Abstract
Purpose
To determine the effect of taurine supplementation on sweating and core temperature responses, including the transition from compensable to uncompensable heat stress, during prolonged low-intensity exercise of a fixed-heat production (~ 200W/m2) in hot conditions (37.5 °C), at both fixed and incremental vapour-pressure.
Methods
Fifteen females (n = 3) and males (n = 12; 27 ± 5 years, 78 ± 9 kg, \(\dot{V}\)O2max 50.3 ± 7.8 mL/kg/min), completed a treadmill walking protocol (~ 200W/m2 heat production [Ḣprod]) in the heat (37.5 ± 0.1 °C) at fixed-(16-mmHg) and ramped-humidity (∆1.5-mmHg/5-min) following 1 week of oral taurine supplementation (50 mg/kg/bm) or placebo, in a double-blind, randomised, cross-over design. Participants were assessed for whole-body sweat loss (WBSL), local sweat rate (LSR), sweat gland activation (SGA), core temperature (Tcore), breakpoint of compensability (Pcrit) and calorimetric heat transfer components. Plasma volume and plasma taurine concentrations were established through pre- and post-trial blood samples.
Results
Taurine supplementation increased WBSL by 26.6% and 5.1% (p = 0.035), LSR by 15.5% and 7.8% (p = 0.013), SGA (1 × 1 cm) by 32.2% and 29.9% (p < 0.001) and SGA (3 × 3 cm) by 22.1% and 17.1% (p = 0.015) during the fixed- and ramped-humidity exercise periods, respectively. Evaporative heat loss was enhanced by 27% (p = 0.010), heat-storage reduced by 72% (p = 0.024) and Pcrit was greater in taurine vs placebo (25.0-mmHg vs 21.7-mmHg; p = 0.002).
Conclusion
Taurine supplementation increased sweating responses during fixed Ḣprod in hot conditions, prior to substantial heat strain and before the breakpoint of compensability, demonstrating improved thermoregulatory capacity. The enhanced evaporative cooling and reduced heat-storage delayed the subsequent upward inflection in Tcore—represented by a greater Pcrit—and offers a potential dietary supplementation strategy to support thermoregulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise increases metabolic heat production (Ḣprod), with dry (conduction, convection and radiation) and evaporative heat exchange providing potential avenues of heat dissipation (Gagge and Gonzalez 1996). In hot environmental conditions, evaporative heat transfer (latent heat transfer; Ėskin) is the main and most modifiable avenue of heat dissipation, which occurs secondary to sweating when ambient vapor pressure permits (Wenger 1972). Indeed, both Ḣprod and the rate of evaporation required to maintain heat balance (Ėreq) drive the thermal sweating response (Cramer and Jay 2014, 2016; Gagnon et al. 2013; Peel et al. 2022). Consequently, when exercising or performing occupational work in the heat, eccrine sweat production is an important physiological mechanism for the maintenance of thermal equilibrium, as it allows for evaporative cooling to offset Ḣprod (Marino et al. 2000; Sawka and Young 2006). Thus, manipulation of factors affecting calorimetric components, such as Ḣprod or any heat loss avenue, will affect thermal balance.
Evaporation of sweat off the skin’s surface, and therefore, the latent heat of vaporisation, is determined by both the efficiency of sweating and the ambient vapour pressure [determined from the relationship between air temperature and relative humidity (RH); Gagge and Gonzalez 1996; Parsons 2007]. Dry, hot environmental conditions allow for a greater capacity to evaporatively cool compared to those with high humidity (Che Muhamed et al. 2016). This is due to the larger vapour pressure gradient between the ambient air and skin’s surface, which permits a superior maximal evaporative heat transfer capacity (Ėmax; Cramer and Jay 2019). Consequently, prolonged exercise in hot and humid environmental conditions causes thermal strain (positive heat storage), due to the reduced capacity for evaporative cooling. Indeed, without sufficient dry and evaporative heat transfer, positive heat storage will ensue, forcing a transition from a compensable to an uncompensable state, denoted by inexorable increases in core temperature (Tcore; Cramer and Jay 2016; Marino et al. 2000). Thermal sweating and the efficiency of evaporative heat transfer can be acutely enhanced through interventions, such as endurance training and heat acclimation (Ravanelli et al. 2018). This training- or acclimation-induced augmentation of the sweating response was reported to delay the upward inflection in Tcore associated with a transition to thermal uncompensability using an ‘inflection protocol’, whereby ambient vapor pressure is manipulated at fixed ambient dry bulb temperature and heat production (Ravanelli et al. 2018). Thus, manipulation of the environmental conditions can be useful in determining capacity to thermoregulate and changes that might occur following interventions, such as dietary supplementation.
Taurine, a sulphur containing amino acid, can be supplemented orally and has been shown to enhance endurance exercise performance in thermoneutral conditions (Balshaw et al. 2013; Waldron et al. 2019, 2018b; Zhang et al. 2004b). These ergogenic effects mimic the magnitude of endurance training responses to heat acclimation (Waldron et al. 2021) and appear to be related to sarcoplasmic reticulum Ca+ handling (Dutka et al. 2014; Hamilton et al. 2006), anti-oxidative effects (Hansen et al. 2006, 2010; Jong et al. 2021; Schaffer et al. 2022; Zhang et al. 2004b) and/or alterations in substrate utilisation, favouring greater relative fat oxidation (Rutherford et al. 2010; Simmonds et al. 2022), which is a feature of the endurance trained phenotype (Lima-Silva et al. 2010). Further, taurine has many other biological roles that could be advantageous to exercise performance in the heat, including cellular osmoregulation (Cuisinier et al. 2002) and vasoactive properties (Maia et al. 2014; Sun et al. 2016; Ulusoy et al. 2017). The osmoregulatory capacity of taurine might be exaggerated following oral supplementation, with higher plasma taurine concentrations increasing osmotic pressure in both central and peripheral sites, thereby acutely drawing fluid into the vascular space and theoretically sustaining, or perhaps expanding, plasma volume. However, it should be noted that taurine has a weaker relationship with plasma volume compared to other osmolytes, such as sodium and chloride (Cuisinier et al. 2002) and the effect of exogenous supplementation on plasma volume in heat stressed, exercising humans has not yet been established. Nonetheless, various beneficial physiological effects of taurine supplementation have been demonstrated in hot conditions, as described below. Indeed, the performance enhancing effect of taurine supplementation was heightened when administered during exercise in the heat, prolonging time to exhaustion by 10% (Page et al. 2019). Here, taurine increased the rate (∼12.7%) and hastened the onset of sweating during exhaustive exercise in the heat, alongside substantial reductions in Tcore (end Tcore of 38.1 °C vs 38.5 °C), demonstrating its potential role in thermoregulation. The early changes in the sweating response were considered to be indicative of a centrally mediated alteration in thermoregulatory set-points, which might relate to its role as a neuromodulator (Hussy et al. 2000; Jia et al. 2008). However, despite this early promising research, it remains necessary to more comprehensively evaluate the potential thermoregulatory role of taurine supplementation in a controlled experimental setting, where sufficient control of calorimetric components is permitted (i.e. Ḣprod and Ėreq). Furthermore, the effect of taurine on the sweating response and subsequent thermoregulation across more prolonged exercise periods is unknown.
The aim of the current study was to determine the effect of an 8-day taurine supplementation period on Tcore and sweating responses [whole-body sweat loss (WBSL), local sweat rate (LSR) and sweat gland activation (SGA)], calorimetric heat transfer components (Ėskin, heat storage), delta plasma volume, and plasma taurine concentrations during prolonged low-intensity exercise of a fixed Ḣprod in the heat at both fixed and increasing vapour pressure. It was hypothesised that taurine supplementation would: (i) induce greater sweating responses across the exercise protocol; (ii) delay the increase in Tcore during the period of increasing vapour pressure (transition to an uncompensable environment); (iii) increase plasma volume and; (iv) result in greater evaporative heat transfer and reduced heat storage, as modelled by partitional calorimetry.
Methods
Participants
Fifteen non-heat acclimated, healthy females (n = 3) and males (n = 12) volunteered to take part in the study [27 ± 5 years, 179 ± 8 cm, 78 ± 9 kg, maximal oxygen uptake (\(\dot{V}\)O2max) 50.3 ± 7.8 mL/kg/min]. Based on the effect sizes (Cohen’s d = 0.7) reported using taurine to improve endurance performance in the heat (Page et al. 2019), G ∗ Power (Version 3.0.10; Universität Düsseldorf, Germany) was used to calculate an appropriate a-priori sample size of 15 to identify significant differences between groups. As part of the health screening questionnaire, participants were asked if they had been exposed to hot ambient temperatures in the previous two months, sufficient to induce heat adaptation and were excluded if so. Participants were asked to refrain from alcohol and caffeine consumption for 24-h and to avoid strenuous exercise and follow a consistent diet for 48-h prior to testing. Use of any performance enhancing or dietary supplements, such as caffeine, was prohibited for the duration of the study. This was verified in the pre-trial screen, along with the opportunity for participants to report any adverse health effects. Written informed consent was obtained from all participants. Institutional ethics approval (JP_30-10-20b) was provided and the study was conducted in accordance with the 2013 Declaration of Helsinki, except for pre-registration on a publicly accessible database.
Design
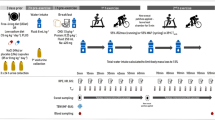
This study adopted a double-blind, randomised, placebo-controlled, cross-over design. Participants reported to the laboratory on five separate occasions; once for pre-screening and familiarisation (visit 1), twice to complete a walking incremental test to establish the work rate-\(\dot{V}\)O2 relationship and \(\dot{V}\)O2max (visits 2 and 4) and twice for the experimental trials, in which they completed a fixed- and ramped-humidity treadmill walking protocol, following 8-days of supplementation with either 50 mg/kg/bm of taurine or 30 mg/kg/bm of maltodextrin (placebo; visits 3 & 5; Fig. 1). All testing sessions took place in an environmental chamber set to 37.5 ± 0.1 °C and 34.2 ± 1.4% RH. The break period of 7-days between conditions was selected to permit complete recovery from the protocols and time to consume the cross-over supplementation. Taurine has a ratio of clearance/bioavailability of ~ 21-h and, therefore, this was considered a sufficient washout period (Ghandforoush-Sattari et al. 2010). All trials were conducted at approximately the same time of day to control for circadian rhythm variation. Randomisation was performed manually via coin toss by an independent person.
Incremental walking test
During visits 2 and 4, participants completed an incremental treadmill walking test to volitional exhaustion on a calibrated treadmill (h/p/cosmos, Am Sportplatz 8, Germany) in hot conditions (37.5 ± 0.1 °C and 34.2 ± 1.5% RH) to determine their \(\dot{V}\)O2max and individual work rates required to elicit Ḣprod of ~ 200 W/m2. The test began at 2 km/h (0.56 m/s) and increased by 1 km/h (0.28 m/s) every 3-min with corresponding gradients of 0% in the first stage, 5% in the second stage, 10% in the third stage, and 2% additional increases thereafter. The incremental test was conducted to volitional exhaustion. Pulmonary oxygen uptake was measured using breath-by-breath expired gas analysis (Jaeger Vyntus CPX, Hoechberg, Germany), with \(\dot{V}\)O2max determined as the highest 30-s mean value, which occurred in the final stage of each participants’ test. The test was designed to progressively increase mechanical work rate, in a square-wave manner, to elicit a range of Ḣprod values, including that required for the treadmill walking protocol in visits 3 and 5 (200 W/m2). Each participant’s Ḣprod for the experimental trials were determined by subtracting the rate of mechanical work (Wk) from the rate of metabolic energy expenditure (Ṁ; Eq. 1).
where: metabolic energy expenditure (Ṁ) was determined using measured \(\dot{V}\)O2 and respiratory exchange ratio (RER) in the final 1-min of each incremental stage (Eq. 2):
To achieve the necessary Ḣprod value, the test was initiated at a mechanical work rate below that required to elicit the desired Ḣprod (200 W/m2) and increased until exhaustion. The Ḣprod (W/m2) at each stage was determined based on participant body surface area (BSA; Eqs. 3 and 4; (Cramer and Jay 2014; DuBois and DuBois 1916).
The mechanical work rate required to elicit each target Ḣprod (W/m2) for the exercise trials during visits 3 and 5 (i.e. ~ 200 W/m2) was subsequently determined based on the linear relationship (y = mx + b), between Ḣprod (W/m2) and work rate during the incremental test (Eq. 5). This equated to ~ 43.1 ± 6.2 W and ~ 34.7 ± 5.5% of \(\dot{V}\)O2max in the taurine condition and ~ 42.1 ± 6.2 W and ~ 34.9 ± 5.6% of \(\dot{V}\)O2max in the placebo condition.
Experimental trials
Pre-trial instrumentation
Participants were required to arrive euhydrated, as determined by a urine osmolality value < 600 mOsm kg/H2O (Portable osmometer, Osmocheck, Vitech, Scientific Ltd). If the reading was > 600 mOsm kg/H2O—the threshold for hypohydration—the participant was asked to drink 500-mL of plain water and wait 30-min. Urine osmolality was then re-determined and if the participant was deemed euhydrated, testing commenced. Participants wore running shorts (90% polyester, 10% elastane), as well as a sports bra for female participants. To measure core body temperature (Tcore), participants were instructed to insert a flexible rectal probe 10 cm past the anal sphincter (Walters Medical, W0001B, England).
Trials (rest, fixed-humidity and ramped-humidity)
Participants initially rested for 30-min in a seated position within the environmental chamber, which was regulated to an ambient dry bulb temperature (Tdb) of 37.5 ± 0.1 °C, RH of 34.2 ± 1.4% and vapour pressure of 16-mmHg. Environmental conditions, such as ambient Tdb, RH and air velocity (m/s), were continuously monitored approximately 120 cm from the exercising participant (Kestrel 5400 Heat Stress Tracker, Kestrel Meters, Boothwyn, PA, US). A large electric fan (SIP 24” Drum Fan, Loughborough, UK) was placed in front of the participant during the rest and exercise periods, providing an airflow of 2.0 ± 0.2 m/s, directed at the torso. During the rest period, skin thermistors (Grant Instruments Ltd., Cambridge, UK) were attached to four sites on the participant’s left side: upper chest, mid-humerus, mid-calf and mid-thigh to measure weighted mean skin temperature (Tsk). Prior to application of the skin thermistors, the skin was dry-shaved. Both Tcore and Tsk temperature were recorded using a data logger, continuously sampling every 5-s (SQ2010; Grant Instruments Ltd., Cambridge, UK). Ramanathan’s equation (Ramanathan 1964) was used to calculate mean Tsk:
After 30-min of rest in the chamber, the participants began walking on the treadmill at an individual-specific speed and gradient intended to elicit a pre-determined fixed Ḣprod (~ 200 W/m2). After 45-min of exercise (fixed-humidity exercise period), the ambient vapour pressure (mmHg) inside the environmental chamber increased by 1.5-mmHg every 5-min for an additional 60-min (ramped-humidity exercise period). The point at which an upward inflection in Tcore was observed was identified as the critical ambient vapour pressure (Pcrit), theoretically indicating the transition from a compensable to an uncompensable state (Kenney and Zeman 2002; Ravanelli et al. 2018); Figs. 2 and 3). The inflection in Tcore at the breakpoint of compensability (Pcrit) was determined using segmental linear regression of the Tcore—ambient vapour pressure relationship, which was averaged to 1-min values during the ramped-humidity exercise period (Graphpad Prism, version 5.01, La Jolla, CA). Participants were provided with 200-mL of plain water [maintained at room temperature (~ 20 °C)] after the rest period and before exercise, and 400-mL between the 45-min fixed-humidity and 60-min ramped-humidity exercise periods. Fluid intake was later accounted for when determining changes in body mass losses at selected trial stages.
During exercise, \(\dot{V}\)O2 was measured using the same breath-by-breath gas analyser. Heart rate (HR) was recorded throughout (Polar Heart Rate Monitor M400, Warwick, UK). Rating of perceived exertion (RPE) was recorded using a 6–20-point Borg scale (Borg 1982), while thermal comfort (TC) was recorded using a 7-point scale [where −3 = “much too cool”, 0 = “comfortable” and 3 = “much too warm” (Bedford 1936)]. Thermal sensation (TS) was recorded using a 9-point scale [where −4 = “very cold”, 0 = “neutral” and 4 = “very hot” (Zhang et al. 2004a)]. Perceptual data (RPE, TC and TS) were recorded at 5-min intervals during the rest and exercise periods.
Sweating measurements
Participants’ body mass was measured at multiple timepoints during the trial. Given the nature of the exercise trials, participants’ body mass was recorded whilst wearing cycling shorts, a sports bra for women, a HR monitor, with the inserted rectal thermistor and the skin thermistors fitted to the skin. Whilst this added some mass to the participant, this was consistent throughout all trials. A force plate (Type 1758A10, Kistler Instruments Ltd, Farnborough, UK) was used at sampling frequency of 1000-Hz and had a coefficient of variation of 0.05% for body mass measurements. Participants’ body mass was measured in the environmental chamber, on a hard, flat surface, immediately pre-exercise and post the 45-min fixed-humidity and 60-min ramped-humidity exercise periods.
Local sweat rate was determined using the absorbent patch technique on the left scapula, via the method reported by Peel et al. (2022). Measurements were taken during the final 5-min of the 45-min fixed-humidity exercise period and the 60-min ramped-humidity exercise period. The patch (Medipore + Pad [3 M]) was 5 cm × 5.5 cm, with an absorbent capacity of ~ 7-g. It was weighed (resolution 0.01-g; Ohaus, Navigator N24120, Nänikon Switzerland) prior to and after the 5-min skin application. Local sweat rate (mg/cm2/min) was determined using Eq. (7).
The modified iodine-paper technique was used to determine SGA on the right scapula, using the method detailed in Peel et al. (2022). In brief, 100% cotton paper (Southworth, Agawam, MA, US) was cut to 9 × 9 cm, saturated with iodine in the preceding 24-h, and then applied to the skin for 5-s at the end of the 45-min fixed-humidity exercise period and the 60-min ramped-humidity exercise period. As recommended by Peel et al. (2022), to establish SGA, the optimal area of sweat gland density within 3 × 3 cm and 1 × 1 cm areas within the 9 × 9 cm iodine paper area was determined. The optimal area was defined as the area (3 × 3 cm and 1 × 1 cm) with the highest density of recruited glands.
Partitional calorimetry
As detailed in supplementary material (Online Resource 1), heat balance parameters, such as Ḣprod, evaporative requirement for heat balance (Ėreq), evaporation at the skin surface (Ėskin) and heat storage (S; Eqs. 7–10) were estimated via partitional calorimetry (Cramer and Jay 2019). Ḣprod was also expressed relative to body surface area (DuBois and DuBois 1916).
where: λ is the latent heat vaporisation of sweat (2426 J/g).
On the assumption that blood entering and leaving the cutaneous circulation was equal to core and skin temperatures, respectively, maximum skin blood flow (SkBF) was determined as (Sawka and Young 2006):
where SH is the specific heat of the blood (~ 1 kcal/°C) and Ḣprod is expressed in kcal/min.
Supplementation
After randomisation to the placebo or experimental condition, all supplements were administered in powder form within gelatine capsules, in a double-blind manner. The capsules contained either 100% isolated taurine or placebo (100% maltodextrin) and were prepared using an analytical balance (Ohaus, Navigator N24120, Nänikon Switzerland). Participants ingested the supplements for a total of 8-days, having 50 mg/kg of body mass per day of taurine or 30 mg/kg of body mass per day of maltodextrin across the 8-day period. On day 7 of supplementation, participants performed the incremental test and ingested the supplements 1.5-h prior to exercise. On day 8 of supplementation, the participants undertook the experimental trial and ingested the supplements 30-min before entering the environmental chamber. Supplement blinding was deemed successful, as participants only guessed which condition they were in correctly 33% of the time. The taurine dosage administered in the current study was informed by published recommendations (Waldron et al. 2018b; Warnock et al. 2017) and because it has previously been demonstrated to be efficacious for thermal sweating during exercise in the heat (Page et al. 2019). The timing of ingestion was designed to elicit peak plasma taurine availability during exercise in both the incremental test and the experimental trial (Ghandforoush-Sattari et al. 2010). Both the taurine and maltodextrin were sourced from Myprotein (Manchester, UK).
Blood sampling
Venous blood samples were taken pre- and post-trial for the measurement of plasma taurine concentration and fingertip capillary blood samples were used to estimate plasma volume changes (Dill and Costill 1974). Both pre- and post-measurements were conducted in a cool room (~ 20 °C). Participants were asked to sit quietly for 10-min prior to any blood sampling, as plasma volume is affected by postural changes (Hagan et al. 1978). Blood was drawn into capillary tubes and microcuvettes (Hemocue Hb 201) for the measurement of haematocrit and haemoglobin concentration, respectively. The capillary tubes were spun in a microcentrifuge (Hawksley Neuation HCT Hematocrit Centrifuge, iFuge-HCT, Hawksley & Sons Ltd., Sussex, England) at 10,000 rev/min for 5-min and separated red cell volume was measured using a haematocrit reader (Hawksley Micro-Haematocrit Reader, Hawksley & Sons Ltd., Sussex, England). All samples were taken and measured in duplicate, with the mean value recorded for analysis. Venous blood samples were obtained via venipuncture from an antecubital vein and were drawn into three EDTA-treated vacutainer tubes (6-mL). These tubes were immediately placed on ice for 15-min before being centrifuged at 3000 rev/min for 15-min at 4 °C. The plasma was pipetted into 1.5-mL eppendorfs and stored in a − 80 °C freezer for subsequent analysis of taurine concentration.
Blood analysis
Plasma taurine concentration was measured using high performance liquid chromatography (HPLC). 100-µL of plasma sample was depleted through the addition of 400-µL of methanol and vortexed for 10-min before being centrifuged at 3000 rev/min for 5-min. The supernatant was speed vacuum concentrated to dryness at 7 °C and reconstituted in 100 µL of 0.4 M (pH 9) sodium bicarbonate buffer before being spiked with aspartic acid standard. The samples were analysed for taurine content using an agilent 1100 system utilising a pre-column derivatisation process and utilising OPA reagent. The samples were separated using a C18 column and ran using a gradient elution of 40-mM sodium phosphate buffer (pH 7.8) and ACN:MeOH:H2O (45:45:10) at 40 °C at a flow rate of 1 mL/min. Taurine that was successfully derivatised was detected using the fluorescence detector excitation 240-nm an emissions 450-nm with a PMT gain: 10 and a peak width of 0.5-min. Peak heights were used for quantifications.
Statistical analysis
A two-way repeated measures analysis of variance (RM-ANOVA) was conducted with time (rest, fixed-humidity and ramped-humidity exercise periods) and condition (taurine and placebo) as the independent variables [WBSL, LSR, SGA, Tcore, Tsk, TC, TS, RPE, HR, plasma (tau) concentration]. A Greenhouse–Geisser correction was applied when the assumption of sphericity was violated. Post hoc analysis was conducted with Bonferroni correction to identify significant pairwise comparisons if significant interaction effects were observed. Data were checked for normality using the Shapiro–Wilk test (Shapiro and Wilk 1965). Two-tailed paired samples t tests were used to identify significant differences between trials (\(\dot{V}\)O2peak, Pcrit, Tcore at Pcrit, delta Tsk during ramped-humidity, Ėskin, and heat storage during fixed-humidity and plasma volume). A Wilcoxon Signed-Rank test was performed on non-parametric data [delta Tcore during ramped-humidity, skin blood flow (SkBF)]. All statistical analysis was conducted in SPSS (IBM SPSS Statistics for Windows, IBM Corp, Version 24.0. Armonk, New York). Data are expressed as means ± SD throughout and a significance level of p < 0.05 was accepted across all tests. The magnitude of effects was calculated using Cohen’s d and partial eta squared (ηp2) using the following criteria of 0.2 and 0.02 (small effect); 0.5 and 0.13 (medium effect); and 0.8 and 0.26 (large effect) to denote differences, respectively (Cohen 1988).
Results
Thermo-physiological responses
Pcrit was greater in the taurine condition compared to placebo (t(13) = 3.817, p = 0.002, Cohen’s d = 0.97; Fig. 4). However, there was no difference in Tcore at the point of inflection (t(13) = −0.046, p = 0.964, Cohen’s d = −0.01) or in delta Tcore during the ramped-humidity exercise period (p = 0.624) between conditions.
Critical ambient vapour pressure (Pcrit)—the point at which an upward inflection in core temperature was observed—indicating the transition from a compensable to an uncompensable state during the ramp-humidity exercise period in taurine and placebo conditions (mean ± SD). Asterisk: significantly greater than placebo (p < 0.05)
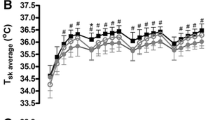
At rest and during the fixed-humidity exercise period, Tcore increased with time across both conditions (F(1.508, 21.117) = 236.585, p < 0.001, ηp2 = 0.944); however, there was no main effect of condition (F(1, 14) = 0.398, p = 0.538, ηp2 = 0.028) or an interaction between condition and time (F(1.394, 19.103) = 0.394, p = 0.601, ηp2 = 0.027). During the fixed-humidity exercise period, mean Tsk increased with time in both conditions (F(1.150, 16.106) = 15.779, p < 0.001, ηp2 = 0.530); however, there was no main effect of condition (F(1, 14) = 0.975, p = 0.340, ηp2 = 0.065) or an interaction effect with time (F(1.227, 17.173) = 3.668, p = 0.065, ηp2 = 0.208). There was also no difference in the change in mean Tsk within the ramped-humidity exercise period (t(14) = −0.435, p = 0.670, Cohen’s d = −0.08). Heart rate increased with time in both conditions during the whole trial (F(1.281, 17.937) = 95.916, p < 0.001, ηp2 = 0.873); however, there was no main effect of condition (F(1, 14) = 2.134, p = 0.166, ηp2 = 0.132) or an interaction effect (F(2.354, 32.956) = 3.101, p = 0.051, ηp2 = 0.181; Fig. 5).
Heart rate (top), skin temperature (middle) and core temperature (bottom) plotted across time in the experimental trials (mean ± SD). A Start, B rest mean, C fixed-humidity start, D fixed-humidity stage 1 mean, E fixed-humidity stage 2 mean, F fixed-humidity stage 3 mean, G ramped-humidity start, H ramped-humidity mean, I end. A, B are intentionally missing for skin temperature
There was no difference in \(\dot{V}\)O2peak taken during the incremental walking test in visits 2 and 4 (Taurine = 50.6 ± 8.0 mL/kg/min; Placebo = 50.1 ± 7.8 mL/kg/min; t(14) = 1.886, p = 0.080, Cohen’s d = 0.08) between conditions.
Sweating measurements
All sweating variables are displayed in Fig. 6. Whole-body sweat loss (F(1, 14) = 5.425, p = 0.035, ηp2 = 0.279), local sweat rate (F(1, 14) = 8.124, p = 0.013, ηp2 = 0.367) and sweat gland activation (optimal 3 × 3 cm [F(1, 14) = 7.750, p = 0.015, ηp2 = 0.356] and 1 × 1 cm [F(1, 14) = 22.525, p < 0.001, ηp2 = 0.617]) were significantly increased in the taurine condition relative to placebo. There was a time effect for WBSL, LSR and SGA (1 × 1 cm; F(1, 14) = 17.438, p < 0.001, ηp2 = 0.555; F(1, 14) = 35.639, p < 0.001, ηp2 = 0.718; F(1, 14) = 4.806, p = 0.046, ηp2 = 0.256, respectively), but not for SGA (3 × 3 cm; F(1, 14) = 4.059, p = 0.064, ηp2 = 0.225). However, there was no condition × time interaction effect for WBSL (F(1, 14) = 1.607, p = 0.226, ηp2 = 0.103), LSR (F(1, 14) = 0.077, p = 0.786, ηp2 = 0.005), SGA (3 × 3 cm; F(1, 14) = 0.035, p = 0.854, ηp2 = 0.003) and SGA (1 × 1 cm; F(1, 14) = 0.182, p = 0.676, ηp2 = 0.013).
Partitional calorimetry and skin blood flow
Evaporation from the skin surface (Ėskin; t(14) = 3.002, p = 0.010, Cohen’s d = 0.79) was increased and total heat storage decreased (t(14) = –2.537, p = 0.024, Cohen’s d = −0.87) in the taurine condition relative to placebo during the fixed-humidity exercise period (Fig. 7). Calculated SkBF (Taurine = 4.07 ± 1.02; Placebo = 4.31 ± 1.70; p = 0.650) did not differ between conditions during the fixed-humidity exercise period.
Perceptual measurements
Thermal comfort, TS and RPE increased with time in both conditions (F(2.044, 28.614) = 18.623, p < 0.001, ηp2 = 0.571; F(1.901, 26.618) = 22.955, p < 0.001, ηp2 = 0.621; F(1.834, 25.669) = 28.562, p < 0.001, ηp2 = 0.671, respectively); however, there was no main effect for condition (F(1, 14) = 0.329, p = 0.575, ηp2 = 0.023; F(1, 14) = 1.072, p = 0.318, ηp2 = 0.071; F(1, 14) = 0.639, p = 0.437, ηp2 = 0.044, respectively). There was also no interaction effect for TC (F(2.681, 37.536) = 1.753, p = 0.177, ηp2 = 0.111) or TS (F(3.263, 45.686) = 1.800, p = 0.156, ηp2 = 0.114); however, there was for RPE (F(2.445, 34.225) = 3.910, p = 0.023, ηp2 = 0.218). Post-hoc pairwise analysis revealed that RPE was only higher in the taurine condition in the last stage of the ramped-humidity exercise period (Taurine = 11 ± 2; Placebo = 10 ± 2; p = 0.035).
Plasma volume and plasma (tau) concentration
Change in plasma volume across the trial period (Taurine = 0.19 ± 8.44%; Placebo = −1.86 ± 7.67%; t(14) = 0.903, p = 0.382, Cohen’s d = 0.25), did not differ between conditions. Plasma taurine concentration, both corrected and uncorrected for plasma volume, was higher (F(1, 10) = 5.266, p = 0.045, ηp2 = 0.345; F(1, 10) = 6.389, p = 0.030, ηp2 = 0.390, respectively) in the taurine vs placebo condition. There was a condition x time interaction effect for corrected and uncorrected plasma taurine concentration (F(1, 10) = 20.212, p = 0.001, ηp2 = 0.669; F(1, 10) = 20.918, p = 0.001, ηp2 = 0.677, respectively). Post-hoc pairwise analysis revealed that both corrected and uncorrected plasma taurine concentration was only higher in the taurine condition post-trial (254 ± 198 µM vs 82 ± 59 µM, p = 0.011; 257 ± 183 µM vs 87 ± 70 µM, p = 0.006).
Discussion
We investigated the effects of 8-day oral taurine supplementation on sweating responses, Tcore, calorimetric heat transfer components and delta plasma volume during prolonged low-intensity exercise of a fixed Ḣprod at both fixed and incremental ambient vapour pressure. In acceptance of our hypothesis, taurine supplementation increased parameters of sweating (WBSL, LSR and SGA), which delayed the upward inflection of Tcore, denoted by a greater Pcrit in the taurine condition compared to placebo. However, contrary to our hypothesis, there was no effect on plasma volume or Tcore and the augmentation of the sweating response appeared to occur earlier following exercise onset, with a trend for larger changes in WBSL and LSR observed during the fixed-humidity compared to the ramped-humidity exercise period. Despite these latter results, calorimetric modelling during fixed-humidity exercise demonstrated increased latent heat dissipation (Ėskin) and, consequently, decreased heat storage in the taurine condition compared to placebo, which is of critical importance to the findings of the current study. Thus, oral taurine supplementation appears to acutely elicit thermoregulatory benefits during low-intensity, fixed Ḣprod exercise in the heat, which is related to enhanced evaporative heat exchange, secondary to an accentuated sweating response early in the exercising period.
The increases in WBSL (~ 26.6%) demonstrated during the initial fixed-humidity exercise period are similar to changes reported in WBSL in response to heat acclimation (23%; Poirier et al. 2016), thus suggesting that oral taurine supplementation also stimulates a systemic sweating response. While increases in LSR (~ 15.5%) were considerably lower than demonstrated with heat acclimation (30%; (Ravanelli et al. 2018), it is comparable with the 12.7% increase reported during higher intensity cycling in the heat following an acute taurine dose (Page et al. 2019). Interestingly, a greater recruitment of sweat glands was found in the current study following taurine supplementation compared to placebo [1 × 1 cm (32.2%) and 3 × 3 cm (22.1%)], which is in a similar range to the changes reported following 8 days of heat acclimation involving 90-min treadmill walking at 70% maximum HR in environmental conditions of 38 °C and 65% RH (27.9%; Ravanelli et al. 2018). These changes observed using the absorbent patch and modified iodine-paper techniques are above their established coefficient of variation (12.8 ± 4.8% and 15.9–24.1 ± 9.6–10.8%, respectively) when exercising at 200 W/m2 Ḣprod (Peel et al. 2022), demonstrating a genuine increase in the response. It is noteworthy that the augmented sweating responses reported herein during low-intensity exercise occurred prior to any substantial changes in Tcore, indicating a marked temporal mismatch between the internal thermal stimulus and the enhanced sudomotor responses. However, it should be noted that changes in Tcore and sweating do not always correspond precisely, due to interplay with additional drivers of the sweating response (Bain et al. 2011; Cramer et al. 2012; Jay et al. 2011). Indeed, there were substantially lower effects of taurine on WBSL (5.1% increase), LSR (7.8% increase) and SGA (1 × 1 cm, 29.9%; 3 × 3 cm, 17.1%) during the ramped-humidity exercise period, compared to earlier in the trial. Therefore, advancing upon previous reports (Page et al. 2019), we demonstrate here that oral taurine supplementation stimulates early onset sweating through increased recruitment of sweat glands during exercise in the heat. This apparent early onset of sweating is somewhat different to strategies that have established effects on thermal sweating, such as heat acclimation, where greater magnitude and more sustained sweating responses are apparent (Ravanelli et al. 2018). Nevertheless, the potential importance of this finding to human thermoregulation is underscored by the translation to an improved evaporative cooling capacity (Ėskin) in the taurine condition compared to placebo (595 W vs 470 W; 27% difference) and lowered rate of heat storage (30 W vs 108 W; 72% difference).
Owing to the nature of the current experimental protocol, we were also able to determine the effect of taurine supplementation on the breakpoint of compensability during the ramped-humidity exercise period. This threshold is important because, without changes to the exercising intensity or thermal stress, an early transition (such as that found with unacclimated people; Ravanelli et al. 2018) will lead to progressive increases in Tcore until critical temperatures are reached. We revealed that taurine delayed the point of uncompensable heat stress, denoted by a rightward shift in the Pcrit value (Fig. 4; 25.0-mmHg vs 21.7-mmHg). A compensable state was maintained to a higher ambient vapour pressure, most likely due to the earlier greater evaporative cooling. Therefore, the early effects of taurine on evaporative heat transfer in the fixed-humidity trial appear to cause a latent enhancement in heat tolerability, which manifested only in response to a progressive increase in the ambient humidity during the ramped segment. Despite this, Tcore was not significantly different between conditions at the breakpoint of compensability (Pcrit) or at any timepoint throughout the trial. There was, however, a trend for a lower core temperature in the taurine condition during both exercise periods, indicating a sustained reduction in heat storage, as demonstrated above.
A novel aspect, and advantage, of the current study was the experimental control of Ḣprod and Ėreq, which are known drivers of thermal sweating (Cramer and Jay 2014, 2016; Gagnon et al. 2013; Peel et al. 2022). This control of the work intensities, metabolic profile and environmental constraints was important, since it is feasible that the 8-day taurine supplementation period could have affected the metabolic response to exercise (Rutherford et al. 2010; Simmonds et al. 2022; Zhang et al. 2004b) and subsequent self-paced work-rates if other exercise models were adopted. Given that the supplementation periods occurred prior to the fixed-humidity and ramped-humidity trials, poor control of these factors would have been sufficient to explain any changes in sweating response. That taurine supplementation did not meaningfully change \(\dot{V}\)O2max or the \(\dot{V}\)O2-WR relationship in the incremental tests that preceded the heat trials, as well as maintaining its effect on thermal sweating responses under control of Ḣprod and Ėreq, experimentally rules out changes in whole-body metabolism as a viable explanation. However, it remains possible that taurine elicited an endurance training-like effect, as reported previously (Waldron et al. 2018a, 2019; Zhang et al. 2004b) that was not recognised by the limited assessment of these characteristics herein. Indeed, endurance training is known to induce partial heat acclimation (Kobayashi et al. 1980), with lower Tcore, increased SGA and sweat rates following 8-weeks of aerobic training (Ravanelli et al. 2018). Therefore, it remains possible that a currently unrecognised enhancement of the endurance phenotype is partly responsible for a change in the sweating response after taurine supplementation.
Given the involvement of numerous physiological mechanisms in stimulating eccrine sweat production (Shibasaki and Crandall 2010) and the wide-spread bioavailability of taurine (HuxTable 1992), there are some potential mechanisms that require further investigation. A primary biological role of taurine is as an osmolyte (Cuisinier et al. 2002; HuxTable 1992), with taurine transporters (TauT) ubiquitously expressed in many tissues, including the kidney (Baliou et al. 2020; Han et al. 2000, 2006; Ito et al. 2010). Thus, taurine has potential to affect fluid regulation at the cellular and organ level. Indeed, exercise-induced changes in endogenous plasma taurine concentrations are related to osmoregulatory function during endurance exercise (Cuisinier et al. 2001; Ward et al. 1999), where it is actively extruded from skeletal muscle cells (Graham et al. 1991, 1995) to maintain intracellular osmolality (Lang et al. 1998; Sejersted and Sjøgaard 2000; Stutzin et al. 1999). However, endogenous taurine (i.e. without oral supplementation) has not been reported to affect plasma volume (Cuisinier et al. 2002) and, herein, we also found no difference in the plasma volume changes across the exercise period. However, given that the taurine condition lost a greater amount of fluid through sweating, and fluid ingestion was equal between conditions, the matching of plasma volume changes between conditions perhaps indicates a regulatory role of exogenous taurine in maintaining plasma volume, despite the additional fluid losses. Given that taurine is extruded from myocytes to the extracellular space during exercise to prevent cell swelling (Stutzin et al. 1999), a greater osmotic gradient and fluid availability in the extracellular compartments to maintain plasma volume is entirely feasible. The consequence of these findings on all fluid compartments during exercise in the heat is uncertain, however, and requires further research. It is unfortunate that plasma volume measurements were not more frequent, as earlier transient changes might have occurred in tandem with the early sweating onset but will not have been identifiable. Theoretically, plasma volume maintenance may have augmented sweating via preservation of SkBF (Nagashima et al. 1998; Nielsen et al. 1984), fluid availability and supply to the sweat gland (Fortney et al. 1981; Wong and Hollowed 2017) or a change in osmoreceptor or baroreceptor signalling (Mack et al. 1995; Shibasaki et al. 2003).
Estimated whole-body SkBF was not different between conditions. This result was unanticipated, as taurine acts peripherally, as a vaso-relaxant, with TauT abundantly expressed in vascular smooth muscle (Liao et al. 2007). Supplementation has been demonstrated to improve both endothelium dependant and independent vasodilation (Maia et al. 2014; Sun et al. 2016; Ulusoy et al. 2017), through increased nitric oxide bioavailability, restoration of vascular redox homeostasis and calcium activated potassium channel opening action (Maia et al. 2014; Ulusoy et al. 2017). Its apparent homeostatic function appears to play a role in vascular tone, by promoting both vasodilation and vasoconstriction, to increase blood flow during ischemia, hypoxia or heat stress and maintain blood pressure (Nishida and Satoh 2009). An association between sweating and SkBF has been established (Van Beaumont and Bullard 1965; Nadel et al. 1971a, 1971b; Brengelmann et al. 1973), suggesting a functional inter-relationship (Wong and Hollowed 2017). However, findings by Ravanelli et al. (2017) demonstrate that increases in SkBF are not a prerequisite for increases in LSR, at least acutely. This is supported by the results herein, as the similarity in estimated whole-body SkBF between the taurine and placebo condition demonstrates no apparent vascular effect, despite the concomitant increased sweating response. While this may be the case, attenuation of SkBF through arterial occlusion (MacIntyre et al. 1968; Collins et al. 1959) or pharmacological blockade (Wingo et al. 2010) reduces the sweating response during heat stress, suggesting a requirement of SkBF for sustained sweating. Future analysis of cutaneous vascular conductance using laser Doppler flowmetry would more conclusively establish whether additional SkBF is required to continue to supply the sweat gland during prolonged periods of sweating and whether taurine supplementation facilitates this.
This study characterised multiple measures of the sweating response (WBSL, LSR and SGA), which demonstrated changes of a large magnitude after taurine supplementation. This enhancement occurring in the earlier segments of the current trial, prior to any marked heat strain, requires mechanistic reasoning and may be explained by a centrally mediated alteration in thermoregulatory set-point. Indeed, whilst taurine supplementation did not significantly affect Tcore, there were clear effects on the domain of compensability in the current study, and the enhanced early sweating response occurred alongside increased sweat gland recruitment, providing further evidence for an alteration in the thermoregulatory feedback loop. This has been suggested previously in exercising humans to explain early onset of sweating in response to high-dose (50 mg/kg/bm) taurine supplementation (Page et al. 2019). Additionally, another potential mechanism for the increased sweating observed is through taurine antagonism of antidiuretic hormone (ADH) or arginine vasopressin (AVP). Vasopressin is an antidiuretic hormone produced in the supraoptic nucleus of the hypothalamus and released by the posterior pituitary gland in response to plasma hyperosmolality (Cunningham and Sawchenko 1991; Bourque et al. 1994; Richard and Bourque 1995). Both potential mechanisms can be linked to taurine’s role as a neuromodulator, where it acts as a glycine and GABA receptor agonist and appears to have multiple roles in maintaining homeostasis during periods of perturbation (Jia et al. 2008; Schmieden et al. 1992; Hussy et al. 1997). Indeed, it functions to protect neurons from toxicity by modulating thalamic network activity under conditions of homeostatic derangement, which are associated with severe pathological conditions (Jia et al. 2008) and may be extended to transient states of heat stress. It has been identified in the animal model that GABA and taurine are released from some hypothalamic cells into the cerebrospinal fluid during thermal strain, which coincides with reductions in Tcore (Frosini et al. 2000). Following oral supplementation, the increased plasma taurine is available to cross the blood–brain barrier via TauT (Kang 2002), and act on hypothalamic regions of the brain, potentially interacting with a specific taurine binding site (i.e. putative Taurinergic pathway) or GABA receptors (Frosini et al. 2003; Quéva et al. 2003). Whilst it remains speculative, in the exercising, thermally-stressed human, we propose that the established cryogenic effect of these pathways (Frosini et al. 2003; Elhussiny et al. 2021) may translate to enhanced sudomotor function, as the major effector response to heat stress. Similarly, taurine’s release from the hypothalamus in response to plasma hypoosmolality and its agonism of glycine receptors is suggested to exert an inhibitory effect on ADH secretion (Hussy et al. 1997; Miyata et al. 1997; Deleuze et al. 1998), thereby promoting increased fluid loss. Due to their many similarities, it has been suggested that ADH may facilitate fluid reabsorption at the sweat gland, as it does in the kidney, through its action on vasopressin subtype 2 receptors (V2 receptors), promoting fluid and sodium reabsorption to maintain fluid homeostasis (Agu 2017; Hew-Butler 2010; Baker 2019). As such, exogenous taurine supplementation may supress the release of ADH and attenuate water reabsorption at the sweat gland, leading to greater fluid loss. In the rat model, subcutaneous injection of ADH reduced initial sweat rate by 50%, suggesting a role for ADH in regulating the sweating response (Quatrale and Speir 1970). Further, in exercising humans, a positive association between plasma ADH and sweat sodium concentrations has been reported, indicating it may have a potential role in fluid retention at the sweat gland (Hew-Butler 2010). Nevertheless, several studies both augmenting and suppressing ADH have observed no significant change in sweat rate during exercise or heat exposure (Pearcy et al. 1956; Senay and Van Beaumont 1969; Ratner and Dobson 1964; Gibiński et al. 1979; Taussig and Braunstein 1973; Allen and Roddie 1974; Hew-Butler et al. 2014). However, none of these studies have investigated the effect of ADH on the sweating response during exercise in the heat when fluid intake is regulated, and other drivers of the sweating response are controlled (e.g. Ḣprod and Ėreq). Therefore, this remains a plausible pathway in which exogenous taurine supplementation augments the thermal sweating response and requires further investigation. Whilst the above mechanisms of action provide a potential explanation for the current results, it is reasonable to suggest that the increased sweating response occurs owing to a combination of several factors, which is consistent with the numerous biological roles ascribed to taurine (HuxTable 1992). In-vivo investigation of the above central mechanisms is likely to be challenging but could be addressed in future research.
High environmental heat stress alongside physical exertion where compensatory limits for heat dissipation are exceeded, pose a risk for serious heat illness due to inexorable increases in Tcore (Howe and Boden 2007; Epstein and Yanovich 2019). Athletes and military personnel are often required to perform endurance exercise in such conditions (Ely et al. 2008; Racinais et al. 2015; World and Booth 2008; Parsons et al. 2019) and, therefore, a strategy (e.g. taurine supplementation) to help offset this rise in Tcore, and the risk of heat illness could be important in reducing its prevalence. To the authors’ knowledge, there are no known serious side effects of taurine supplementation at doses of up to 10-g (Shao and Hathcock 2008). Therefore, supplementing 50 mg/kg/bm of taurine prior to heat exposure might be a useful strategy to offset the deleterious effects of heat stress among healthy populations. However, further research is required to better understand the efficacy of its thermoregulatory role in applied settings. Thus, findings that oral taurine supplementation can acutely increase the sweating response, augment evaporative heat transfer, and reduce heat storage during low-intensity exercise in the heat has many promising future applications.
Conclusion
Eight days of oral taurine supplementation (50 mg/kg/bm) increased sweating responses (WBSL, LSR and SGA) during low-intensity exercise of a fixed Ḣprod in the heat. The subsequent enhanced evaporative cooling (Ėskin) and reduced heat storage delayed the upward inflection in Tcore—represented by a greater Pcrit—demonstrated during the exercise period of incremental ambient vapour pressure. Despite this, there was only a trend towards a lower Tcore throughout the trial, and reduced changes in WBSL and LSR during the ramped-humidity exercise period. This apparent early augmentation of the sweating response appears to offer thermoregulatory benefits for latter parts of an exercising period. These findings have potential implications for both athletes and military personnel performing exercise in hot environmental conditions that permit sufficient latent heat transfer. The experimental control of the thermal drivers of sweating (Ḣprod and Ėreq) suggests that other mechanisms are likely to be responsible for the observed increase in sweating. We suggest several possibilities to direct future investigations, which will help to elucidate the mechanistic actions of taurine during exercising-heat stress.
Abbreviations
- ADH:
-
Antidiuretic hormone
- AVP:
-
Arginine vasopressin
- BSA:
-
Body surface area
- Ė max :
-
Maximal evaporative heat transfer capacity
- Ė req :
-
Evaporation required to maintain heat balance
- Ė skin :
-
Evaporative heat transfer
- Ḣ prod :
-
Heat production
- HR:
-
Heart rate
- LSR:
-
Local sweat rate
- Ṁ :
-
Metabolic energy expenditure
- P crit :
-
Breakpoint of uncompensability
- RER:
-
Respiratory exchange ratio
- RH:
-
Relative humidity
- RM-ANOVA:
-
Repeated measures analysis of variance
- RPE:
-
Rating of perceived exertion
- SGA:
-
Sweat gland activation
- TauT:
-
Taurine transporters
- TC:
-
Thermal comfort
- T core :
-
Core temperature
- T db :
-
Dry bulb temperature
- TS:
-
Thermal sensation
- T sk :
-
Skin temperature
- \(\dot{V}\)O2max :
-
Maximal oxygen uptake
- V2 receptors:
-
Vasopressin subtype 2 receptors
- WBSL:
-
Whole-body sweat loss
- Wk:
-
Mechanical work
References
Agu KA (2017) Can sweat glands act as temporary or permanent replacement for the excretory function of kidney? Emerg Life Sci Res 3:37–41
Allen JA, Roddie I (1974) The effect of antidiuretic hormone on human sweating. J Physiol 236(2):403
Bain AR, Deren TM, Jay O (2011) Describing individual variation in local sweating during exercise in a temperate environment. Eur J Appl Physiol 111(8):1599–1607
Baker LB (2019) Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 6(3):211–259
Baliou S, Kyriakopoulos AM, Goulielmaki M, Panayiotidis MI, Spandidos DA, Zoumpourlis V (2020) Significance of taurine transporter (TauT) in homeostasis and its layers of regulation. Mol Med Rep 22(3):2163–2173
Balshaw TG, Bampouras TM, Barry TJ, Sparks SA (2013) The effect of acute taurine ingestion on 3-km running performance in trained middle-distance runners. Amino Acids 44(2):555–561
Bedford T (1936) The warmth factor in comfort at work. A physiological study of heating and ventilation, vol 76
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377
Bourque CW, Oliet SH, Richard D (1994) Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol 15(3):231–274
Brengelmann GL, Wyss C, Rowell LB (1973) Control of forearm skin blood flow during periods of steadily increasing skin temperature. J Appl Physiol 35(1):77–84
Che Muhamed AM, Atkins K, Stannard SR, Mündel T, Thompson MW (2016) The effects of a systematic increase in relative humidity on thermoregulatory and circulatory responses during prolonged running exercise in the heat. Temperature 3(3):455–464
Cohen J (1988) The effect size. In: Statistical power analysis for the behavioral sciences, pp 77–83
Collins K, Sargent F, Weiner J (1959) The effect of arterial occlusion on sweat-gland responses in the human forearm. J Physiol 148(3):615
Cramer MN, Jay O (2014) Selecting the correct exercise intensity for unbiased comparisons of thermoregulatory responses between groups of different mass and surface area. J Appl Physiol 116(9):1123–1132
Cramer MN, Jay O (2016) Biophysical aspects of human thermoregulation during heat stress. Auton Neurosci 196:3–13
Cramer MN, Jay O (2019) Partitional calorimetry. J Appl Physiol 126(2):267–277
Cramer MN, Bain AR, Jay O (2012) Local sweating on the forehead, but not forearm, is influenced by aerobic fitness independently of heat balance requirements during exercise. Exp Physiol 97(5):572–582
Cuisinier C, Ward RJ, Francaux M, Sturbois X, De Witte P (2001) Changes in plasma and urinary taurine and amino acids in runners immediately and 24 h after a marathon. Amino Acids 20:13–23
Cuisinier C, Michotte de Welle J, Verbeeck RK, Poortmans JR, Ward R, Sturbois X, Francaux M (2002) Role of taurine in osmoregulation during endurance exercise. Eur J Appl Physiol 87(6):489–495
Cunningham ET Jr, Sawchenko PE (1991) Reflex control of magnocellular vasopressin and oxytocin secretion. Trends Neurosci 14(9):406–411
Deleuze C, Duvoid A, Hussy N (1998) Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol 507(2):463
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37(2):247–248
DuBois D, DuBois EF (1916) Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17(6–2):863–871
Dutka TL, Lamboley CR, Murphy RM, Lamb GD (2014) Acute effects of taurine on sarcoplasmic reticulum Ca2+ accumulation and contractility in human type I and type II skeletal muscle fibers. J Appl Physiol 117(7):797–805
Elhussiny MZ, Tran PV, Pham CV, Nguyen LT, Haraguchi S, Gilbert ER, Cline MA, Bungo T, Furuse M, Chowdhury VS (2021) Central GABAA receptor mediates taurine-induced hypothermia and possibly reduces food intake in thermo-neutral chicks and regulates plasma metabolites in heat-exposed chicks. J Therm Biol 98:102905
Ely MR, Martin DE, Cheuvront SN, Montain SJ (2008) Effect of ambient temperature on marathon pacing is dependent on runner ability. Med Sci Sports Exerc 40(9):1675–1680
Epstein Y, Yanovich R (2019) Heatstroke. N Engl J Med 380(25):2449–2459
Fortney S, Nadel E, Wenger C, Bove J (1981) Effect of blood volume on sweating rate and body fluids in exercising humans. J Appl Physiol 51(6):1594–1600
Frosini M, Sesti C, Palmi M, Valoti M, Fusi F, Mantovani P, Bianchi L, Della Corte L, Sgaragli G (2000) Heat-stress-induced hyperthermia alters CSF osmolality and composition in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 279(6):2095–2103
Frosini M, Sesti C, Saponara S, Ricci L, Valoti M, Palmi M, Machetti F, Sgaragli G (2003) A specific taurine recognition site in the rabbit brain is responsible for taurine effects on thermoregulation. Br J Pharmacol 139(3):487–494
Gagge A, Gonzalez R (1996) Handbook of physiology. Environmental physiology. American Physiological Society, Bethesda, pp 45–84
Gagnon D, Jay O, Kenny GP (2013) The evaporative requirement for heat balance determines whole-body sweat rate during exercise under conditions permitting full evaporation. J Physiol 591(11):2925–2935
Ghandforoush-Sattari M, Mashayekhi S, Krishna CV, Thompson JP, Routledge PA (2010) Pharmacokinetics of oral taurine in healthy volunteers. J Amino Acids 2010:346237
Gibiński K, Kozłowski S, Chwalbińska-Moneta J, Giec L, Żmudziński J, Markiewicz A (1979) ADH and thermal sweating. Eur J Appl Physiol Occup Physiol 42:1–13
Graham TE, Kiens B, Hargreaves M, Richter EA (1991) Influence of fatty acids on ammonia and amino acid flux from active human muscle. Am J Physiol Endocrinol Metab 261(2):168–176
Graham TE, Turcotte LP, Kiens B, Richter EA (1995) Training and muscle ammonia and amino acid metabolism in humans during prolonged exercise. J Appl Physiol 78(2):725–735
Hagan R, Diaz F, Horvath S (1978) Plasma volume changes with movement to supine and standing positions. J Appl Physiol 45(3):414–417
Hamilton E, Berg H, Easton C, Bakker AJ (2006) The effect of taurine depletion on the contractile properties and fatigue in fast-twitch skeletal muscle of the mouse. Amino Acids 31(3):273–278
Han X, Budreau A, Chesney R (2000) The taurine transporter gene and its role in renal development. Amino Acids 19(3–4):499–507
Han X, Patters A, Jones D, Zelikovic I, Chesney R (2006) The taurine transporter: mechanisms of regulation. Acta Physiol 187(1–2):61–73
Hansen SH, Andersen ML, Birkedal H, Cornett C, Wibrand F (2006) The important role of taurine in oxidative metabolism. Taurine 6:129–135
Hansen SH, Andersen ML, Cornett C, Gradinaru R, Grunnet N (2010) A role for taurine in mitochondrial function. J Biomed Sci 17(1):1–8
Hew-Butler T (2010) Arginine vasopressin, fluid balance and exercise: is exercise-associated hyponatraemia a disorder of arginine vasopressin secretion? Sports Med 40:459–479
Hew-Butler T, Hummel J, Rider BC, Verbalis JG (2014) Characterization of the effects of the vasopressin V2 receptor on sweating, fluid balance, and performance during exercise. Am J Physiol Regul Integr Comp Phys 30(4):366–375
Howe AS, Boden BP (2007) Heat-related illness in athletes. Am J Sports Med 35(8):1384–1395
Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, Moos F (1997) Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol 502(3):609–621
Hussy N, Deleuze C, Desarménien MG, Moos FC (2000) Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol 62(2):113–134
Huxtable R (1992) Physiological actions of taurine. Physiol Rev 72(1):101–163
Ito T, Oishi S, Takai M, Kimura Y, Uozumi Y, Fujio Y, Schaffer SW, Azuma J (2010) Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. J Biomed Sci 17:1–5
Jay O, Bain AR, Deren TM, Sacheli M, Cramer MN (2011) Large differences in peak oxygen uptake do not independently alter changes in core temperature and sweating during exercise. Am J Physiol Regul Integr Comp Phys 301(3):832–841
Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL (2008) Taurine is a potent activator of extrasynaptic GABAA receptors in the thalamus. J Neurosci 28(1):106–115
Jong CJ, Sandal P, Schaffer SW (2021) The role of taurine in mitochondria health: more than just an antioxidant. Molecules 26(16):4913
Kang Y-S (2002) Taurine transport mechanism through the bloos-brain barrier in spontaneously hypertensive rats. In: Taurine 4: taurine and excitable tissues, pp 321–324
Kenney WL, Zeman MJ (2002) Psychrometric limits and critical evaporative coefficients for unacclimated men and women. J Appl Physiol 92(6):2256–2263
Kobayashi Y, Ando Y, Takeuchi S, Takemura K, Okuda N, Isobe Y, Takaba S, Ohara K (1980) Effects of heat acclimation of distance runners in a moderately hot environment. Eur J Appl Physiol Occup Physiol 45:189–198
Lang F, Busch GL, Völkl H (1998) The diversity of volume regulatory mechanisms. Cell Physiol Biochem 8(1–2):1–45
Liao X-B Zhou X-M, Li J-M, Tan Z-P, Liu L-M, Zhang W, Tan H, Lu Y, Yuan L-Q (2007) Taurine transporter is expressed in vascular smooth muscle cells. Amino Acids 33:639–643
Lima-Silva A, Bertuzzi RC, Pires FO, Gagliardi JF, Barros RV, Hammond J, Kiss MA (2010) Relationship between training status and maximal fat oxidation rate. J Sport Sci Med 9(1):31
MacIntyre B, Bullard R, Banerjee M, Elizondo R (1968) Mechanism of enhancement of eccrine sweating by localized heating. J Appl Physiol 25(3):255–260
Mack G, Nishiyasu T, Shi X (1995) Baroreceptor modulation of cutaneous vasodilator and sudomotor responses to thermal stress in humans. J Physiol 483(2):537–547
Maia AR, Batista TM, Victorio JA, Clerici SP, Delbin MA, Carneiro EM, Davel AP (2014) Taurine supplementation reduces blood pressure and prevents endothelial dysfunction and oxidative stress in post-weaning protein-restricted rats. PLoS ONE 9(8):e105851
Marino FE, Mbambo Z, Kortekaas E, Wilson G, Lambert MI, Noakes TD, Dennis SC (2000) Advantages of smaller body mass during distance running in warm, humid environments. Pflugers Arch 441(2):359–367
Miyata S, Matsushima O, Hatton GI (1997) Taurine in rat posterior pituitary: localization in astrocytes and selective release by hypoosmotic stimulation. J Comp Neurol 381(4):513–523
Nadel ER, Bullard RW, Stolwijk J (1971a) Importance of skin temperature in the regulation of sweating. J Appl Physiol 31(1):80–87
Nadel ER, Mitchell JW, Saltin B, Stolwijk J (1971b) Peripheral modifications to the central drive for sweating. J Appl Physiol 31(6):828–833
Nagashima K, Nose H, Takamata A, Morimoto T (1998) Effect of continuous negative-pressure breathing on skin blood flow during exercise in a hot environment. J Appl Physiol 84(6):1845–1851
Nielsen B, Rowell LB, Bonde-Petersen F (1984) Cardiovascular responses to heat stress and blood volume displacements during exercise in man. Eur J Appl Physiol Occup Physiol 52(4):370–374
Nishida S, Satoh H (2009) Vascular modulation of rat aorta by taurine. Taurine 7:37–46
Page LK, Jeffries O, Waldron M (2019) Acute taurine supplementation enhances thermoregulation and endurance cycling performance in the heat. Eur J Sport Sci 19(8):1101–1109
Parsons K (2007) Human thermal environments: the effects of hot, moderate, and cold environments on human health, comfort and performance. CRC Press, New York
Parsons IT, Stacey MJ, Woods DR (2019) Heat adaptation in military personnel: mitigating risk, maximizing performance. Front Physiol 10:1485
Pearcy M, Robinson S, Miller D, Thomas J Jr, Debrota J (1956) Effects of dehydration, salt depletion and pitressin on sweat rate and urine flow. J Appl Physiol 8(6):621–626
Peel JS, McNarry MA, Heffernan SM, Nevola VR, Kilduff LP, Waldron M (2022) Measurement of thermal sweating at rest and steady-state exercise in healthy adults: Inter-day reliability and relationships with components of partitional calorimetry. PLoS ONE 17(12):e0278652
Poirier MP, Gagnon D, Kenny GP (2016) Local versus whole-body sweating adaptations following 14 days of traditional heat acclimation. Appl Physiol Nutr Metab 41(8):816–824
Quatrale RP, Speir EH (1970) The effect of ADH on eccrine sweating in the rat. J Invest Dermatol 55(5):344–349
Quéva C, Bremner-Danielsen M, Edlund A, Jonas Ekstrand A, Elg S, Erickson S, Johansson T, Lehmann A, Mattsson JP (2003) Effects of GABA agonists on body temperature regulation in GABAB (1)−/− mice. Br J Pharmacol 140(2):315–322
Racinais S, Alonso J-M, Coutts AJ, Flouris AD, Girard O, González-Alonso J, Hausswirth C, Jay O, Lee JK, Mitchell N (2015) Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports 25:6–19
Ramanathan N (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19(3):531–533
Ratner AC, Dobson RL (1964) The effect of antidiuretic hormone on sweating. J Invest Dermatol 43:379–381
Ravanelli N, Jay O, Gagnon D (2017) Sustained increases in skin blood flow are not a prerequisite to initiate sweating during passive heat exposure. Am J Physiol Regul Integr Comp Phys 313(2):140–148
Ravanelli N, Coombs GB, Imbeault P, Jay O (2018) Maximum skin wettedness after aerobic training with and without heat acclimation. Med Sci Sports Exerc 50(2):299–307
Richard D, Bourque CW (1995) Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol 489(2):567–577
Rutherford JA, Spriet LL, Stellingwerff T (2010) The effect of acute taurine ingestion on endurance performance and metabolism in well-trained cyclists. Int J Sport Nutr Exerc Metab 20(4):5
Sawka MN, Young AJ (2006) Physiological systems and their responses to conditions of heat and cold. Acsm’s Adv Exerc Physiol 26:535–563
Schaffer SW, Jong CJ, Ramila K, Ito T, Kramer J (2022) Differences between physiological and pharmacological actions of taurine. Taurine 12. Adv Exp Med Biol 1370:311–321
Schmieden V, Kuhse J, Betz H (1992) Agonist pharmacology of neonatal and adult glycine receptor alpha subunits: identification of amino acid residues involved in taurine activation. EMBO 11(6):2025–2032
Sejersted OM, Sjøgaard G (2000) Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev 80(4):1411–1481
Senay L Jr, Van Beaumont W (1969) Antidiuretic hormone and evaporative weight loss during heat stress. Pflugers Arch 312(3):82–90
Shao A, Hathcock JN (2008) Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regul Toxicol Pharmacol 50(3):376–399
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biome 52(3/4):591–611
Shibasaki M, Crandall CG (2010) Mechanisms and controllers of eccrine sweating in humans. Front Biosci (schol Ed) 2:685
Shibasaki M, Kondo N, Crandall CG (2003) Non-thermoregulatory modulation of sweating in humans. Exerc Sport Sci Rev 31(1):34–39
Simmonds R, Cole J, Tallent J, Jeffries O, Theis N, Waldron M (2022) Physiological and thermoregulatory effects of oral taurine supplementation on exercise tolerance during forced convective cooling. Eur J Sport Sci 22(2):209–217
Stutzin A, Torres R, Oporto M, Pacheco P, Eguiguren AL, Cid LP, Sepúlveda FV (1999) Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am J Physiol Cell Physiol 277(3):C392–C402
Sun Q, Wang B, Li Y, Sun F, Li P, Xia W, Zhou X, Li Q, Wang X, Chen J (2016) Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: randomized, double-blind, placebo-controlled study. Hypertension 67(3):541–549
Taussig LM, Braunstein GD (1973) Effects of vasopressin on sweat rate and composition in patients with diabetes insipidus and normal controls. J Invest Dermatol 60(4):197–202
Ulusoy KG, Kaya E, Karabacak K, Seyrek M, Duvan İ, Yildirim V, Yildiz O (2017) Taurine relaxes human radial artery through potassium channel opening action. Korean J Physiol Pharmacol 21(6):617–623
Van Beaumont W, Bullard RW (1965) Sweating: direct influence of skin temperature. Science 147(3664):1465–1467
Waldron M, Knight F, Tallent J, Patterson S, Jeffries O (2018a) The effects of taurine on repeat sprint cycling after low or high cadence exhaustive exercise in females. Amino Acids 50(6):663–669
Waldron M, Patterson SD, Tallent J, Jeffries O (2018b) The effects of an oral taurine dose and supplementation period on endurance exercise performance in humans: a meta-analysis. Sports Med 48(5):1247–1253
Waldron M, Patterson SD, Jeffries O (2019) Oral taurine improves critical power and severe-intensity exercise tolerance. Amino Acids 51(10):1433–1441
Waldron M, Fowler R, Heffernan S, Tallent J, Kilduff L, Jeffries O (2021) Effects of heat acclimation and acclimatisation on maximal aerobic capacity compared to exercise alone in both thermoneutral and hot environments: a meta-analysis and meta-regression. Sports Med 51(7):1509–1525
Ward RJ, Francaux M, Cuisinier C, Sturbois X, De Witte P (1999) Changes in plasma taurine levels after different endurance events. Amino Acids 16:71–77
Warnock R, Jeffries O, Patterson S, Waldron M (2017) The effects of caffeine, taurine, or caffeine-taurine coingestion on repeat-sprint cycling performance and physiological responses. Int J Sports Physiol Perform 12(10):1341–1347
Wenger CB (1972) Heat of evaporation of sweat: thermodynamic considerations. J Appl Physiol 32(4):456–459
Wingo JE, Low DA, Keller DM, Brothers RM, Shibasaki M, Crandall CG (2010) Skin blood flow and local temperature independently modify sweat rate during passive heat stress in humans. J Appl Physiol 109(5):1301–1306
Wong BJ, Hollowed CG (2017) Current concepts of active vasodilation in human skin. Temperature 4(1):41–59
World MJ, Booth TC (2008) Iraq: the environmental challenge to HM Land Forces. Clin Med (Northfield IL) 8(4):399
Zhang H, Huizenga C, Arens E, Wang D (2004a) Thermal sensation and comfort in transient non-uniform thermal environments. Eur J Appl Physiol 92(6):728–733
Zhang M, Izumi I, Kagamimori S, Sokejima S, Yamagami T, Liu Z, Qi B (2004b) Role of taurine supplementation to prevent exercise-induced oxidative stress in healthy young men. Amino Acids 26(2):203–207
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
JP, MM, SH, RN, LK and MW conceived and designed research. JP conducted the data collection. KC and ED contributed analytical tools. JP, KC, ED and MW analysed the data. JP wrote the manuscript. All authors read, revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Communicated by Narihiko Kondo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peel, J.S., McNarry, M.A., Heffernan, S.M. et al. The effect of 8-day oral taurine supplementation on thermoregulation during low-intensity exercise at fixed heat production in hot conditions of incremental humidity. Eur J Appl Physiol (2024). https://doi.org/10.1007/s00421-024-05478-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00421-024-05478-3