Abstract
Purpose
To determine if 7d of New Zealand blackcurrant (NZBC) extract alters the heat shock, inflammatory and apoptotic response during prolonged exertional-heat stress.
Methods
Ten men (Age: 29 ± 2 years, Stature: 1.82 ± 0.02 m, Mass: 80.3 ± 2.7 kg, V̇O2max: 56 ± 2 mL·kg−1·min−1) ingested two capsules of CurraNZ™ (NZBC extract: 210 mg anthocyanins·day−1) or PLACEBO for 7d prior to 1 h treadmill run (65% V̇O2max) in hot ambient conditions (34 °C/40% RH). Blood samples were collected before (Pre), immediately after (Post), 1 h after (1-Post), and 4 h after (4-Post) exercise. Heat shock proteins (HSP90, HSP70, HSP32) were measured in plasma. HSP and protein markers of inflammatory capacity (TLR4, NF-κB) and apoptosis (BAX/BCL-2, Caspase 9) were measured in peripheral blood mononuclear cells (PBMC).
Results
eHSP32 was elevated at baseline in NZBC(+ 31%; p < 0.001). In PLACEBO HSP32 content in PBMC was elevated at 4-Post(+ 98%; p = 0.002), whereas in NZBC it fell at Post(− 45%; p = 0.030) and 1-Post(− 48%; p = 0.026). eHSP70 was increased at Post in PLACEBO(+ 55.6%, p = 0.001) and NZBC (+ 50.7%, p = 0.010). eHSP90 was increased at Post(+ 77.9%, p < 0.001) and 1-Post(+ 73.2%, p < 0.001) in PLACEBO, with similar increases being shown in NZBC (+ 49.0%, p = 0.006 and + 66.2%, p = 0.001; respectively). TLR4 and NF-κB were both elevated in NZBC at PRE(+ 54%, p = 0.003 and + 57%, p = 0.004; respectively). Main effects of study condition were also shown for BAX/BCL-2(p = 0.025) and Caspase 9 (p = 0.043); both were higher in NZBC.
Conclusion
7d of NZBC extract supplementation increased eHSP32 and PBMC HSP32 content. It also increased inflammatory and apoptotic markers in PBMC, suggesting that NZBC supports the putative inflammatory response that accompanies exertional-heat stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise in hot environments confers direct (thermal) and indirect (splanchnic vasoconstriction) stress on the gastrointestinal mucosa (Rowell 1974), altering the phosphorylation status of tight junction proteins and allowing lipopolysaccharide (LPS) to enter the blood circulation (Zuhl et al. 2014). There LPS binds Toll-like Receptor-4 (TLR4) on surveilling leukocytes, activating the nuclear factor kappa B (NF-κB) cascade and resulting in the production of pro-inflammatory cytokines [tumor necrosis factor-α (TNF-α), Interleukin-1-β (IL-1β), and Interleukin-6 (IL-6)] (Kuennen et al. 2011). Overactivation of this pathway (e.g., systemic inflammatory response syndrome) confers the greater core temperature rise, intravascular coagulation, and multiple organ failure that characterize exertional-heat stroke (EHS) (Lim 2018).
Given the preeminent role of the gastrointestinal tract in the etiology of exertional heatstroke, our group, and others have screened a variety of dietary supplements for protection against gastrointestinal barrier damage during exertional-heat stress. Improvements have been shown following short-term dietary supplementation with curcumin (Szymanski et al. 2018) and glutamine (Zuhl et al. 2015; Zuhl et al. 2014), but not probiotics (Gill et al. 2016), quercetin (Kuennen et al. 2011) or bovine colostrum (McKenna et al. 2017; Morrison et al. 2014). Recently, we reported reductions in gastrointestinal barrier permeability and enterocyte damage following one week of blackcurrant extract supplementation (equivalent to 210 mg of anthocyanins per day; for reference anthocyanins are a class of water-soluble flavonoids that promote antioxidant and anti-inflammatory functions) (Lee et al. 2022). Our interest in this supplement was predicated on data from animal models that reported improvements in serum antioxidant capacity and gut microbiota function (Jurgonski et al. 2014) in addition to other biomarkers of intestinal health (Paturi et al. 2018). Fecal microbiota evidence in humans indicates improved bacterial growth and inactivation of toxic bacterial enzymes following blackcurrant supplementation (Molan et al. 2014). Multiple preclinical trials with another anthocyanin-containing supplement (bilberry) have reported reductions in NF-κB mediated inflammatory cascades in ulcerative colitis patients (Biedermann et al. 2013; Roth et al. 2016).
Despite the improvements in gut barrier integrity (Intestinal Fatty-Acid Binding Protein; I-FABP) and function (lactulose/rhamnose ratio) that were shown following 1wk of NZBC-extract supplementation in our prior work (Lee et al. 2022), changes were not detected in markers of microbial translocation [Lipopolysaccharide Binding Protein (LBP) and Plasma-Soluble Cluster of Differentiation 14 (sCD14)]. Circulating cytokines that are associated with EHS risk (IL-1RA, IL-6, IL-10) were also not improved. Because heat shock proteins (HSP) intersect the otherwise linear relationship between gut barrier integrity and microbial translocation (and impart influence on pro-inflammatory cytokine cascades and oxidative-stress markers), in the present study we measured extracellular HSP (eHSP90, eHSP70, eHSP32) and intracellular HSP (HSP90, HSP70, HSP60, & HSP32) derived from peripheral blood mononuclear cell (PBMC) samples. In our prior work, we have shown the combination of these four HSP to be a thorough means of analyzing the heat shock response and its’ influence on pro- and anti-inflammatory cytokine cascades (Kuennen et al. 2011; Falgiano et al. 2018; Szymanski et al. 2018; Hill et al. 2020; Hill et al. 2020). Given the advent of newer technologies and our interest in inflammatory function (intracellular HSPs reduce pro-inflammatory cytokine cascades, whereas extracellular HSPs are typically pro-inflammatory), in the present study both extracellular and intracellular HSPs were examined. In addition, potential changes in PBMC-associated inflammatory cascades were assessed via measurement of TLR4, myeloid differentiation protein 88 (MyD88), phosphorylated I-κB-α (p-IκB-α), and NF-κB. Alterations in inflammatory status trigger changes in apoptotic markers in PBMC (Shen et al. 2019). For that reason, we also measured common markers of pro- and anti-apoptotic function in PBMC [B-cell lymphoma protein (BCL-2), BCL-2 associated X, apoptosis regulator (BAX), and Caspase 9].
Methods
Participants
Ten recreationally active men (Age: 29 ± 2 years, Stature: 1.82 ± 0.02 m, Mass: 80.3 ± 2.7 kg, VO2max: 56 ± 2 mL·kg−1·min−1) provided written informed consent prior to completing a double-blind placebo-controlled study with randomized, cross over design. The study was approved by the University of Chichester Research Ethics Committee with protocols and procedures conforming to the 2013 Declaration of Helsinki. All participants were non-smokers and negative for cardiovascular, pulmonary, or metabolic disease as defined by the American College of Sports Medicine (Riebe et al. 2015). These data are part of a larger data set, from which information on gastrointestinal barrier permeability, enterocyte damage, and circulating cytokine concentrations have been reported previously (Lee et al. 2022).
Experimental design
Each participant completed one maximal exercise test, one familiarization trial, and two experimental trials. Participants were instructed to abstain from strenuous exercise and alcohol for 48 h prior, and caffeine-containing products on the day of testing. They were also asked to adhere to their normal exercise training schedule. All testing was conducted in the morning following a 12-h overnight fast. On the first visit, each participant completed an incremental exercise test to exhaustion on a motorized treadmill (HP Cosmos, Pulsar, h/p/cosmos Sports & Medical gmbh, Germany) to determine their maximal aerobic capacity (V̇O2max). They also completed a second exercise bout on that date to verify the workload that was required to elicit 65% of individual V̇O2max. The second visit was a FAM trial that familiarized participants with all measurements and study procedures under thermoneutral conditions (18 °C, 40% RH). This included 1 h of treadmill exercise at a workload equivalent to 65% V̇O2max (e.g., the same workload that was completed during their heat stress trials (e.g., Study Visits 3 and 4), which were separated by a minimum of 2 weeks to minimize the likelihood that carry-over effects influenced primary study outcomes.
Dietary supplementation and diet monitoring
Participants used standard food diaries to record their dietary intake for 48 h prior to their first experimental visit. Upon completion of their first experimental visit, participants were given a copy of their food log and asked to replicate this dietary intake for all subsequent visits. Dietary intake was quantified using Nutritics software (Nutritics Ltd, Dublin, Ireland). Participants also completed a food frequency questionnaire that listed the amount and frequency of anthocyanin-containing foods and drinks to estimate their habitual anthocyanin intake (Neveu et al. 2010). Habitual anthocyanin intake from the diet was shown to be low, as reported previously (Lee et al. 2022). Prior to Visits 3 and 4, participants consumed 2 capsules of concentrated NZBC extract (2 × 300 mg active cassis containing 210 mg of anthocyanins, i.e., 35–50% delphinidin-3-rutinoside, 5–20% delphinidin-3-glucoside, 30–45% cyanidin-3-rutinoside, 3–10% cyanidin-3-glucoside per capsule; CurraNZ™, Health Currancy Ltd., Surrey, UK) or 2 capsules of identical-looking placebo capsules (2 × 300 mg microcrystalline cellulose M102) every morning for 7 days (Cook et al. 2015, 2017). One experimenter (M.E.T.W) made up visually identical NZBC and placebo pill packets for each participant, then left them in bulk in the principal investigator’s office (B.J.L) without any personal interaction. Blinding was not broken until after the sample analysis was completed. The 2 experimental conditions (NZBC and PLACEBO) were separated by a 14-day washout period. Trial order was determined using a free online tool (https://www.randomizer.org) and five participants received NZBC extract as the first condition. All experimental trials were conducted in an environmental chamber (TISS Services UK, Medstead, Hampshire, UK) controlled at 34.1 ± 0.1 °C and 40.8 ± 0.2% RH.
Experimental protocol
On the morning of each experimental trial, participants were instructed to drink ~ 400 ml of water and arrived at the laboratory between 06:30 and 08:30. At that time a urine sample was taken for assessment of urine osmolality (Osmocheck PAL-OSMO; Vitech Scientific, Partridge Green, West Sussex, UK) and specific gravity (ATAGO 2791, ATAGO, Tokyo, Japan) to ensure participants were euhydrated (mOsmol−1 ≤ 600; USG ≤ 1.020) (Sawka et al. 2007). Following the recording of nude-body mass, participants inserted a polyethylene thermistor (Edale Instruments, Cambridge, UK) 10 cm past the external anal sphincter and were fitted with a heart rate monitor. Skin thermistors (Edale Instruments, Cambridge, UK) were attached to the mid-belly of the pectoralis major, triceps brachii, rectus femoris, and gastrocnemius for calculation of mean skin temperature (Ramanathan 1964). Standard equations were used to calculate mean body temperature and physiological strain index (PSI) during exercise (Kenney 1998; Moran et al. 1998).
Participants next rested in a supine position for 20-min, after which baseline physiologic measurements (HR, skin and rectal temperatures) were recorded. Participants next entered the environmental chamber where they rested for 5 min prior to 60 min of treadmill running at 65% V̇O2max (1% incline). For reference, in Fig. 1 the measurement following 20-min of seated rest aligns to 0-min and the resting measurement taken inside the environmental chamber aligns to the 5-min mark. Following these two resting measurements, heart rate, rectal and skin temperatures were recorded at 10-min intervals throughout exercise. Bottled water (chamber temperature) was available ad libitum during each trial, and the volume of ingested fluid was recorded. On completion of exercise, participants towel-dried and nude-body mass was reassessed. The difference in pre-to-post-exercise body mass was used to calculate sweat rate (corrected for water ingestion but not respiratory water loss).
Blood collection
Before exercise (Pre), within 20 min following exercise cessation (Post), 1 h after exercise (1-Post), and 4 h after exercise (4-Post) blood samples were drawn from an antecubital vein using standard venipuncture techniques. From these blood samples, extracellular HSP were measured at Pre, Post, and 1-Post using standard ELISA kits (Proteintech, Manchester, UK). Peripheral blood mononuclear cells (PBMC) were isolated from 5 ml EDTA blood that had been layered upon 3 ml Ficoll (10,771, Sigma Aldrich, Gillingham, UK) inside of Leucosep tubes (163290P, Greiner Bio-One, Stonehouse, UK) prior to centrifugation at 800 g for 20 min. Isolated PBMC were washed thrice in 10 ml PBS at 400 g for 10 min. Clean PBMC were transferred into sterile 1.7 ml micro-eppendorf tubes and frozen at − 80 °C until later batch analysis (described below).
Immunoblotting and protein expression
PBMC were lysed in RIPA buffer from Bio-Rad (Hercules, CA, USA) supplemented with protease inhibitor mix (0.1%) from Bio-Rad (Hercules, CA, USA) on ice for 1 h. Insoluble material was removed, and protein concentrations were determined by Bradford assay from Bio-Rad (Hercules, CA, USA). 50 μg of total protein from each sample was size-separated on an 8–10% sodium dodecyl sulfate–polyacrylamide gel using electrophoresis, after which proteins were electro-transferred to PVDF membranes. Membranes were blocked in TBST-5% non-fat milk powder for > 90 min, after which they were probed at 4ºC overnight with target antibodies from Santa Cruz Biotechnologies (Santa Cruz, CA, USA) or Abcam (Cambridge, MA, USA) or Enzo Life Sciences (Farmingdale, NY, USA), as indicated in Supplemental Table 1. Unless stated otherwise, protein loading was confirmed with anti-β-Actin primary antibody from Santa Cruz Biotechnologies (Santa Cruz, CA, USA) at a dilution of 1:400 in TBST-5% non-fat milk powder. Bound antibodies were detected by horseradish peroxidase-conjugated secondary antibodies from Santa Cruz Biotechnologies (Santa Cruz, CA, USA) or Abcam (Cambridge, MA, USA) at a dilution of 1:5000 in TBST-5% non-fat milk powder for 1 h at room temperature on an orbital platform at 250RPM. Protein signal intensities were determined by chemiluminescence using the Clarity Western ECL substrate kit from Bio-Rad (Hercules, CA, USA) and imaged using the ChemiDoc Touch from Bio-Rad (Hercules, CA, USA). Relative signal intensities were normalized to β-Actin and quantified using Image Lab from Bio-Rad (Hercules, CA, USA). A complete list of all primary antibodies that were utilized to generate Western Blots in this experiment is provided in Supplemental Table 1.
Statistical analysis
Statistical analyses were performed using STATISTICA for Windows (version 7.1; StatSoft Inc., Tulsa, OK, USA). Unless stated otherwise, text and table data are presented as mean ± SEM for N = 10. As reported previously (Lee et al. 2022), two-tailed paired t tests were used to determine if dietary intake, urine specific gravity, water ingestion, and sweat rate were different between conditions (PLACEBO or NZBC). Two-factor RM-ANOVAs [where study condition (PLACEBO or NZBC) and exercise time (0–60 min) served as the repeated measures factors] were used to examine potential differences in core and shell temperatures, HR, and PSI over the 60 min exercise bout. Statistical analysis of extracellular HSP were conducted on the absolute concentrations, after correction for plasma volume change. Western blot data were analyzed with two-factor RM-ANOVAs, where intervention (PLACEBO or NZBC) and blood sample timepoint (Pre, Post, 1-Post, 4-Post) served as the repeated measures factors. Statistical significance was set at p ≤ 0.05. Significant main and interaction effects were further evaluated by way of Duncan’s New Multiple Range Test. To ensure clear articulation of study findings, the two study conditions (PLACEBO and NZBC) were also separated and one-way RM-ANOVAs were run on each (to examine the main effect of time), followed by Duncan’s New Multiple Range Test (where appropriate). Effect sizes were calculated for all significant main and interaction effects [partial eta squared (ηp2)]. For reference, ηp2 values of 0.01, 0.09, and 0.25 are considered to be small, medium, and large effect sizes; respectively (Cohen 1992).
Power analysis
A power analysis was conducted (G*PowerVersion 3.1; Dusseldorf, North Rhine-Westphalia, Germany) using the means and SDs of a prior research study that examined the effect of short-term dietary quercetin supplementation on exertional-heat stress-mediated changes in protein expression in PBMC (Kuennen et al. 2011). It was determined that with an α-level of p ≤ 0.05, eight participants would result in a 92% probability (i.e., 1–β) of detecting differences in protein expression in PBMC.
Results
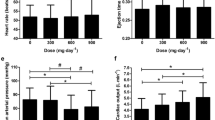
Cardiovascular and thermoregulatory responses
Cardiovascular and thermoregulatory responses were not different between study conditions (all interaction effects p > 0.05). Significant main effects (of time) indicate exercise conferred elevations in heart rate (F = 796.90, p < 0.0001, ηp2 = 0.989) (Fig. 1A), rectal temperature (F = 387.40, p < 0.0001, ηp2 = 0.977) (Fig. 1B), skin temperature (F = 37.86, p < 0.0001, ηp2 = 0.808) (Fig. 1C), and physiological strain (F = 345.40, p < 0.0001, ηp2 = 0.975) (Fig. 1D). As reported previously (Lee et al. 2022), fluid intake and whole-body sweat rate were also not different between study conditions (p = 0.938 and 0.465; respectively).
eHSP32
The interaction effect for eHSP32 was significant (F = 9.445, p = 0.002, ηp2 = 0.512) (Fig. 2A). Post-hoc analysis indicated that eHSP32 was elevated at baseline in NZBC as compared to PLACEBO baseline expression (+ 31.3%; p < 0.001). When conditions were run separately the main effect of time was significant in PLACEBO (F = 3.775, p = 0.043, ηp2 = 0.295), where eHSP32 was shown elevated above baseline at Post (+ 20.1%; p = 0.021). While the main effect of time was also significant in NZBC (F = 5.287, p = 0.016, ηp2 = 0.370), in this condition eHSP32 was shown elevated at baseline before falling below Pre-exercise values at Post (− 21.9%; p = 0.014) and 1-Post (− 21.5%; p = 0.012) exercise.
eHSP70
The interaction effect for eHSP70 was not significant (F = 2.290, p = 0.130, ηp2 = 0.203) (Fig. 2B). A significant main effect (of time) was shown in both PLACEBO (F = 8.374, p = 0.003, ηp2 = 0.482) and NZBC (F = 4.731, p = 0.022, ηp2 = 0.345). eHSP70 increased at Post in PLACEBO (+ 55.6%; p = 0.001) and at Post in NZBC (+ 50.7%; p = 0.010).
eHSP90α
The interaction effect for eHSP90α was not significant (F = 0.593, p = 0.563, ηp2 = 0.062) (Fig. 2C). A significant main effect (of time) was shown in both PLACEBO (F = 21.292, p < 0.001, ηp2 = 0.703) and NZBC (F = 9.786, p = 0.001, ηp2 = 0.521). eHSP90α increased at Post (+ 77.9%; p < 0.001) and 1-Post (73.2%; p < 0.001) exercise in PLACEBO. It also increased at Post (+ 49.0%; p = 0.006) and 1-Post (66.2%; p = 0.001) exercise in NZBC.
Extracellular heat shock response. Impact of 7d of New Zealand blackcurrant (NZBC) extract supplementation (equivalent to 210 mg anthocyanin·day−1) on extracellular concentrations of (A) eHSP32, (B) eHSP70, and (C) eHSP90. eHSP were assayed from plasma samples collected before (PRE), after (POST), and 1 h after (1-POST), and 4 h after 60 min of submaximal treadmill exercise (workload) performed under hot (37 °C), dry (25% RH) ambient conditions. Data for subjects (N = 10) are presented as box plots that display individual data points (closed circles), the 25 and 75th interquartile ranges (boxes), and the median (mid-line). Whiskers illustrate the highest and lowest value at each timepoint. Data were analyzed with two-factor RM-ANOVAs, where intervention (PLACEBO or NZBC) and blood sample timepoint (Pre, Post, 1-POST) served as the repeated measures factors. Statistical significance was set at p ≤ 0.05. Significant main effects and interactions were further evaluated using Duncan’s post hocs. #Significant between condition effect. *Significant within condition effect
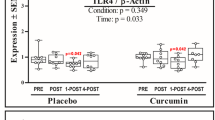
HSP32
The interaction effect for HSP32 was significant (F = 3.339, p = 0.034, ηp2 = 0.271) (Fig. 3A). Post-hoc analysis indicated HSP32 levels in PLACEBO and NZBC differed at Post (p = 0.012), 1-Post (p = 0.029), and 4-Post exercise (p = 0.001). Interestingly, in PLACEBO HSP32 was elevated at Post (+ 12.8%) and markedly elevated at 4-Post (+ 98.2%; p = 0.002). Whereas in NZBC, HSP32 fell at Post (-44.8%) and 1-Post (-48.2%) exercise before returning to baseline values at 4-Post.
HSP60
The interaction effect for HSP60 was not significant (F = 1.549, p = 0.225, ηp2 = 0.147) (Fig. 3B). The main effects of condition and time were not significant, and there were also no main effects of exercise time when the two conditions were analyzed separately (all p > 0.05).
HSP70
The interaction effect for HSP70 was significant (F = 3.322, p = 0.035, ηp2 = 0.270) (Fig. 3C). Post-hoc analysis indicated that HSP70 was elevated at baseline in NZBC as compared to PLACEBO baseline expression (+ 40%; p = 0.010). Although HSP70 was also visually elevated at POST and 1-POST exercise in NZBC, these levels did not achieve statistical significance (p = 0.062 and 0.090; respectively). When conditions were run separately there was a significant main effect (of time) in PLACEBO (F = 3.382, p = 0.033, ηp2 = 0.273), wherein HSP70 increased above baseline levels at 4-Post (+ 54.9%; p = 0.007).
HSP90
The interaction effect for HSP90 was not significant (F = 0.796, p = 0.508, ηp2 = 0.091) (Fig. 3D). The main effects of condition and time were not significant, and there were also no main effects of exercise time when the two conditions were analyzed separately (all p > 0.05).
Intracellular heat shock tesponse. Impact of 7d of New Zealand blackcurrant (NZBC) extract supplementation (equivalent to 210 mg anthocyanin·day−1) on intracellular concentrations of (A) HSP32, (B) HSP60, (C) HSP70, and (D) HSP90. Changes in protein expression were determined in peripheral blood mononuclear cells (PBMC), which were isolated from plasma samples that were collected before (PRE), after (POST), 1 h after (1-POST), and 4 h after (4-POST) 60 min of submaximal treadmill exercise (workload) performed under hot (37 °C), dry (25% RH) ambient conditions. Data for subjects (N = 10) are presented as box plots that display individual data points (closed circles), the 25 and 75th interquartile ranges (boxes), and the median (mid-line). Whiskers illustrate the highest and lowest value at each timepoint. Data were analyzed with two-factor RM-ANOVAs, where intervention (PLACEBO or NZBC) and blood sample timepoint (Pre, Post, 1-POST, 4-POST) served as the repeated measures factors. Statistical significance was set at p ≤ 0.05. Significant main effects and interactions were further evaluated using Duncan’s post hocs. #Significant between condition effect. *Significant within condition effect
TLR4
The interaction effect for TLR4 was significant (F = 3.654, p = 0.025, ηp2 = 0.289) (Fig. 4A). Post-hoc analysis indicated TLR4 was elevated at baseline in NZBC as compared to PLACEBO baseline expression (+ 54%; p = 0.003). Under PLACEBO TLR4 was shown increased above baseline at 4post (+ 50.8%, p = 0.017) whereas in NZBC TLR4 fell below baseline at Post (− 37.7%, p = 0.020) before returning to baseline values by 1-Post exercise.
p-IκBa
Neither main effect nor the interaction effect were significant for p-IκBa (Fig. 4B). When conditions were run separately the main effect of time was significant in PLACEBO only (F = 3.163, p = 0.043, ηp2 = 0.283), wherein p-IκBa rose above baseline at 1-Post (+ 35.1%; p = 0.011).
NF-κB
The interaction effect for NF-κB was significant (F = 3.087, p = 0.046, ηp2 = 0.278) (Fig. 4C). Post-hoc analysis indicated NF-κB was elevated at baseline (+ 57%; p = 0.004) in NZBC. When conditions were run separately the main effect of time was significant in NZBC only (F = 3.904, p = 0.019, ηp2 = 0.303), wherein NZBC was shown to be reduced below baseline at Post (− 39.9%, p = 0.008), 1-Post (− 37.1%, p = 0.012), and 4-Post (− 30.4%, p = 0.028) exercise.
p-AMPK
The main effect of condition was significant (F = 11.380, p = 0.008, ηp2 = 0.558) for p-AMPK, reflecting ~ 100% increase in p-AMPK at all time points in NZBC (Fig. 4D). However, neither the main effect of time (F = 2.517, p = 0.079, ηp2 = 0.219) nor the interaction effect (F = 1.577, p = 0.218, ηp2 = 0.149) achieved statistical significance. When conditions were run separately the main effect (of time) was not significant in either study condition.
BAX/BCL-2 ratio
The interaction effect for BAX/BCL-2 was not significant (F = 1.737, p = 0.183, ηp2 = 0.162). However, there was a significant main effect of condition (F = 7.163, p = 0.025, ηp2 = 0.443) reflecting greater BAX/BCL-2 in NZBC as compared to PLACEBO (Fig. 4E). When conditions were run separately, the main effect of time was significant in NZBC only (F = 4.486, p = 0.011, ηp2 = 0.333), where BAX/BCL-2 was shown to be elevated above baseline at Post (+ 43.5%; p = 0.006), 1-Post (+ 46.2%; p = 0.006), and 4-Post (+ 31.3%; p = 0.020) exercise.
Caspase 9
The main effect of condition was significant (F = 5.514, p = 0.043, ηp2 = 0.380), reflecting ~ 41% greater Caspase 9 in NZBC as compared to PLACEBO (Fig. 4F). The interaction effect was also significant (F = 3.064, p = 0.045, ηp2 = 0.254), where post-hoc analysis reflected greater Caspase 9 content in NZBC at baseline (+ 90%; p = 0.001). It is worthwhile to point out that when conditions were run separately the main effect of time was only significant in the NZBC condition (F = 3.715, p = 0.023, ηp2 = 0.292), wherein Caspase 9 had fallen below baseline levels at 4-Post (− 34.7%; p = 0.004).
Inflammation and apoptosis. Impact of 7d of New Zealand blackcurrant (NZBC) extract supplementation (equivalent to 210 mg anthocyanin·day−1) on intracellular concentrations of (A) TLR4 (B) p-IκBa, (C) NF-κB, (D) p-AMPK, (E) BAX/BCL-2, and (F) Caspase 9. Changes in protein expression were determined in peripheral blood mononuclear cells (PBMC), which were isolated from plasma samples that were collected before (PRE), after (POST), 1 h after (1-POST), and 4 h after (4-POST) 60 min of submaximal treadmill exercise (workload) performed under hot (37 °C), dry (25% RH) ambient conditions. Data for subjects (N = 10) are presented as box plots that display individual data points (closed circles), the 25 and 75th interquartile ranges (boxes), and the median (mid-line). Whiskers illustrate the highest and lowest value at each timepoint. Data were analyzed with two-factor RM-ANOVAs, where intervention (PLACEBO or NZBC) and blood sample timepoint (Pre, Post, 1-POST, 4-POST) served as the repeated measures factors. Statistical significance was set at p ≤ 0.05. Significant main effects and interactions were further evaluated using Duncan’s post hocs. #Significant between condition effect. *Significant within condition effect
Discussion
In our prior work, we reported decreased gut barrier permeability and damage following one week of NZBC-extract supplementation, but microbial translocation and circulating cytokine concentrations were not shown to be altered (Lee et al. 2022). Because HSP intersect the otherwise linear relationship between gut barrier integrity and microbial translocation via their influence on pro-inflammatory cytokine cascades, in the present study we examined extracellular (eHSP90, eHSP70, eHSP32) and intracellular HSP (HSP90, HSP70, HSP60, HSP32) that had been derived from PBMC samples. For reference, prior work has shown that human cells secrete relatively low levels of HSP under normal physiologic conditions (Ogura et al. 2008), but plasma levels are greatly increased following exercise and/or thermal stress exposure (Jay et al. 2022; Periard et al. 2012). Dietary supplements that promote gastrointestinal health, including Rhodiola rosea (Jafari et al. 2022) and probiotics (Gill et al. 2016), have not been shown to alter basal eHSP concentrations or influence eHSP responses following endurance exercise (Marshall et al. 2017; Shanely et al. 2014).
As such, the elevated eHSP32 and HSP32 that were shown following NZBC-extract supplementation in the present study were unexpected. However, because NZBC-extract supplementation was also shown to elevate TLR4 and NF-κB content, we postulate that elevated eHSP32 may help coordinate the inflammatory response in PBMCs under basal conditions (Asea et al. 2000; Jay et al. 2022). This hypothesis is supported by the elevated NF-κB and TLR4 that were shown in PBMC under basal conditions following NZBC supplementation. For reference, eHSP90 binds to TLR4 expressed on NK cells and monocytes, activating these leukocytes to increase NF-κB mediated production of pro-inflammatory cytokines and improve their cidal capacity (Zhang et al. 2017). Similar findings have been reported for eHSP32, which has been shown to be elevated in chronic inflammatory conditions such as diabetes mellitus (Bao et al. 2010) and hemophagocytic lymphohistiocytosis (Miyazaki et al. 2010).
While plasma concentrations of eHSP70 and eHSP90 were also shown to increase following exertional-heat stress, this increase was equivalent across PLACEBO and NZBC-extract conditions. To our knowledge this study is the first to examine eHSP90 in conjunction with exertional-heat stress, whereas eHSP70 release has been relatively well characterized as an intensity-dependent phenomenon (Periard et al. 2012). Given that prior work, the modest (~ 150%) increase in eHSP70 that was shown in the present study was likely due to our moderate exercise intensity (60-min treadmill at 65% V̇O2max), whereas long-duration exercise has been shown to provoke greater eHSP70 responses (~ 2200% in an ironman triathlon and ~ 1674% in an 100 km run) (Gomez-Merino et al. 2006).
Overall, the changes in intracellular HSP that were shown in the present study were more subdued than the changes that were reported in eHSP. Of these, changes in HSP32 were the most interesting, where PBMC HSP32 content following NZBC-extract supplementation was shown reduced at Post, 1-Post, and 4-Post exercise. This contrasted with the PLACEBO condition, where HSP32 had nearly doubled (+ 98%) by 4 h post-exercise. Reduced HSP32 content following anthocyanin-rich NZBC-extract supplementation was unexpected because anthocyanin’s have previously been shown to increase HSP32 content and promote anti-inflammatory and immune supportive effects (Funes et al. 2020). Other dietary supplements that exhibit strong antioxidant effects have also been shown to upregulate HSP32, protecting mice from kidney damage following thermal burn injury (Guo et al. 2021). However, reduced HSP32 is not unprecedented, as Chen et al. (2022) reported lower HSP32 in the skeletal muscle of exercising Kunming mice following supplementation with vitamin C and L. plantarum KSFY01 (a probiotic with antioxidant effects) (Chen et al. 2022). Given that HSP32 helps maintain homeostasis during exertional-heat stress (Taylor et al. 2012) and also affords protection against ischemia–reperfusion injury (Zeynalov et al. 2009), we think a plausible explanation is that HSP32 was increased under PLACEBO conditions to combat the stress associated with reductions in gut barrier function because the lactulose/rhamnose ratio and I-FABP were both shown elevated following PLACEBO supplementation but not under NZBC-extract supplementation (Lee et al. 2022). Although they utilized a slightly different model than our own, Peng (2000) has also shown a reduced-HSP32 response in lymphocytes that were collected from human subjects following 5 weeks of antioxidant supplementation, then subjected to ex vivo heat shock (42.5 °C for 45 min) (Peng et al. 2000).
Prior reports have examined changes in HSP70 content following short-term dietary supplementation with probiotics and glutamine. The 45% increase in HSP70 expression in PBMCs following 1wk of NZBC-extract supplementation that was shown in the present study is similar to the 35% increase in HSP70 expression following a single dose (0.9 g/kg of fat-free mass) of dietary glutamine supplementation (Zuhl et al. 2015). Likely owing to the difference in cell/tissue type, 8 weeks of 500 mg/d vitamin C supplementation has been shown to elevate the HSP70 content of skeletal muscle ~ sixfold (Khassaf et al. 2003). Collectively, these data support greater basal activation of the heat shock response to promote enhanced physiologic function and greater stress tolerance. Interestingly in all three of these studies HSP70 did not increase further with exercise heat stress. Whereas HSP70 did increase with exercise under PLACEBO supplementation in the present study, suggesting thermal tolerance may have been lower under PLACEBO supplementation. Evidence has suggested that anthocyanin-rich supplements increase HSP70 induction in the GI tract of mice (Murakami et al. 2015) and there is also some corresponding evidence from anthocyanin-supplemented Caco-2 models that were subjected to severe levels of oxidative stress (Bei et al. 2008). A meta-analysis on heat acclimation-induced intracellular HSP70 in humans has also provided evidence supporting the relationship between the HSP70 content of PBMCs and corresponding changes in gastrointestinal barrier function (Nava and Zuhl 2020). In this context, the upregulation of basal HSP70 in PBMCs following NZBC supplementation may provide some indirect evidence of HSP induction in the gut, possibly explaining the improvements in the lactulose/rhamnose ratio and I-FABP responses that were shown in our prior work (Lee et al. 2022).
Extracellular HSPs are known to promote pro-inflammatory cascades that reciprocally inhibit apoptotic drive. In our prior report on NZBC extract and exertional-heat stress (Lee et al. 2022), we did not detect any changes in inflammatory cytokine production, so in the present study we wanted to determine if inflammatory markers were altered in PBMC. Interestingly, we found that TLR4 and NF-κB were both elevated at baseline under NZBC-extract supplementation. These changes did not elevate inflammatory signaling with exercise, as circulating cytokine concentrations were not different between NZBC and PLACEBO conditions (Lee et al. 2022), and PBMC NF-κB content actually fell Post-exercise. TLR4 content did rise with exercise, but only under PLACEBO conditions. As such, the mechanism to explain such changes remains unclear.
However, similar to what has been reported for eHSP70, recent work suggests that TLR4 may also be regulated by exercise intensity (Ducharme et al. 2024). In addition to membrane-bound protein levels, recent research has shown that assessment of circulating/soluble TLR4 may offer greater insight into TLR4 expression and signaling in response to exercise (Ducharme et al. 2024;Ducharme et al. 2023; Perkins et al. 2023). As soluble TLR4 has been shown to function as a decoy receptor to TLR4 ligands and thus reduced TLR4-mediated signaling (Ducharme et al. 2023; Iwami et al. 2000) it is possible that an increased concentration of both membrane-bound and soluble TLR4 may help to explain the absence of increased inflammatory signaling with exercise under NZBC. Acute exercise has been shown to change the fraction of PBMC subpopulations that express TLR4, which could potentially explain the increase in TLR4 expression with moderate exercise that was shown in the present study. For example, acute exercise has been shown to increase shear stress that can demarginalize NK cells, effectively increasing the number of circulating peripheral blood NK cells (Millard et al. 2013). As such, an increase in TLR4-expressing NK cells could be a plausible explanation for the rise in TLR4 expression that was shown under PLACEBO conditions in the present study, one that would not necessitate an increase in inflammatory cytokine production.
It is well established that high-intensity exercise increases intestinal permeability (Pals et al. 1997), leading to activation of the NF-κB pro-inflammatory pathway and transcription of pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) (Selkirk et al. 2008). An in vitro study that explored the effect of anthocyanin-rich blackcurrant supplementation on THP-1 cells reported pre-incubation with NZBC inhibits NF-κB mediated pro-inflammatory cascades and reduces LPS-stimulated TNF-α by ~ 50% (Lyall et al. 2009). However, in that study, total NF-κB content was unchanged (Lyall et al. 2009). Another in vitro study reported that blackcurrant juice lowers NF-κB activity and LPS-mediated inflammation in cultured macrophages (Huebbe et al. 2012). The in vivo data from the present study do not agree with those prior in vitro findings, suggesting that differences in the blackcurrant-dosing strategy and reduced-NZBC anthocyanin bioavailability across human gastrointestinal tissues may help to explain these discordant findings.
BCL-2 and BAX are intracellular proteins that restrain and promote apoptosis, respectively (Shen et al. 2019). They play key roles in the maintenance of mitochondrial membrane function and cytochrome C release (Slee et al. 1999). After release of cytochrome C from the mitochondria, Caspase-9 binds to apoptotic peptidase activating factor-1 to form the apoptosome that leads to Caspase-9 activation and production of downstream executioner caspases like Caspase-3 and Caspase-7 (Slee et al. 1999). Researchers commonly use the ratio of BAX to BCL-2 (BAX:BCL-2) as an index of apoptotic drive and measure Caspase-9 to verify this outcome. In the present study, although BAX:BCL-2 was not different between study conditions at baseline, under NZBC BAX:BCL-2 was shown to rise at Post (+ 44%), 1-Post (+ 46%), and 4-Post (+ 31%) exercise. Caspase 9, which is one of the primary coordinators of apoptotic cell death, was also elevated at baseline in NZBC. When taken together, these data suggest PBMCs experienced a greater apoptotic drive under NZBC.
The literature on inflammatory cytokines and apoptosis is somewhat less clear, as they can be pro- or anti-apoptotic depending on the concentration and cell type. Crosstalk between cytokines adds further confusion. For example, IL-6 acts as an initiator to produce anti-inflammatory cytokines (IL-1RA and IL-10) (Steensberg et al. 2003) that help to offset elevated levels of other pro-inflammatory cytokines (TNF-α and IL-1β) (Schindler et al. 1990). Interestingly, multiple NF-κB-dependent genes that are induced by TNF-α have been shown to protect cells against apoptosis (Baichwal and Baeuerle 1997). Likewise, elevations in HSP70 have been shown to inhibit heat-induced apoptosis via prevention of BAX translocation into the mitochondria (Stankiewicz et al. 2005). Antioxidant supplementation in animal heat-stroke models provides strong evidence that elevations in HSP70 content help to reduce NF-κB-mediated signaling cascades and also result in less cleaved Caspase 3, which is the final downstream caspase prior to apoptotic cell death (Shen et al. 2019). In conjunction with HSP70, animal models also provide evidence that geldanamycin-mediated elevations in p-AMPK help to reduce apoptosis and defend against lethal heat stroke (Tsai et al. 2016). This is intriguing when one considers that p-AMPK and HSP70 were both shown to be upregulated at baseline following NZBC-extract supplementation, but failed to increase further in response to exertional-heat stress. As such, it is possible that the lack of increase in these mediators, in conjunction with the lack of increase in inflammatory cytokine production during exertional-heat stress under NZBC supplementation may have contributed to the elevated apoptotic drive we report here.
Limitations
One limitation of this research is the focus on males. Future work should examine NZBC responses across both biologic sexes (men and women). It may also be worthwhile to examine NZBC in conjunction with other polyphenol substances (such as curcumin: Szymanski et al. 2018) and through the use of more novel investigative techniques (such as microRNA). In addition, it is worthwhile to note that a greater expression of TLR4 and NF-κB at baseline under NZBC may suggest a greater percentage of TLR4 expressing cells within the PBMC fraction being sampled. Since the percentage of TLR4 expressing cells within the PBMC fraction were not evaluated in the present study, this represents an additional potential limitation of our manuscript.
Conclusion
The present study provides evidence that 7d intake of anthocyanin-rich NZBC increases extracellular and intracellular HSP32 concentrations under basal conditions, resulting in a lesser heat shock response when those persons are subjected to 1 h of exertional-heat stress. Elevated levels of TLR4 and NF-κB were also shown at baseline following NZBC-extract supplementation, but the overall impact of these changes is questionable because in our prior work (Lee et al. 2022) we did not detect any differences between study conditions in IL-1RA, IL-6, or IL-10.
Data availability
Depending on the rationale and scenario, the reader could email the corresponding author for access to study data.
References
Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6(4):435–442. https://doi.org/10.1038/74697
Baichwal VR, Baeuerle PA (1997) Apoptosis: activate NF-κB or die? Curr Biol 7(2):R94–R96. https://doi.org/10.1016/S0960-9822(06)00046-7
Bao W, Song F, Li X, Rong S, Yang W, Zhang M, Yao P, Hao L, Yang N, Hu FB, Liu L (2010) Plasma heme oxygenase-1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS ONE 5(8):e12371. https://doi.org/10.1371/journal.pone.0012371
Bei R, Calandra L, Cardile V, Fauci LL, Galvano F, Renis M, Scifo C, Tomasello B, Vanella L (2008) Response of cell cycle/stress-related protein expression and DNA damage upon treatment of CaCo2 cells with anthocyanins. Br J Nutr 100(1):27–35. https://doi.org/10.1017/S0007114507876239
Biedermann L, Mwinyi J, Scharl M, Frei P, Zeitz J, Kullak-Ublick GA, Vavricka SR, Fried M, Weber A, Humpf HU, Peschke S, Jetter A, Krammer G, Rogler G (2013) Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—an open pilot study. J Crohns Colitis 7(4):271–279. https://doi.org/10.1016/j.crohns.2012.07.010
Chen Q, Liu C, Zhang Y, Wang S, Li F (2022) Effect of Lactobacillus plantarum KSFY01 on the exercise capacity of D-galactose-induced oxidative stress-aged mice. Front Microbiol 13:1030833. https://doi.org/10.3389/fmicb.2022.1030833
Cohen J (1992) Statistical power analysis. Curr Dir Psychol Sci 1(3):98–101. https://doi.org/10.1111/1467-8721.ep10768783
Cook MD, Myers SD, Blacker SD, Willems MET (2015) New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur J Appl Physiol 115(11):2357–2365. https://doi.org/10.1007/s00421-015-3215-8
Cook MD, Myers SD, Gault ML, Willems MET (2017) Blackcurrant alters physiological responses and femoral artery diameter during sustained isometric contraction. Nutrients 9(6):556
Ducharme JB, Fennel ZJ, McKenna ZJ, Nava RC, Deyhle MR (2023) Stimulated myotube contractions regulate membrane-bound and soluble TLR4 to prevent LPS-induced signaling and myotube atrophy in skeletal muscle cells. Am J Physiol Cell Physiol 325(1):C300-c313. https://doi.org/10.1152/ajpcell.00007.2023
Ducharme JB, McKenna ZJ, Specht JW, Fennel ZJ, Berkemeier QN, Deyhle MR (2024) Divergent mechanisms regulate TLR4 expression on peripheral blood mononuclear cells following workload-matched exercise in normoxic and hypoxic environments. J Appl Physiol (1985) 136(1):33–42. https://doi.org/10.1152/japplphysiol.00626.2023
Falgiano PA, Gillum TL, Schall ZJ, Strag HR, Kuennen MR (2018) Dietary curcumin supplementation does not alter peripheral blood mononuclear cell responses to exertional heat stress. Eur J Appl Physiol 118(12):2707–2717. https://doi.org/10.1007/s00421-018-3998-5
Funes SC, Rios M, Fernandez-Fierro A, Covian C, Bueno SM, Riedel CA, Mackern-Oberti JP, Kalergis AM (2020) Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front Immunol 11:1467. https://doi.org/10.3389/fimmu.2020.01467
Gill SK, Allerton DM, Ansley-Robson P, Hemmings K, Cox M, Costa RJ (2016) Does short-term high dose probiotic supplementation containing lactobacillus casei attenuate exertional-heat stress induced endotoxaemia and cytokinaemia? Int J Sport Nutr Exerc Metab 26(3):268–275. https://doi.org/10.1123/ijsnem.2015-0186
Gomez-Merino D, Drogou C, Guezennec CY, Burnat P, Bourrilhon C, Tomaszewski A, Milhau S, Chennaoui M (2006) Comparison of systemic cytokine responses after a long distance triathlon and a 100-km run: relationship to metabolic and inflammatory processes. Eur Cytokine Netw 17(2):117–124
Guo S, Guo L, Fang Q, Yu M, Zhang L, You C, Wang X, Liu Y, Han C (2021) Astaxanthin protects against early acute kidney injury in severely burned rats by inactivating the TLR4/MyD88/NF-kappaB axis and upregulating heme oxygenase-1. Sci Rep 11(1):6679. https://doi.org/10.1038/s41598-021-86146-w
Hill GW, Gillum TL, Lee BJ, Romano PA, Schall ZJ, Hamilton AM, Kuennen MR (2020) Prolonged treadmill running in normobaric hypoxia causes gastrointestinal barrier permeability and elevates circulating levels of pro- and anti-inflammatory cytokines. Appl Physiol Nutr Metab 45(4):376–386. https://doi.org/10.1139/apnm-2019-0378%M31505122
Huebbe P, Giller K, de Pascual-Teresa S, Arkenau A, Adolphi B, Portius S, Arkenau CN, Rimbach G (2012) Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br J Nutr 108(2):234–244. https://doi.org/10.1017/S0007114511005642
Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y (2000) Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol 165(12):6682–6686. https://doi.org/10.4049/jimmunol.165.12.6682
Jafari M, Juanson Arabit JG, Courville R, Kiani D, Chaston JM, Nguyen CD, Jena N, Liu ZY, Tata P, Van Etten RA (2022) The impact of Rhodiola rosea on biomarkers of diabetes, inflammation, and microbiota in a leptin receptor-knockout mouse model. Sci Rep 12(1):10581. https://doi.org/10.1038/s41598-022-14241-7
Jay D, Luo Y, Li W (2022) Extracellular heat shock protein-90 (eHsp90): everything you need to know. Biomolecules. https://doi.org/10.3390/biom12070911
Jurgonski A, Juskiewicz J, Zdunczyk Z, Matusevicius P, Kolodziejczyk K (2014) Polyphenol-rich extract from blackcurrant pomace attenuates the intestinal tract and serum lipid changes induced by a high-fat diet in rabbits. Eur J Nutr 53(8):1603–1613. https://doi.org/10.1007/s00394-014-0665-4
Kenney WL (1998) Heat flux and storage in hot environments. Int J Sports Med 19(Suppl 2):S92-95. https://doi.org/10.1055/s-2007-971966
Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ (2003) Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol 549(Pt 2):645–652. https://doi.org/10.1113/jphysiol.2003.040303
Kuennen M, Gillum T, Dokladny K, Bedrick E, Schneider S, Moseley P (2011) Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Physiol Regul Integr Comp Physiol 301(2):R524-533. https://doi.org/10.1152/ajpregu.00039.2011
Lee BJ, Flood TR, Hiles AM, Walker EF, Wheeler LEV, Ashdown KM, Willems MET, Costello R, Greisler LD, Romano PA, Hill GW, Kuennen MR (2022) Anthocyanin-rich blackcurrant extract preserves gastrointestinal barrier permeability and reduces enterocyte damage but has no effect on microbial translocation and inflammation after exertional heat stress. Int J Sport Nutr Exerc Metab 32(4):265–274. https://doi.org/10.1123/ijsnem.2021-0330
Lim CL (2018) Heat sepsis precedes heat toxicity in the pathophysiology of heat stroke—a new paradigm on an ancient disease. Antioxidants (Basel). https://doi.org/10.3390/antiox7110149
Lyall KA, Hurst SM, Cooney J, Jensen D, Lo K, Hurst RD, Stevenson LM (2009) Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am J Physiol Regul Integr Comp Physiol 297(1):R70-81. https://doi.org/10.1152/ajpregu.90740.2008
Marshall H, Chrismas BCR, Suckling CA, Roberts JD, Foster J, Taylor L (2017) Chronic probiotic supplementation with or without glutamine does not influence the eHsp72 response to a multi-day ultra-endurance exercise event. Appl Physiol Nutr Metab 42(8):876–883. https://doi.org/10.1139/apnm-2017-0131
McKenna Z, Berkemeier Q, Naylor A, Kleint A, Gorini F, Ng J, Kim JK, Sullivan S, Gillum T (2017) Bovine colostrum supplementation does not affect plasma I-FABP concentrations following exercise in a hot and humid environment. Eur J Appl Physiol 117(12):2561–2567. https://doi.org/10.1007/s00421-017-3743-5
Millard AL, Valli PV, Stussi G, Mueller NJ, Yung GP, Seebach JD (2013) Brief exercise increases peripheral blood NK cell counts without immediate functional changes, but impairs their responses to ex vivo stimulation. Front Immunol 4:125. https://doi.org/10.3389/fimmu.2013.00125
Miyazaki T, Kirino Y, Takeno M, Hama M, Ushihama A, Watanabe R, Takase K, Tachibana T, Matsumoto K, Tanaka M, Yamaji S, Ideguchi H, Tomita N, Fujita H, Ohno S, Ueda A, Ishigatsubo Y (2010) Serum HO-1 is useful to make differential diagnosis of secondary hemophagocytic syndrome from other similar hematological conditions. Int J Hematol 91(2):229–237. https://doi.org/10.1007/s12185-010-0495-y
Molan AL, Liu Z, Plimmer G (2014) Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother Res 28(3):416–422. https://doi.org/10.1002/ptr.5009
Moran DS, Shitzer A, Pandolf KB (1998) A physiological strain index to evaluate heat stress. Am J Physiol 275(1):R129-134. https://doi.org/10.1152/ajpregu.1998.275.1.R129
Morrison SA, Cheung SS, Cotter JD (2014) Bovine colostrum, training status, and gastrointestinal permeability during exercise in the heat: a placebo-controlled double-blind study. Appl Physiol Nutr Metab 39(9):1070–1082. https://doi.org/10.1139/apnm-2013-0583
Murakami A, Nesumi A, Maeda-Yamamoto M, Yamaguchi H, Yashima K, Miura M, Nakano T, Nekoshima K (2015) Anthocyanin-rich tea Sunrouge upregulates expressions of heat shock proteins in the gastrointestinal tract of ICR mice: a comparison with the conventional tea cultivar Yabukita. J Food Drug Anal 23(3):407–416. https://doi.org/10.1016/j.jfda.2014.11.004
Nava R, Zuhl MN (2020) Heat acclimation-induced intracellular HSP70 in humans: a meta-analysis. Cell Stress Chaperones 25(1):35–45. https://doi.org/10.1007/s12192-019-01059-y
Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A (2010) Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010. https://doi.org/10.1093/database/bap024
Ogura Y, Naito H, Akin S, Ichinoseki-Sekine N, Kurosaka M, Kakigi R, Sugiura T, Powers SK, Katamoto S, Demirel HA (2008) Elevation of body temperature is an essential factor for exercise-increased extracellular heat shock protein 72 level in rat plasma. Am J Physiol Regul Integr Comp Physiol 294(5):R1600-1607. https://doi.org/10.1152/ajpregu.00581.2007
Pals KL, Chang RT, Ryan AJ, Gisolfi CV (1997) Effect of running intensity on intestinal permeability. J Appl Physiol 82(2):571–576. https://doi.org/10.1152/jappl.1997.82.2.571
Paturi G, Butts CA, Monro JA, Hedderley D (2018) Effects of blackcurrant and dietary fibers on large intestinal health biomarkers in rats. Plant Foods Hum Nutr 73(1):54–60. https://doi.org/10.1007/s11130-018-0652-7
Peng J, Jones GL, Watson K (2000) Stress proteins as biomarkers of oxidative stress: effects of antioxidant supplements. Free Radical Biol Med 28(11):1598–1606. https://doi.org/10.1016/S0891-5849(00)00276-8
Periard JD, Ruell P, Caillaud C, Thompson MW (2012) Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones 17(3):375–383. https://doi.org/10.1007/s12192-011-0313-3
Perkins RK, Miranda ER, Varshney P, Farabi SS, Quinn LT, Haus JM (2023) Effects of acute aerobic exercise on circulating sTLR and sRAGE profiles in normal- and abnormal-glucose-tolerant individuals. Physiol Rep 11(22):e15859. https://doi.org/10.14814/phy2.15859
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human bodY. J Appl Physiol 19:531–533. https://doi.org/10.1152/jappl.1964.19.3.531
Riebe D, Franklin BA, Thompson PD, Garber CE, Whitfield GP, Magal M, Pescatello LS (2015) Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc 47(11):2473–2479. https://doi.org/10.1249/mss.0000000000000664
Roth S, Spalinger MR, Gottier C, Biedermann L, Zeitz J, Lang S, Weber A, Rogler G, Scharl M (2016) Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS ONE 11(5):e0154817. https://doi.org/10.1371/journal.pone.0154817
Rowell LB (1974) Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54(1):75–159. https://doi.org/10.1152/physrev.1974.54.1.75
Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS (2007) American college of sports medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39(2):377–390. https://doi.org/10.1249/mss.0b013e31802ca597
Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA (1990) Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75(1):40–47
Selkirk GA, McLellan TM, Wright HE, Rhind SG (2008) Mild endotoxemia, NF-kappaB translocation, and cytokine increase during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol 295(2):R611-623. https://doi.org/10.1152/ajpregu.00917.2007
Shanely RA, Nieman DC, Zwetsloot KA, Knab AM, Imagita H, Luo B, Davis B, Zubeldia JM (2014) Evaluation of Rhodiola rosea supplementation on skeletal muscle damage and inflammation in runners following a competitive marathon. Brain Behav Immun 39:204–210. https://doi.org/10.1016/j.bbi.2013.09.005
Shen HH, Tseng YS, Kuo NC, Kung CW, Amin S, Lam KK, Lee YM (2019) Alpha-lipoic acid protects cardiomyocytes against heat stroke-induced apoptosis and inflammatory responses associated with the induction of Hsp70 and activation of autophagy. Mediators Inflamm 2019:8187529. https://doi.org/10.1155/2019/8187529
Slee EA, Adrain C, Martin SJ (1999) Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ 6(11):1067–1074. https://doi.org/10.1038/sj.cdd.4400601
Stankiewicz AR, Lachapelle G, Foo CPZ, Radicioni SM, Mosser DD (2005) Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing bax translocation*. J Biol Chem 280(46):38729–38739. https://doi.org/10.1074/jbc.M509497200
Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285(2):E433-437. https://doi.org/10.1152/ajpendo.00074.2003
Szymanski MC, Gillum TL, Gould LM, Morin DS, Kuennen MR (2018) Short-term dietary curcumin supplementation reduces gastrointestinal barrier damage and physiological strain responses during exertional heat stress. J Appl Physiol 124(2):330–340. https://doi.org/10.1152/japplphysiol.00515.2017
Taylor L, Hillman AR, Midgley AW, Peart DJ, Chrismas B, McNaughton LR (2012) Hypoxia-mediated prior induction of monocyte-expressed HSP72 and HSP32 provides protection to the disturbances to redox balance associated with human sub-maximal aerobic exercise. Amino Acids 43(5):1933–1944. https://doi.org/10.1007/s00726-012-1265-3
Tsai YC, Lam KK, Peng YJ, Lee YM, Yang CY, Tsai YJ, Yen MH, Cheng PY (2016) Heat shock protein 70 and AMP-activated protein kinase contribute to 17-DMAG-dependent protection against heat stroke. J Cell Mol Med 20(10):1889–1897. https://doi.org/10.1111/jcmm.12881
Zeynalov E, Shah ZA, Li RC, Dore S (2009) Heme oxygenase 1 is associated with ischemic preconditioning-induced protection against brain ischemia. Neurobiol Dis 35(2):264–269. https://doi.org/10.1016/j.nbd.2009.05.010
Zhang G, Liu Z, Ding H, Zhou Y, Doan HA, Sin KWT, Zhu ZJ, Flores R, Wen Y, Gong X, Liu Q, Li YP (2017) Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun 8(1):589. https://doi.org/10.1038/s41467-017-00726-x
Zuhl MN, Lanphere KR, Kravitz L, Mermier CM, Schneider S, Dokladny K, Moseley PL (2014) Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol 116(2):183–191. https://doi.org/10.1152/japplphysiol.00646.2013
Zuhl M, Dokladny K, Mermier C, Schneider S, Salgado R, Moseley P (2015) The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell Stress Chaperones 20(1):85–93. https://doi.org/10.1007/s12192-014-0528-1
Acknowledgements
The authors report no conflict of interest. Partial support for this work was provided by the Department of Health and Human Performance within the Congdon School of Health Sciences. Supply of PLACEBO and supplement (CurraNZ™) for this study was obtained from Health Currancy Ltd. (United Kingdom). Health Currancy Ltd had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results. We wish to thank the participants for their effort and dedication during the experiments. We would also like to thank the Department of Physical Therapy (Congdon School of Health Sciences) for the use of shared lab space and LJK for his lifetime of guidance and support.
Funding
Open access funding provided by the Carolinas Consortium.
Author information
Authors and Affiliations
Contributions
The study was designed by MRK, BJL, and METW. Physiological data were collected by BJL, TRF, LEVW, RC, and EFW. Biochemical analysis was conducted by BJL, TRF, MRK and GWH with assistance from NJC, and EPH. NJC, EPH, MRK, and TLG generated the first manuscript draft. Data interpretation and further manuscript preparation/revision were undertaken by BJL, MRK, TLG, and METW. METW is an editor of the European Journal of Applied Physiology and recused himself from all editorial decisions about this paper. All authors read and approved of the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Conrad, N.J., Heckler, E.P., Lee, B.J. et al. New Zealand blackcurrant extract modulates the heat shock response in men during exercise in hot ambient conditions. Eur J Appl Physiol (2024). https://doi.org/10.1007/s00421-024-05439-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00421-024-05439-w