oxidase gene from Phaseolus coccineus L. enhances gibberellin inactivation and induces dwarfism in Solanum species. Plant Cell Rep 27:463–470
Ewing EE, Struik PC (1992) Tuber formation in potato: induction, initiation, and growth. Hortic Rev 14:189–198
Gómez-Cadenas A, Zentella R, Walker-Simmons MK, Ho TH (2001) Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellin signalling molecules. Plant Cell 13:667–679
Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, GongF Phillips AL, Hedden P, Sun TP, Thomas SG (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414
Hannapel DJ, Chen H, Rosin FM, Banerjee AK, Davies PJ (2004) Molecular controls of tuberization. Am J Potato Res 81:263–274
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5:523–530
Hendriks T, Vreugdenhil D, Stiekema WJ (1991) Patatin and four serine proteinase inhibitor genes are differentially expressed during potato tuber development. Plant Mol Biol 17:385–394
Hirayama T, Shinozaki K (2010) Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J 61:1041–1052
Ho THD, Gómez-Cadenas A, Zentella R, Casaretto JA (2003) Crosstalk between gibberellin and abscisic acid in cereal aleurone. J Plant Growth Regul 22:185–194
Holsters M, DeWarle D, Depicker A, Messens E, Van Montague M, Schell J (1978) Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet 163:181–187
Hossain MA, Cho JI, Han M, Ahn CH, Jeon JS, An G, Park PB (2010a) The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signalling in rice. J Plant Physiol 167:1512–1520
Hossain MA, Lee Y, Cho JI, Ahn CH, Lee SK, Jeon JS, Kang H, Lee CH, An G, Park PB (2010b) The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signalling in rice. Plant Mol Biol 72:557–566
Hsieh TH, Li CW, Su RC, Cheng CP, Sanjaya, Tsai YC, Chan MT (2010) A tomato bZIP transcription factor, SlAREB, is involved in water deficit and salt stress response. Planta 231:1459–1473
Huijser C, Kortstee A, Pego J, Weisbeek P, Wisman E, Smeekens S (2000) The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: involvement of abscisic acid in sugar responses. Plant J 23:577–585
Iriti M, Picchi V, Rossoni M, Gomarasca S, Ludwig N, Gargano M, Faoro F (2009) Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ Exp Bot 66:493–500
Jackson SD (1999) Multiple signalling pathways control tuber induction in potato. Plant Physiol 119:1–8
Kang J, Choi H, Im M, Kim SY (2002) Arabidopsis basic leucine zipper proteins mediate stress-responsive abscisic acid signaling. Plant Cell 14:343–357
Kelen M, Cubek Demiralay E, Sen S, Ozkan G (2004) Separation of abscisic acid, indole-3-acetic acid, gibberellic acid in 99 R (Vitis berlandieri x Vitis rupestris) and Rose Oil (Rosa damascena Mill.) by Reversed Phase Liquid Chromatography. Turk J Chem 28:603–610
Kim S, Kang JY, Cho DI, Park JH, Kim SY (2004) ABF2, an ABRE binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J 40:75–87
Kloosterman B, Navarro C, Bijsterbosch G, Lange T, Prat S, Visser RGF, Bachem GWB (2007) StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant J 52:362–373
Kobayashi F, Maeta E, Terashima A, Takumi S (2008) Positive role of a wheat HvABI5 ortholog in abiotic stress response of seedlings. Physiol Plant 134:74–86
Koda Y, Okazawa Y (1983) Influences of environmental, hormonal and nutritional factors on potato tuberization in vitro. Jpn J Crop Sci 52:582–591
Krauss A, Marschner H (1982) Influence of nitrogen nutrition, daylength, and temperature on contents of gibberellic and abscisic acid and on tuberization in potato plants. Potato Res 25:13–21
Kumar D, Wareing PF (1972) Factors controlling stolon development in the potato plant. New Phytol 71:639–648
Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23:587–596
Lo S-F, Yang S-Y, Chen K-T, Hsing Y-I, Zeevaart JAD, Chen L-J, Yu S-M (2008) A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 20:2603–2618
Martínez-García JF, García-Martínez JL, Bou J, Prat S (2001) The interaction of gibberellins and photoperiod in the control of potato tuberization. J Plant Growth Regul 20:377–386
Menzel BM (1980) Tuberization in potato (Solanum tuberosum) cultivar Sebago at high temperatures: responses to gibberellins and growth inhibitors. Ann Bot 46:259–266
Muñiz García MN, Giammaria V, Grandellis C, Téllez-Iñón MT, Ulloa RM, Capiati DA (2012) Characterization of StABF1, a stress-responsive bZIP transcription factor from Solanum tuberosum L. that is phosphorylated by StCDPK2 in vitro. Planta 235:761–778
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8:4321–4325
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RTPCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351
Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee HS, Sun TP, Kamiya Y, Choi G (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19:1192–1208
Okazawa Y (1960) Studies on the relation between the tuber formation of potato and its natural gibberellin content. Proc Crop Sci Soc Jpn 29:121–124
Orellana S, Yañez M, Espinoza A, Verdugo I, González E, Ruiz-Lara S, Casaretto JA (2010) The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress-related genes in tomato. Plant Cell Environ 33:2191–2208
País SM, González MA, Téllez-Iñón MT, Capiati DA (2009) Characterization of potato (Solanum tuberosum) and tomato (Solanum lycopersicum) protein phosphatases type 2A catalytic subunits and their involvement in stress responses. Planta 230:13–25
País SM, Muñíz García MN, Téllez-Iñón MT, Capiati DA (2010) Protein phosphatases type 2A mediate tuberization signaling in Solanum tuberosum L. leaves. Planta 232:37–49
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res 29:e45
Raices M, Ulloa RM, MacIntosh GC, Crespi M, Tellez-Inon MT (2003) StCDPK1 is expressed in potato stolon tips and is induced by high sucrose concentration. J Exp Bot 54:2589–2591
Railton ID, Wareing PF (1973) Effects of daylength on endogenous gibberellins in leaves of Solanum andigena. Physiol Plant 28:88–94
Rodríguez-Falcón M, Bou J, Prat S (2006) Seasonal control of tuberization in potato: conserved elements with the flowering response. Annu Rev Plant Biol 57:151–180
Romano E, Proite K, Soares A, Torres A, Arieta J, Jach G, Mentaberry AN, Monte D (2001) New binary vectors for plant co-transformation. Physiol Mol BiolPlants 7:133–137
Sarkar D (2008) The signal transduction pathways controlling in planta tuberization in potato: an emerging synthesis. Plant Cell Rep 27:1–8
Sarkar D (2010) Photoperiodic inhibition of potato tuberization: an update. Plant Growth Regul 62:117–125
Schoonheim PJ, Costa Pereira DD, De Boer AH (2009) Dual role for 14-3-3 proteins and ABF transcription factors in gibberellic acid and abscisic acid signalling in barley (Hordeum vulgare) aleurone cells. Plant Cell Environ 32:439–447
Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun TP, Koshiba T, Kamiya Y, Yamaguchi S, Nambara E (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48:354–366
Smith OE, Rappaport L (1969) Gibberellins, inhibitors, and tuber formation in the potato (Solanum tuberosum). Am Potato J 46:185–191
Thomas SG, Sun TP (2004) Update on gibberellin signaling. A tale of the tall and the short. Plant Physiol 135:668–676
Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97:11632–11637
Vreugdenhil D, Struik PC (1989) An integrated view of the hormonal regulation of tuber formation in potato (Solanum tuberosum). Physiol Plant 75:525–531
Xu X, van Lammeren AAM, Vermeer E, Vreugdenhil D (1998) The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol 117:575–584
Yáñez M, Cáceres S, Orellana S, Bastías A, Verdugo I, Ruiz-Lara S, Casaretto JA (2009) An abiotic stress-responsive bZIP transcription factor from wild and cultivated tomatoes regulates stress-related genes. Plant Cell Rep 28:1497–1507
Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, JikumaruY Nambara E, Kamiya Y, Sun TP (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19:3037–3057
Acknowledgments
We thank Dra. María Mercedes Rivero for her generous gift of the A. tumefaciens strain EHA101, Dr. Fernando Bravo-Almonacid and Dra. María Eugenia Segretin for providing the plasmids for plant transformation, and Dra. María Teresa Tellez-Iñón for reading the manuscript and providing valuable comments. This work was supported by grants from CONICET, University of Buenos Aires and FONCYT-ANPCYT.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muñiz García, M.N., Stritzler, M. & Capiati, D.A. Heterologous expression of Arabidopsis ABF4 gene in potato enhances tuberization through ABA-GA crosstalk regulation. Planta 239, 615–631 (2014). https://doi.org/10.1007/s00425-013-2001-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-2001-2