Abstract
Purpose
Dietary nitrate (NO3−) supplementation can lower systolic blood pressure (SBP) and improve exercise performance. Salivary flow rate (SFR) and pH are key determinants of oral NO3− reduction and purported to peak in the afternoon. We tested the hypotheses that NO3−-rich beetroot juice (BR) would increase plasma [nitrite] ([NO2−]), lower SBP and improve exercise performance to a greater extent in the afternoon (AFT) compared to the morning (MORN) and evening (EVE).
Method
Twelve males completed six experimental visits in a repeated-measures, crossover design. NO3−-depleted beetroot juice (PL) or BR (~ 13 mmol NO3−) were ingested in the MORN, AFT and EVE. SFR and pH, salivary and plasma [NO3−] and [NO2−], brachial SBP and central SBP were measured pre and post supplementation. A severe-intensity exercise tolerance test was completed to determine cycling time to exhaustion (TTE).
Results
There were no between-condition differences in mean SFR or salivary pH. The elevation in plasma [NO2−] after BR ingestion was not different between BR-MORN, BR-AFT and BR-EVE. Brachial SBP was unchanged following BR supplementation in all conditions. Central SBP was reduced in BR-MORN (− 3 ± 4 mmHg), BR-AFT (− 4 ± 3 mmHg), and BR-EVE (− 2 ± 3 mmHg), with no differences between timepoints. TTE was not different between BR and PL at any timepoint.
Conclusion
Acute BR supplementation was ineffective at improving TTE and brachial SBP and similarly effective at increasing plasma [NO2−] and lowering central SBP across the day, which may have implications for informing NO3− supplementation strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diurnal variation in acute cardiovascular events is well established, with epidemiological data revealing increased incidence of strokes (Elliot 1998; Sheppard et al. 2015), myocardial infarctions (Cohen et al. 1997; Fabbian et al. 2017), and sudden cardiac death (Cohen et al. 1997) in the morning. Typical morning behaviours, including arousal from sleep, sudden postural changes, increased activity and psychological stress, instigate increases in sympathetic tone, vasoconstriction and peripheral arterial resistance, which contribute to the ‘morning surge’ in blood pressure (BP) (Kario 2010). Both peripheral and central BP exhibit parallel circadian rhythms, with lower values manifesting during night-time sleep, followed by abrupt increases with morning wakening before attaining peak values in the late afternoon (Douma and Gumz 2018). Diurnal fluctuations in exercise performance across a range of sport and exercise settings is also well established, with performance purported to attain peak and nadir levels in the afternoon and morning, respectively (Chtourou et al. 2011, 2012; Hammouda et al. 2012; Lericollais et al. 2009; Martin et al. 1999; Souissi et al. 2004; Hill 2014). Although BP and exercise performance exhibit a diurnal variation, the efficacy of dietary interventions to modulate these diurnal responses is unclear.
Dietary nitrate (NO3−) supplementation has been reported to improve various aspects of cardiovascular function, including lowering resting brachial BP and arterial stiffness variables, and to improve exercise performance (Jackson et al. 2018; Bahrami et al. 2021; Li et al. 2020; Senefeld et al. 2020). These effects have been linked to increased circulating plasma [nitrite] ([NO2−]), a substrate for nitric oxide (NO) production via the so-called NO3−–NO2−–NO pathway (Kapil et al. 2020). After ingestion, approximately 25% of NO3− enters the enterosalivary circulation, being absorbed by, and concentrated in, the salivary glands on the first-pass (Govoni et al. 2008). Subsequently, NO3−-rich saliva is secreted into the oral cavity wherein NO3− undergoes second-pass metabolism by anaerobic bacteria on the tongue which reduce salivary NO3− to NO2− (Doel et al. 2005; Duncan et al. 1995). Once swallowed, a portion of this NO2− is reduced to NO and other reactive nitrogen intermediates in the stomach (Benjamin et al. 1994), with some NO2− and reactive nitrogen intermediates entering systemic circulation for later NO generation (Kapil et al. 2020). Whilst NO3− supplementation has the potential to improve BP, vascular function, and exercise performance, it is currently unclear whether such effects are consistent across the day.
The efficacy of the NO3−–NO2−–NO pathway to elicit physiological effects is dependent on NO3− transport into the oral cavity and the host oral microbiome for NO3− reduction (Govoni et al. 2008; Bailey et al. 2016; Hezel and Weitzberg 2015; Jansson et al. 2008; Lundberg 2012). Indeed, when salivary NO3− uptake and secretion or oral NO3− reduction are impaired, the increase in plasma [NO2−] and lowering in BP after NO3− supplementation are attenuated (Govoni et al. 2008; Bailey et al. 2016; McDonagh et al. 2015). On the other hand, secretion of NO3− into the oral cavity and exposure to the oral NO3−-reducing anaerobes will be enhanced by increasing salivary flow rate (SFR). However, although previous research has shown that music stimuli can elevate saliva secretion and salivary NO2− generation (Jin et al. 2018), it is presently unclear whether diurnal variation in SFR impacts salivary and plasma [NO3−] and [NO2−].
In addition to SFR, salivary pH can impact oral NO3− reduction. Specifically, increasing salivary pH after NO3− supplementation has been reported to increase salivary and plasma [NO2−] (Cocksedge et al. 2023). Since both unstimulated SFR (Dawes 1975, 1972) and salivary pH (Choi et al. 2017; Ferguson and Fort 1974) exhibit circadian rhythms, with an acrophase in the afternoon, oral NO3− reduction and the resultant increases in salivary and plasma [NO2−] after NO3− supplementation may be greatest in the afternoon. Consistent with this postulate, oral NO3− reduction was reported to be enhanced in the afternoon compared with the morning during a mouth rinse with a KNO3− solution (Rowland et al. 2021). However, it is unclear whether enhanced oral NO3− reduction in the afternoon during a brief mouth rinse is reproducible after NO3− ingestion and whether this effect translates into greater plasma [NO2−], and more pronounced reductions in BP and improvements in exercise tolerance.
The purpose of this study was to investigate the effect of dietary NO3− supplementation on NO3− metabolism, peripheral and central BP, pulse wave variables, and exercise performance, and the extent to which any improvements in these variables after NO3− supplementation exhibited a diurnal variation. It was hypothesised that NO3− supplementation would increase salivary and plasma [NO2−], and improve brachial and central BP, pulse wave variables and exercise performance to a greater extent when ingested in the afternoon compared to the morning and evening.
Methods
Participants
Twelve young healthy males [mean ± SD: age: 23 ± 4 years, stature: 1.80 ± 0.09 m, body mass: 75.8 ± 10.9 kg, \(\dot{V}{\text{O}}_{{{\text{2peak}}}}\): 50.4 ± 8.5 ml.kg.min−1, gas exchange threshold (GET): 118 ± 32 W, peak aerobic power (PAP): 323 ± 68 W] volunteered to participate in this study. None of the participants were tobacco smokers (Bailey et al. 2016) or vapers or taking any medication known to interfere with stomach acid production (e.g., proton pump inhibitors). No participants had any pre-existing medical conditions such as hypertension or diabetes. All participants were classified as recreationally active (McKay et al. 2022). Experimental testing was approved by Loughborough University Research Ethics Approvals Human Participants Sub Committee (ethics code: R18-P145) and confirmed with the principles of the Declaration of Helsinki, apart from registration in a database. Participants gave their written informed consent to participate.
Pre-visit standardisation
Participants recorded their dietary intake 24 h prior to their first session and were asked to replicate this before subsequent visits. Each participant was given a list of NO3−- and thiocyanate-rich foods (Dewhurst-Trigg et al. 2018) to abstain from eating 24 h before sessions and asked to avoid caffeine and alcohol ingestion in the 12 h and 24 h before each visit, respectively. All visits were conducted in a postprandial state. Since SFR is reduced in a state of hypohydration (Ship and Fischer 1997), participants were provided with 40 mL·kg−1 body mass−1 of fluid to consume in the 24 h before each visit (Minshull and James 2013) and instructed to consume 500 mL of water 1 h before testing to ensure euhydration on arrival. During testing sessions, participants were given 300 mL of water in 2 equal boluses to ensure euhydration was maintained. Since antibacterial mouthwash disrupts oral NO3− reduction (Govoni et al. 2008), participants were required to abstain from using mouthwash 48 h prior to each testing session. Participants were instructed to maintain their habitual exercise patterns for the duration of the study but were required to avoid strenuous exercise in the 24 h prior to each visit.
Experimental design
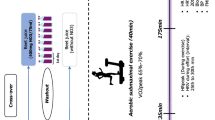
Participants reported to the laboratory on eight occasions. During the first visit, participants were familiarised with all the experimental procedures and completed a ramp incremental test for determination of GET, PAP and \(\dot{V}{\text{O}}_{{{\text{2peak}}}}\). GET is a non-invasive estimate of the lactate threshold and demarcates the boundary between the moderate and heavy intensity exercise domains. PAP was the maximum power output attained during the incremental ramp test. \(\dot{V}{\text{O}}_{{{\text{2peak}}}}\) is defined as the highest volume of oxygen uptake during the incremental ramp test. During visit two, participants were familiarised with the time to exhaustion (TTE) exercise protocol. In the six main experimental visits, baseline measures of SFR and pH, BP and vascular function (pulse wave analysis) were obtained, in sequence, and salivary and plasma samples were collected for later assessment of [NO3−] and [NO2−]. Urine and serum osmolality were also evaluated at baseline to assess hydration status. Subsequently, participants ingested 2 × 70 mL of concentrated NO3−-rich (BR; 13 mmol NO3−) or NO3−-depleted (PL; ~ 0.04 mmol NO3−) beetroot juice (Beet It, James White Drinks Ltd., Ipswich, UK) with 30 g of cornflakes and 125 mL of semi-skimmed milk. Saliva measurements were repeated 1 h post beetroot ingestion. All baseline measurements were then repeated 2.5 h following beetroot ingestion to coincide with the peak plasma [NO2−] (Wylie et al. 2013). Finally, participants completed the TTE exercise test. The six experimental conditions, PL and BR in the morning (started at 08:00; PL-MORN and BR-MORN), afternoon (started at 12:00; PL-AFT, BR-AFT) and evening (started at 15:00; PL-EVE and BR-EVE) were administered in a randomised, repeated-measures, crossover experimental design. PL and BR supplement administration was randomised (counterbalancing not possible due to the number of sequence permutations) and double-blinded (supplement bags labelled 1 and 2 by an independent investigator). Supplement ingestion occurred at 09:00, 13:00 and 16:00 in the MORN, AFT and EVE, respectively.
Measurements
Hydration status
Urine samples obtained at baseline was analyzed immediately to evaluate urine osmolality (Osmocheck, Vitech Scientific, UK), with a reading of < 700 mOsmol/kg required for visit continuation (Sawka et al. 2007). Additionally, 5 mL of venous blood was collected into a serum tube and left to clot at room temperature, with serum separated by centrifugation (3500 × g at 4 °C for 15 min) and frozen at − 80 °C for later analysis of osmolality via freezing-point depression (Gonotec 225 Osmomat 030 Cryoscopic Osmometer; Gonotec, Germany). Serum osmolality values ranging between 285 and 295 mOsmol.kg H2O−1 were taken to imply euhydration (Knepper et al. 2015).
Saliva collection
Prior to sample collection, participants rinsed their oral cavity with room-temperature tap water to remove any food debris. Following 2 min rest, unstimulated saliva samples were collected via passive drool and spit into pre-weighed sterile containers every 20 s for 2 min. This process was then repeated after 2 min. Samples were subsequently weighed for determination of SFR before salivary pH was measured in duplicate using a microFET electrode (Sentron, Leek, The Netherlands), with the measured pH value accepted once readings on the pH meter were stable for 5 s. A 3-point calibration of the pH probe was undertaken prior to analysis using buffers with known pH (4.01, 7.00, 10.01). 1 mL aliquots were then frozen at − 80 °C for later analysis of salivary [NO3−] and [NO2−]. Given that salivary [NO3−] and [NO2−] are influenced by SFR (Granli et al. 1989), salivary [NO3−] and [NO2−] data were also normalised to SFR to report salivary [NO3−] and [NO2−] flux per min.
Blood pressure
Participants were required to rest supine for 10 min. Thereafter, BP of the brachial artery was measured using an automated sphygmomanometer (Omron Healthcare, Kyoto, Japan). In total, five measurements were taken at 2 min intervals, with the mean of all five readings used for analysis. MAP was calculated as ([(2 × DBP) + SBP]/3).
Aortic blood pressure and pulse wave variables
Following 20 min supine rest, pulse wave analysis was assessed at the radial artery using applanation tonometry methods (SphygmoCor; Atcor Medical, Sydney, Australia) to determine central BP and indices of arterial stiffness. Pulse wave analysis calibrated to brachial BP uses a validated generalised transfer function to derive corresponding central aortic pressures (Chen et al. 1997). All tonometry data were recorded by a single investigator. A minimum of two recordings were taken at each time interval and the two measurements with the highest quality index (> 80%) were accepted for analysis. Pulse wave analysis indices of interest included: aortic systolic and diastolic BP, augmentation pressure (AP: the amplitude of the reflected wave), augmentation index (AI: the reflected wave amplitude divided by pulse pressure expressed as a percentage) and, due to the known influence of HR on AI, AI adjusted for heart rate of 75 bpm (AI@HR75).
Blood collection
Following 30 min supine rest, a tourniquet was applied around the upper arm prior to sample collection. Blood samples were subsequently drawn from an antecubital vein via venepuncture into 6 mL lithium heparin vacutainers. Samples were centrifuged at 3000 × g and 4 °C for 10 min, within 2 min of collection. Plasma was subsequently extracted and immediately frozen at − 80 °C for later analysis of [NO3−] and [NO2−].
Exercise procedures
All exercise tests were performed on an electronically-braked cycle ergometer (Lode Excalibur Sport, Groningen, The Netherlands). During the first laboratory visit, participants completed a ramp incremental test involving 4 min of baseline cycling at 20 W followed by a linear 30 W/min increase in work rate until task failure. Task failure was recorded once the pedal rate fell ≥ 10 rpm below self-selected cadence (70–100 rpm) for ≥ 5 s. The saddle and handlebar height and configuration were recorded and reproduced in subsequent tests. Breath-by-breath pulmonary gas exchange data were collected continuously during the incremental test and averaged over consecutive 10 s periods (Vyntus CPX metabolic cart, Vyaire Medical, Chicago, USA). Participants wore a face mask and breathed through a low dead space, low resistance, digital volume transducer assembly. The inspired and expired gas volume and gas concentration signals were continuously sampled via a capillary line connected to the mouthpiece. The gas analyser was calibrated prior to testing with gases of known concentration. The turbine volume transducer was calibrated automatically and manually using a 3 L syringe (Hans Rudolph, Kansas City, Missouri). \(\dot{V}{\text{O}}_{{{\text{2peak}}}}\) was taken as the highest 30 s mean value attained prior to the participant’s volitional exhaustion. GET, was determined from a cluster of measurements including (1) the first disproportionate increase in CO2 production (\(\dot{V}{\text{CO}}_{{\text{2}}}\)) from visual inspection of individual plots of \(\dot{V}{\text{CO}}_{{\text{2}}}\) vs. \(\dot{V}{\text{O}}_{{\text{2}}}\), (2) an increase in expired ventilation (\(\dot{V}{\text{E}}\))/\(\dot{V}{\text{O}}_{{\text{2}}}\) with no increase in \(\dot{V}{\text{E}}\)/\(\dot{V}{\text{CO}}_{{\text{2}}}\), and (3) an increase in end-tidal O2 tension with no fall in end-tidal CO2 tension. The TTE protocols involved 4 min cycling at 20 W followed by a step increase in work rate equivalent to 75%Δ (GET + 75% of the difference between the work rate at GET and PAP), with account taken for the mean response time for \(\dot{V}{\text{O}}_{{\text{2}}}\) during the ramp protocol (i.e., two-thirds of the ramp rate (20 W) deducted from the work rate at GET and PAP to account for the muscle-to-lung gas transit time). The test was terminated once pedal cadence fell ≥ 10 rpm below the self-selected cadence for ≥ 5 s. This exercise protocol was replicated during visits 3–8 and TTE was recorded.
[NO3 −] and [NO2 −] determination
All glassware, utensils and surfaces were rinsed thoroughly with deionised water to remove residual NO3− and NO2− prior to analysis. Plasma samples were deproteinised prior to [NO3−] determination. Firstly, 500 μL of 0.18 N NaOH was added to 100 µL of sample followed by 5 min incubation at room temperature. Subsequently, samples were treated with 300 μL aqueous ZnSO4 (5% w/v) and vortexed for 30 s before undergoing an additional 10 min incubation period at room temperature. Samples were then centrifuged at 21,000 × g for 10 min and the supernatant was removed for subsequent analysis. The [NO3−] of the deproteinised plasma sample was determined by its reduction to NO in the presence of 0.8% (w/v) vanadium chloride (VCl3) in 1 M HCl via 50 μL injections into the septum of the air-tight purge vessel. The spectral emission of electronically excited nitrogen dioxide, derived from the reaction of NO with ozone, was detected by a thermoelectrically cooled, red-sensitive photomultiplier tube housed in a gas-phase chemiluminescence NO analyser (Sievers NOA 280i, Analytix Ltd, Durham, UK). All samples were analyzed in duplicate. The [NO3−] was determined by plotting signal (mV) area against a calibration plot of sodium nitrate standards. Prior to plasma [NO2−] determination, samples were deproteinised using ice-cold ethanol. Specifically, 500 μL of ethanol was added to 500 μL of sample followed by 15 min incubation. Samples were then centrifuged at 21,000 × g for 10 min and the supernatant was removed for subsequent analysis. Plasma [NO2−] was determined by its reduction to NO in the presence of glacial acetic acid and aqueous sodium iodide (4% w/v) and calibrated using sodium nitrite standards. To determine plasma [NO2−], 200 μL of deproteinised plasma was injected into the purge vessel. Origin Lab was used to smooth the NO analyser signal and objectively identify the peaks to derive the NO2− concentration data. After thawing at room temperature, saliva samples were centrifuged for 10 min at 21,000 × g and the supernatant was then removed and diluted at least 100-fold with deionised water for subsequent analysis. [NO3−] and [NO2−] were determined using the same reagents described above for the respective plasma analyses.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 27. Shapiro Wilk’s test was used to check data normality. Baseline data and data containing one factor (condition [PL-MORN, BR-MORN, PL-AFT, BR-AFT, PL-EVE, BR-EVE] including hydration biomarkers, SFR, salivary pH, and TTE) were analyzed using one-way repeated-measures ANOVAs. Plasma [NO3−] and [NO2−], BP and vascular function were initially analysed using two-way repeated-measures ANOVAs (condition [PL-MORN, BR-MORN, PL-AFT, BR-AFT, PL-EVE, BR-EVE] × time [0 h and 2.5 h]). Salivary [NO3−] and [NO2−] were initially analyzed using two-way repeated-measures ANOVAs (condition [PL-MORN, BR-MORN, PL-AFT, BR-AFT, PL-EVE, BR-EVE] × time [0 h, 1 h, 2.5 h]). Significant ANOVA interaction effects were followed up with post hoc Dunnett’s tests for comparisons to baseline control for the salivary data and Holm-Bonferroni corrected paired-samples t tests were used for all other variables. To calculate effect sizes, partial eta squared (np2) was used for the omnibus tests and Cohen’s dz (t/√n) for paired-samples t tests. All data are displayed as mean ± SD unless otherwise stated. Statistical significance was accepted at P ≤ 0.05.
Results
Hydration biomarkers
For all participants, urine osmolality on arrival was < 700 mOsmol.kg H2O−1 in all six conditions. Serum osmolality was between 285 and 295 mOsmol kg H2O−1 across all visits and not different between conditions (P > 0.050, np2 = 0.03).
Salivary flow rate and pH
There were no inter-condition differences in SFR or salivary pH at baseline (both P > 0.050). Mean SFR and pH between 1 and 2.5 h did not differ between conditions (np2 = 0.14, np2 = 0.16, both P > 0.050, Fig. 1, respectively).
Mean salivary flow rate (SFR; upper panel) and salivary pH (lower panel) from 1 to 2.5 h following ingestion of nitrate-depleted and nitrate-rich beetroot juice in the morning (PL-MORN and BR-MORN), afternoon (PL-AFT and BR-AFT) and evening (PL-EVE and BR-EVE). The bars represent the group mean ± SEM responses with the filled circles representing individual participants. No differences observed between conditions (P > 0.050)
Salivary [NO3 −] and [NO2 −]
There were no inter-condition differences in salivary [NO3−] at baseline (P > 0.050). There was a main effect for condition (P < 0.001, np2 = 0.79) and time (P < 0.001, np2 = 0.84), and a condition × time interaction effect (P < 0.001, np2 = 0.71) for salivary [NO3−]. Compared to baseline, salivary [NO3−] was unchanged at 1 h and 2.5 h in PL-MORN (P > 0.050) but reduced at 2.5 h vs baseline in PL-AFT (P = 0.034) and PL-EVE (P = 0.018). Salivary [NO3−] was elevated above baseline at all time points in BR conditions (all P < 0.001), with no differences between BR-MORN, BR-AFT and BR-EVE at 1 h (dz ≤ 0.60) or 2.5 h (dz ≤ 0.05, both P > 0.050, Fig. 2). Normalising salivary [NO3−] relative to SFR did not alter any of the observed effects compared to absolute salivary [NO3−].
Salivary nitrate concentration ([NO3−], upper panel) and salivary nitrite concentration ([NO2−], lower panel) at baseline, 1 h and 2.5 h following ingestion of nitrate-depleted and nitrate-rich beetroot juice in the morning (PL-MORN and BR-MORN), afternoon (PL-AFT and BR-AFT) and evening (PL-EVE and BR-EVE). Data presented as the group mean ± SEM responses with the filled circles representing individual participants. *Denotes higher than PL-MORN, PL-AFT and PL-EVE in BR-MORN, BR-AFT and BR-EVE (P < 0.050). #denotes salivary [NO3−] lower than baseline at 2.5 h in PL-AFT and PL-EVE (P < 0.050)
There were no inter-condition differences in salivary [NO2−] at baseline (P > 0.050). There was a main effect for condition (P < 0.001, np2 = 0.74) and time (P < 0.001, np2 = 0.79), and a condition × time interaction effect (P < 0.001, np2 = 0.66) for salivary [NO2−]. Salivary [NO2−] was unchanged between 0 and 2.5 h in PL-MORN, PL-AFT and PL-EVE (all P > 0.050). Salivary [NO2−] was increased above baseline at all time points in BR-MORN, BR-AFT and BR-EVE (all P < 0.001), with no inter-condition differences at 1 h (dz ≤ 0.16) or 2.5 h (dz ≤ 0.42, both P > 0.050, Fig. 2). Normalising salivary [NO2−] to SFR did not change any of the observed effects compared to absolute salivary [NO2−].
Plasma [NO3 −] and [NO2 −]
Plasma [NO3−] was not different between conditions at baseline (P > 0.050). There was a main effect for condition (P < 0.001, np2 = 0.95) and time (P < 0.001, np2 = 0.96), and a condition × time interaction effect (P < 0.001, np2 = 0.92). Plasma [NO3−] remained stable between baseline and 2.5 h in PL-MORN, PL-AFT and PL-EVE (all P > 0.050) but was elevated above baseline at 2.5 h in BR-MORN (640 ± 141 µM), BR-AFT (663 ± 79 µM) and BR-EVE (626 ± 154 µM) (all P < 0.001), with no differences between the BR conditions (P > 0.050, dz ≤ 0.31, Fig. 3).
Plasma nitrate concentration ([NO3−], upper panel) and plasma nitrite concentration ([NO2−], lower panel) 2.5 h following ingestion of nitrate-depleted and nitrate-rich beetroot juice in the morning (PL-MORN and BR-MORN), afternoon (PL-AFT and BR-AFT) and evening (PL-EVE and BR-EVE). The bars represent the group mean ± SD responses with the filled circles representing individual participants. *Denotes higher than PL-MORN, PL-AFT and PL-EVE in BR-MORN, BR-AFT and BR-EVE (P < 0.050)
Plasma [NO2−] was not different between conditions at baseline (P > 0.050). There was a main effect for condition (P < 0.001, np2 = 0.73) and time (P < 0.001, np2 = 0.80), and a condition × time interaction effect (P < 0.001, np2 = 0.76). Plasma [NO2−] was unchanged between 0 and 2.5 h in PL-MORN (P > 0.050) but decreased in PL-AFT (P = 0.027) and PL-EVE (P = 0.050). Plasma [NO2−] was increased above baseline 2.5 h post supplement ingestion in BR-MORN (642 ± 289 nM), BR-AFT (670 ± 314 nM) and BR-EVE (675 ± 355 nM) (all P < 0.001), with no differences between conditions (P > 0.050, dz ≤ 0.11, Fig. 3).
Brachial artery blood pressure
Systolic blood pressure
There were no inter-condition differences in brachial SBP at baseline (P > 0.050). There was a main effect for time (P = 0.007, np2 = 0.50), but no main effect for condition (P > 0.050, np2 = 0.05) or condition × time interaction (P > 0.050, np2 = 0.09, Table 1).
Diastolic blood pressure
There were no inter-condition differences in brachial DBP at baseline (P > 0.050). There was a condition × time interaction (P = 0.005, np2 = 0.25), but no main effect for condition (P > 0.050, np2 = 0.03) or time (P > 0.050, np2 = 0.11). Follow up post-hoc analysis revealed that brachial DBP was unchanged over time in PL-MORN (dz = 0.46), PL-AFT (dz = 0.60) and PL-EVE (dz = 0.67), and in BR-MORN (dz = 0.08), BR-AFT (dz = 0.51), and BR-EVE (dz = 0.34, all P > 0.050, Table 1).
Mean arterial pressure
There were no inter-condition differences in brachial MAP at baseline (P > 0.050) or any main effects for condition (P > 0.050, np2 = 0.05) or time (P > 0.050, np2 = 0.02), or a condition × time interaction (P > 0.050, np2 = 0.11, Table 1).
Aortic blood pressure and arterial stiffness
Central systolic blood pressure
There were no inter-condition differences in central SBP at baseline (P > 0.050). There was a main effect for time (P = 0.011, np2 = 0.49) and condition × time interaction (P = 0.007, np2 = 0.27) but no main effect for condition (P > 0.050, np2 = 0.04). Central SBP was unchanged over time in PL-MORN (dz = 0.09), PL-AFT (dz = 0.19) and PL-EVE (dz = 0.28, all P > 0.050). Central SBP was lower at 2.5 h compared to baseline within BR-MORN (P = 0.030, dz = 0.88), BR-AFT (P = 0.009, dz = 1.19) and BR-EVE (P = 0.046, dz = 0.69), with no differences between these conditions (P > 0.050, dz ≤ 0.44, Table 2).
Central diastolic blood pressure
There were no inter-condition differences in central DBP at baseline (P > 0.050). There was no main effect for condition (P > 0.050, np2 = 0.02) or time (P > 0.050, np2 = 0.02), but there was a condition × time interaction effect (P = 0.011, np2 = 0.25). Post hoc analysis revealed no significant differences between conditions or over time (P > 0.050, Table 2).
Augmentation pressure, augmentation index and augmentation index normalised to heart rate
There were no inter-condition differences in AP, AI or AI@HR75 at baseline (all P > 0.050). There was no main effect for condition (P > 0.050, np2 = 0.06) or time (P > 0.050, np2 = 0.05) but there was a significant condition × time interaction (P = 0.029, np2 = 0.22) for AP. AP was lower at 2.5 h vs baseline in BR-AFT (P = 0.045, np2 = 0.89), but no differences were observed in the other conditions (all P > 0.050, Table 2). There was no main effect for condition (P > 0.050, np2 = 0.08) or time (P > 0.050, np2 = 0.17) for AI; however, there was a condition × time interaction (P = 0.022, np2 = 0.23). Post hoc analysis revealed no significant differences between conditions (P > 0.050, Table 2). There was a main effect for time (P = 0.024, np2 = 0.42) for AI@HR75, but no main effect for condition (P > 0.050, np2 = 0.04) or condition × time interaction (P > 0.050, np2 = 0.19, Table 2).
Exercise performance
Exercise TTE did not differ between PL-MORN (307 ± 96 s), BR-MORN (308 ± 71 s), PL-AFT (321 ± 81 s), BR-AFT (311 ± 68 s), PL-EVE (306 ± 76 s) and BR-EVE (318 ± 83 s), (P > 0.050, np2 = 0.02; Fig. 4).
Time to exhaustion (TTE) during severe-intensity cycling exercise following ingestion of nitrate-depleted or nitrate-rich beetroot juice in the morning (PL-MORN and BR-MORN), afternoon (PL-AFT and BR-AFT) and evening (PL-EVE and BR-EVE) (upper panel). The bars represent the group mean ± SEM responses with the filled circles representing individual participants. No differences observed between conditions (P > 0.050)
Discussion
This study assessed whether the time-of-day an acute dose of dietary NO3− was administered influenced its efficacy to lower BP and improve exercise performance in healthy adults. The principal novel findings from this study were: (1) SFR, salivary pH, BP and exercise performance did not exhibit a marked circadian rhythm; (2) salivary and plasma [NO3−] and [NO2−] were increased by a similar magnitude after BR ingestion in the morning, afternoon and evening; (3) BR consumption lowered central SBP by a similar magnitude across the day but did not reduce brachial SBP; and (4) severe-intensity cycling TTE was not improved with BR supplementation irrespective of the time of day ingested. These findings improve understanding of the effects of acute BR supplementation on BP, vascular function and exercise performance in healthy young men by evaluating the potential for time-specific effects of BR supplementation on these health indices.
Salivary flow rate and pH
Previous studies have shown both unstimulated SFR (Dawes 1975, 1972) and salivary pH (Choi et al. 2017; Ferguson and Fort 1974) exhibit circadian variability, being lowest during sleep and the early morning and peaking mid-afternoon. Contrary to previous findings, SFR and salivary pH did not exhibit a circadian rhythm in the current study with these variables not being significantly different across the morning, afternoon, and evening assessment points. It is well documented that food and fluid consumption can alter SFR and salivary pH (Ship and Fischer 1997; Brunstrom et al. 2000; Watanabe and Dawes 1988). Since fluid consumption and food intake was standardised over the 24 h preceding each testing session and participants were objectively determined to be euhydrated in the current study, the lack of diurnal variation on SFR and salivary pH is unlikely to be a result of altered dietary intake. However, it is plausible that our hydration protocol may have overridden the underlying daily rhythm in SFR. Moreover, both SFR and salivary pH were highly variable between-participants, which likely impeded the detection of any subtle changes in these variables across the day. We concede that a larger sample size may have been necessary to detect subtle changes in SFR and salivary pH. It should also be acknowledged that the methods used to collect and measure SFR and pH may not have been sufficiently sensitive to detect small diurnal variability in these responses. Previous studies have used passive drool techniques or fitted oral collection devices to collect saliva and have collected samples for 11–12 successive days between ~ 07:00 and 22:00 to evaluate SFR (Dawes 1975, 1972). Similarly, a previous study reporting circadian-like patterns in salivary pH captured continuous changes over 48 h using custom-made intraoral appliances (Choi et al. 2017). In contrast, the current study only measured SFR and salivary pH on nine occasions between 08:00 and 18:30 and with different techniques, which may account for the lack of a significant within-day variability in SFR or salivary pH herein.
Dietary nitrate metabolism
Salivary and plasma [NO3−] and [NO2−] were not different between pre-supplementation baseline measures during the morning, afternoon, and evening experimental testing sessions. Consistent with previous research (Bailey et al. 2016; Cocksedge et al. 2023; Burleigh et al. 2018; Woessner et al. 2016), both salivary and plasma [NO3−] and [NO2−] increased following the acute ingestion of BR in the present study. However, contrary to the experimental hypothesis, the increases in salivary and plasma [NO3−] and [NO2−] after BR supplementation were consistent across the morning, afternoon, and evening. These findings align with previous research which has shown stability in plasma and urinary [NO3−] (Ringqvist et al. 2000) and plasma [NOx] (Tangphao et al. 1999) over a 24 h period. The lack of diurnal variation in the evaluated NO3− metabolism biomarkers in the current study may be partially attributed to the absence of a circadian rhythm in SFR and salivary pH. However, it is worth acknowledging that there may be diurnal variation in salivary and plasma [NO3−] and [NO2−] if assessed using more ecologically valid experimental designs (i.e., where individuals maintain their habitual dietary intake and activity levels) due to the NO3− content of the diet with different meals.
Blood pressure and vascular function
Despite evidence of circadian rhythms in BP reported in the literature (Douma and Gumz 2018; Williams et al. 2013; Jankowski et al. 2013; Boggia et al. 2016), no differences in baseline pre-supplementation brachial BP were observed between the morning, afternoon, and evening in healthy young men in the present study. Previous studies assessing 24 h BP have reported that the amplitudes of basal resting rhythms are small (3–6 mmHg peak-to-trough) in healthy young men and women (Scheer et al. 2010). Moreover, and also contrary to the experimental hypothesis, brachial SBP was not lowered in the morning, afternoon or evening after acute BR ingestion. Numerous previous studies have assessed the potential of NO3− supplementation to lower BP, with a lowering in brachial SBP after NO3− consumption observed in several (Bahadoran et al. 2017; Kapil et al. 2010; Larsen et al. 2006; Siervo et al. 2013; Bailey et al. 2010), but not all, previous studies (Siervo et al. 2013; Cermak et al. 2012; Zoughaib et al. 2023; Shepherd et al. 2015; Walker et al. 2019). However, a principal original contribution of the current study was evaluating the efficacy of NO3− supplementation to lower brachial BP at three different time points across the day and the observation that brachial SBP was not lowered in the morning, afternoon or evening after acute BR ingestion. The factors that regulate BP across the day are multifaceted (including but not limited to renal haemodynamics, the nervous system and mental/emotional stress) and highly complex (Smolensky et al. 2017), and as such it is unclear why reductions in brachial BP were not observed in the present study.
In contrast to the peripheral brachial artery SBP response, aortic SBP was lowered from the pre-supplementation baseline after BR supplementation by a similar magnitude in the morning, afternoon and evening. Lower aortic SBP after acute NO3− supplementation has been reported in some (Pekas et al. 2021; Kukadia et al. 2019; Hughes et al. 2016; Kim et al. 2019), but not all (Floyd et al. 2019) previous studies. In studies assessing both aortic and brachial SBP, a concurrent lowering in central and peripheral SBP has been reported (Pekas et al. 2021; Hughes et al. 2016; Kim et al. 2019), however, some studies have observed a reduction in central but not peripheral SBP (Kukadia et al. 2019; Mills et al. 2020). In contrast with the findings of the current study, both central and peripheral SBP have been reported to be lowered after acute NO3− supplementation in the morning (Pekas et al. 2021; Hughes et al. 2016; Kim et al. 2019), with studies reporting a greater effect on central than peripheral SBP not specifying the time of the day the assessments were completed (Kukadia et al. 2019; Mills et al. 2020). Both the lowering of central SBP and the increases in plasma [NO2−] after BR ingestion were consistent across timepoints in the current study. Therefore, while previous studies have reported a strong agreement between plasma [NO2−] and brachial SBP after acute NO3− supplementation, the current study suggests that central, but not brachial, SBP is modulated by circulating plasma [NO2−]. The mechanisms for the lowering of brachial SBP after NO3− ingestion have been considered to be linked to the reduction of circulating plasma NO2− to NO leading to elevated cyclic guanosine monophosphate signalling leading to vasodilation (Kapil et al. 2020, 2010). However, NO2− can directly elicit vasodilation via s-nitrosylation (Bryan et al. 2005). In addition to increasing plasma [NO2−], acute BR ingestion has been demonstrated to increase plasma S-nitrosothiol concentrations (Abu-Alghayth et al. 2021) which have been suggested to more closely reflect the improvement in vascular function after NO3− ingestion than plasma [NO2−] (Pinheiro et al. 2015). It has also been suggested that the lowering in BP with elevated NO2− can occur via mechanisms independent of NO-cGMP signalling, and instead related to a novel, alternative redox pathway (mediated by hydrogen peroxide, persulfides and oxidation of protein kinase G1α), which culminates in NO-independent vasorelaxation (Feelisch et al. 2020). Therefore, further research is required to assess the mechanisms for the lowering in SBP after acute BR ingestion in humans and the extent to which this may differ in the control of central and peripheral SBP.
Acute BR ingestion largely did not modulate indices of arterial stiffness in the current study. Specifically, AP and AI were unaltered after BR ingestion in the morning or evening, but there was a small lowering in AP after BR ingestion in the afternoon. The clinical relevance of this finding in healthy young normotensive males is unclear (Wojciechowska et al. 2006). The general lack of improvement in pulse wave variables after acute NO3− ingestion is consistent with most (Pekas et al. 2021; Kim et al. 2019; Liu et al. 2013), but not all (Hughes et al. 2016), previous studies reporting the effects of BR on AP and AI in the morning. The current study extends these previous observations by assessing the effects of BR supplementation on these variables in the afternoon and evening. It is possible that chronic NO3− supplementation is required to improve pulse wave variables (Li et al. 2020) since arterial remodelling, including changes in the timing and/or magnitude of reflected waves from the peripheral arterial tree, may be necessary to elicit changes in central haemodynamics. Longer term NO3− supplementation and the potential for greater overall NO exposure could positively modulate endothelial homeostasis (Carlström et al. 2018), and vascular gene expression to support vascular function (Rammos et al. 2015). In turn, such effects could contribute to lower vascular resistance and associated pulse wave variables. Therefore, our findings suggest that acute BR ingestion is more likely to lower central versus peripheral SBP, and to not influence indices of arterial stiffness in healthy young males. This finding is consistent with research in pre-diabetic and diabetic individuals after daily ingestion of dietary NO3− for 6 months (Faconti et al. 2019). Although exact mechanisms of action are unclear, lowered SBP yet unaltered arterial stiffness with NO3− consumption has been postulated to be due in part to increased venodilation leading to decreased preload (Mills et al. 2020).
Exercise performance
There were no changes in high-intensity cycling TTE between the morning, afternoon and evening timepoints after PL ingestion in the current study. This observation conflicts with previous studies reporting diurnal variation in maximal voluntary contractions (Chtourou et al. 2012; Martin et al. 1999), Wingate test performance (Chtourou et al. 2012, 2011; Hammouda et al. 2012; Lericollais et al. 2009; Souissi et al. 2004) and exhaustive severe-intensity cycling exercise (Hill 2014), with performance typically peaking in the afternoon and being lowest in the morning. Acute ingestion of BR providing ~ 13 mmol NO3− did not improve TTE during high-intensity cycling in the current study. While the existing literature generally supports a small but significant effect of NO3− supplementation to improve performance across a range of exercise settings (Senefeld et al. 2020), and acute NO3− supplementation has previously been reported to enhance performance during continuous high-intensity exercise tests (Wylie et al. 2013), there are also previous studies reporting no ergogenic effects in such settings (Cocksedge et al. 2020). It has previously been reported that sprint cycling performance is impaired in the morning compared to the afternoon and that acute BR ingestion (providing ~ 6.5 mmol NO3−) can improve sprint cycling performance in the morning such that it is not different from the afternoon (Dumar et al. 2021). However, a limitation of that study was the lack of an appropriate placebo supplement to ensure the participants were blinded to the treatment conditions. The current study expands on this previous study by indicating that acute BR ingestion did not improve performance compared to PL in the morning, afternoon or evening. The lack of an ergogenic effect in the current study is unlikely to be due to an insufficient NO3− dose (Wylie et al. 2013). There is some evidence to suggest that NO3− supplementation may be more likely to improve continuous high-intensity exercise performance after multiple-day supplementation, due to the greater time course needed to increase muscle [NO2−] (Kadach et al. 2023; Gilliard et al. 2018; Wylie et al. 2019) and subsequently impact skeletal muscle contractile function (Cermak et al. 2012; Jones et al. 2018), which may account for the lack of ergogenic effect of acute BR ingestion in the current study.
Potential implications and experimental considerations
The findings of the current study suggest that acute BR supplementation is more likely to lower central SBP than brachial artery SBP across the course of the day. Whilst the measurement of brachial artery BP is well-established and provides strong clinical prognostic value, the importance of assessing central aortic BP and indices of aortic wave reflections has been clearly established in recent years (McEniery et al. 2014; Siervo et al. 2013). Indeed, the coronary arteries are exposed to central rather than peripheral pressures, which may account for observations that cardiovascular events may be more closely related to central pressures (McEniery et al. 2014). Moreover, the greater effect of BR ingestion on lowering central compared to brachial SBP is consistent with some studies reporting that central SBP is more likely to respond to antihypertensive treatments than brachial SBP (McEniery et al. 2014). The clinical relevance of the observed reductions in central SBP in healthy young men is currently unknown and warrants additional investigation. However, central SBP readings > 125 mmHg are associated with a significant increase in atherosclerotic cardiovascular outcomes, and for every 10 mmHg increase in central SBP the risk of an adverse cardiovascular outcomes increases by 11.7% (Kwon et al. 2022). This is important since resolving the time of day that administration of antihypertensive interventions elicits the optimal effects on cardiovascular health and cardioprotection remains unclear and an active area or research in cardiovascular medicine (Mackenzie et al. 2022; Hermida et al. 2010).
Limitations
A challenge of administering NO3− acutely is that the second-pass metabolism of NO3− delays the attainment of peak plasma [NO2−] until ~ 2–4 h post ingestion, and assessments of BP and exercise performance are recommended to take place upon attainment of peak plasma [NO2−] (Wylie et al. 2013). To accommodate for the slow plasma [NO2−] pharmacokinetics after NO3− ingestion, a limitation of the current study is that BP, vascular function and exercise performance measures were assessed early afternoon (~ 12:00–13:00), mid-afternoon (~ 16:00–17:00) and early evening (~ 19:00–20:00) after, respectively, ingesting BR in the morning (~ 09:00), early afternoon (~ 13:00) and mid-afternoon (~ 16:00). Therefore, the morning BP surge was not assessed in the current study, and the exercise test may not have been conducted early enough in the day to detect previously reported morning decrements in performance. Moreover, research in chronobiology has investigated the effect of chronotype; an individual’s predisposition towards morningness and eveningness, on responses to exercise (Vitale and Weydahl 2017). Studies have shown that circadian rhythms of physiological variables such as temperature are shifted dependent on chronotype characterisation, with biological rhythms in morningness types showing earlier peaks and troughs compared to eveningness types (Baehr et al. 2000; Bailey and Heitkemper 2001). Since chronotype was not characterized for the participants in the current study, this may have contributed to the observation of marked diurnal variability in exercise tolerance in the current study.
Conclusion
In young healthy males, we observed no significant circadian variability in SFR, salivary pH, salivary and plasma [NO3−] and [NO2−], brachial or central BP or high-intensity exercise TTE across the non-supplemented baseline assessments. Acute NO3−-rich BR consumption resulted in similar increases in salivary and plasma [NO3−] and [NO2−] and reductions in central SBP in the morning, afternoon, and evening. In contrast, brachial SBP was unchanged following BR supplementation in the morning, afternoon, and evening and TTE was not improved at any of the timepoints assessed after BR ingestion. These findings improve our understanding of the effect of BR supplementation on BP, vascular function and exercise performance and suggest that central SBP is consistently lowered across the day after BR supplementation in healthy adults.
Data availability
Data are available upon reasonable request.
Abbreviations
- AFT:
-
Afternoon
- BP:
-
Blood pressure
- BR:
-
Nitrate-rich beetroot juice
- EVE:
-
Evening
- GET:
-
Gas exchange threshold
- NO:
-
Nitric oxide
- NO2 − :
-
Nitrite
- NO3 − :
-
Nitrate
- MORN:
-
Morning
- PAP:
-
Peak aerobic power
- PL:
-
Nitrate-depleted beetroot juice
- SBP:
-
Systolic blood pressure
- SFR:
-
Salivary flow rate
- TTE:
-
Time to exhaustion
- \(\dot{V}{\text{CO}}_{{\text{2}}}\) :
-
Carbon dioxide production
- \(\dot{V}{\text{E}}\) :
-
Expired ventilation
- \(\dot{V}{\text{O}}_{{\text{2}}}\) :
-
Oxygen consumption
- \(\dot{V}{\text{O}}_{{{\text{2peak}}}}\) :
-
Peak aerobic capacity
References
Abu-Alghayth M, Vanhatalo A, Wylie LJ, McDonagh STJ, Thompson C, Kadach S, Kerr P, Smallwood MJ, Jones AM, Winyard PG (2021) S-nitrosothiols, and other products of nitrate metabolism, are increased in multiple human blood compartments following ingestion of beetroot juice. Redox Biol 43:101974. https://doi.org/10.1016/j.redox.2021.101974
Baehr EK, Revelle W, Eastman CI (2000) Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res 9:117–127. https://doi.org/10.1046/j.1365-2869.2000.00196.x
Bahadoran Z, Mirmiran P, Kabir A, Azizi F, Ghasemi A (2017) The nitrate-independent blood pressure-lowering effect of beetroot juice: a systematic review and meta-analysis. Adv Nutr: Int Rev J 8:830–838. https://doi.org/10.3945/an.117.016717
Bahrami LS, Arabi SM, Feizy Z, Rezvani R (2021) The effect of beetroot inorganic nitrate supplementation on cardiovascular risk factors: a systematic review and meta-regression of randomized controlled trials. Nitric Oxide 115:8–22. https://doi.org/10.1016/j.niox.2021.06.002
Bailey SL, Heitkemper MM (2001) Circadian rhythmicity of cortisol and body temperature: Morningness-eveningness effects. Chronobiol Int 18:249–261. https://doi.org/10.1081/CBI-100103189
Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM (2010) Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol 109:135–148. https://doi.org/10.1152/japplphysiol.00046.2010
Bailey SJ, Blackwell JR, Wylie LJ, Holland T, Winyard PG, Jones AM (2016) Improvement in blood pressure after short-term inorganic nitrate supplementation is attenuated in cigarette smokers compared to non-smoking controls. Nitric Oxide 61:29–37. https://doi.org/10.1016/j.niox.2016.10.002
Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H (1994) Stomach NO synthesis. Nature 368:502–502. https://doi.org/10.1038/368502a0
Boggia J, Luzardo L, Lujambio I, Sottolano M, Robaina S, Thijs L, Olascoaga A, Noboa A, Struijker-Boudier HA, Safar ME, Staessen JA (2016) The diurnal profile of central hemodynamics in a general Uruguayan population. Am J Hypertens 6:737–746. https://doi.org/10.1093/ajh/hpv169
Brunstrom J, Tribbeck P, MacRae AW (2000) The role of mouth state in the termination of drinking behavior in humans. Physiol Behav 68:579–583. https://doi.org/10.1016/S0031-9384(99)00210-3
Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M (2005) Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1:290–297. https://doi.org/10.1038/nchembio734
Burleigh MC, Liddle L, Monaghan C, Muggeridge DJ, Sculthorpe N, Butcher JP, Henriquez FL, Allen JD, Easton C (2018) Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic Biol Med 120:80–88. https://doi.org/10.1016/j.freeradbiomed.2018.03.023
Carlström M, Lundberg JO, Weitzberg E (2018) Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol 224:13080. https://doi.org/10.1111/apha.13080
Cermak NM, Gibala MJ, Van Loon LJC (2012) Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab 1:64–71. https://doi.org/10.1123/ijsnem.22.1.64
Chen CH, Nevo E, Fetics B, Pak PH, Yin FCP, Maughan WL, Kass DA (1997) Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Circulation 95:1827–1836. https://doi.org/10.1161/01.CIR.95.7.1827
Choi JE, Lyons KM, Kieser JA, Waddell NJ (2017) Diurnal variation of intraoral pH and temperature. BDJ Open 3:170015. https://doi.org/10.1038/bdjopen.2017.15
Chtourou H, Zarrouk N, Chaouachi A, Dogui M, Behm DG, Chamari K, Hug F, Souissi N (2011) Diurnal variation in Wingate-test performance and associated electromyographic parameters. Chronobiol Int 8:706–713. https://doi.org/10.3109/07420528.2011.596295
Chtourou H, Chaouachi A, Driss T, Dogui M, Behm DG, Chamari K, Souissi N (2012) The effect of training at the same time of day and tapering period on the diurnal variation of short exercise performances. J Strength Cond Res 3:697–708. https://doi.org/10.1519/JSC.0b013e3182281c87
Cocksedge SP, Breese BC, Morgan PT, Nogueira L, Thompson C, Wylie LJ, Jones AM, Bailey SJ (2020) Influence of muscle oxygenation and nitrate-rich beetroot juice supplementation on O2 uptake kinetics and exercise tolerance. Nitric Oxide 99:25–33. https://doi.org/10.1016/j.niox.2020.03.007
Cocksedge SP, Causer AJ, Winyard PJ, Jones AM, Bailey SJ (2023) Oral temperature and pH influence dietary nitrate metabolism in healthy adults. Nutrients 15:784. https://doi.org/10.3390/nu15030784
Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA (1997) Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol 11:1512–1516. https://doi.org/10.1016/S0002-9149(97)00181-1
Dawes C (1972) Circadian rhythms in human salivary flow rate and composition. J Physiol 220:529–545. https://doi.org/10.1113/jphysiol.1972.sp009721
Dawes C (1975) Circadian rhythms in the flow rate and composition of unstimulated and stimulated human submandibular saliva. J Physiol 244:535–548. https://doi.org/10.1113/jphysiol.1975.sp010811
Dewhurst-Trigg R, Yeates T, Blackwell JR, Thompson C, Linoby A, Morgan PT, Clarke I, Connolly LJ, Wylie LJ, Winyard PG, Jones AM, Bailey SJ (2018) Lowering of blood pressure after nitrate-rich vegetable consumption is abolished with the co-ingestion of thiocyanate-rich vegetables in healthy normotensive males. Nitric Oxide 74:39–46. https://doi.org/10.1016/j.niox.2018.01.009
Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP (2005) Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci 113:14–19. https://doi.org/10.1111/j.1600-0722.2004.00184.x
Douma LG, Gumz ML (2018) Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 119:108–114. https://doi.org/10.1016/j.freeradbiomed.2017.11.024
Dumar AM, Huntington AF, Rogers RR, Kopec TJ, Williams TD, Ballmann CG (2021) Acute beetroot juice supplementation attenuates morning-associated decrements in supramaximal exercise performance in trained sprinters. Int J Environ Res Public Health 18:412. https://doi.org/10.3390/ijerph18020412
Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N (1995) Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1:546–551. https://doi.org/10.1038/nm0695-546
Elliott WJ (1998) Circadian variation in the timing of stroke onset: a meta-analysis. Stroke 29:992–996. https://doi.org/10.1161/01.STR.29.5.992
Fabbian F, Bhatia S, de Giorgi A, Maietti E, Bhatia S, Shanbhag A, Deshmukh A (2017) Circadian periodicity of ischemic heart disease: a systematic review of the literature. Heart Fail Clin 4:673–680. https://doi.org/10.1016/j.hfc.2017.05.003
Faconti L, Mills CE, Govoni V, Gu H, Morant S, Jiang B, Cruickshank JK, Webb AJ (2019) Cardiac effects of 6 months’ dietary nitrate and spironolactone in patients with hypertension and with/at risk of type 2 diabetes, in the factorial design, double-blind, randomized controlled VaSera trial. Br J Clin Pharmacol 85:169–180. https://doi.org/10.1111/bcp.13783
Feelisch M, Akaike T, Griffiths K, Ida T, Prysyazhna O, Goodwin JJ, Gollop ND, Fernandez BO, Minnion M, Cortese-Krott MM, Borgognone A, Hayes RM, Eaton P, Frenneaux MP, Madhani M (2020) Long-lasting blood pressure lowering effects of nitrite are NO-independent and mediated by hydrogen peroxide, persulfides, and oxidation of protein kinase G1α redox signalling. Cardiovasc Res 116:51–62. https://doi.org/10.1093/cvr/cvz202
Ferguson DB, Fort A (1974) Circadian variations in human resting submandibular saliva flow rate and composition. Arch Oral Biol 19:47–55. https://doi.org/10.1016/0003-9969(74)90224-6
Floyd CN, Lidder S, Hunt J, Omar SA, McNeill K, Webb AJ (2019) Acute interaction between oral glucose (75 g as Lucozade) and inorganic nitrate: decreased insulin clearance, but lack of blood pressure-lowering. Br J Clin Pharmacol 85:1443–1453. https://doi.org/10.1111/bcp.13913
Gilliard CN, Lam JK, Cassel KS, Park JW, Schechter AN, Piknova B (2018) Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide 75:1–7. https://doi.org/10.1016/j.niox.2018.01.010
Govoni M, Jansson EÅ, Weitzberg E, Lundberg JO (2008) The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19:333–337. https://doi.org/10.1016/j.niox.2008.08.003
Granli T, Dahl R, Brodin P, Bøckman OC (1989) Nitrate and nitrite concentrations in human saliva: variations with salivary flow-rate. Food Chem Toxicol 27:675–680. https://doi.org/10.1016/0278-6915(89)90122-1
Hammouda O, Chtourou H, Chahed H, Ferchichi S, Chaouachi A, Kallel C, Miled A, Chamari K, Souissi N (2012) High intensity exercise affects diurnal variation of some biological markers in trained subjects. Int J Sports Med 11:886–891. https://doi.org/10.1055/s-0032-1301887
Hermida RC, Ayala DE, Mojón A, Fernández JR (2010) Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiol Int 27:1629–1651. https://doi.org/10.3109/07420528.2010.510230
Hezel M, Weitzberg E (2015) The oral microbiome and nitric oxide homoeostasis. Oral Dis 21:7–16. https://doi.org/10.1111/odi.12157
Hill D (2014) Morning-evening differences in response to exhaustive severe-intensity exercise. Appl Physiol, Nutr, Metab 39(2):248–254. https://doi.org/10.1139/apnm-2013-0140
Hughes WE, Ueda K, Treichler DP, Casey DP (2016) Effects of acute dietary nitrate supplementation on aortic blood pressure and aortic augmentation index in young and older adults. Nitric Oxide 59:21–27. https://doi.org/10.1016/j.niox.2016.06.007
Jackson JK, Patterson AJ, MacDonald-Wicks LK, Oldmeadow C, McEvoy MA (2018) The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: a systematic review and meta-analysis of human evidence. Nutr Rev 76:348–371. https://doi.org/10.1093/nutrit/nuy005
Jankowski P, Bednarek A, Olszanecka A, Windak A, Kawecka-Jaszcz K, Czarnecka D (2013) Twenty-four-hour profile of central blood pressure and central-to- peripheral systolic pressure amplification. Am J Hypertens 1:27–33. https://doi.org/10.1093/ajh/hps030
Jansson EÅ, Huang L, Malkey R, Govoni M, Nihlén C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO (2008) A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol 4:411–417. https://doi.org/10.1038/nchembio.92
Jin L, Zhang M, Xu J, Xia D, Zhang C, Wang J, Wang S (2018) Music stimuli lead to increased levels of nitrite in unstimulated mixed saliva. Sci China Life Sci 61:1099–1106. https://doi.org/10.1007/s11427-018-9309-3
Jones AM, Thompson C, Wylie LJ, Vanhatalo A (2018) Dietary nitrate and physical performance. Annu Rev Nutr 38:303–328. https://doi.org/10.1146/annurev-nutr-082117-051622
Kadach S, Park JW, Stoyanov Z, Black MI, Vanhatalo A, Burnley M, Walter PJ, Cai H, Schechter AN, Piknova B, Jones AM (2023) 15 N-labeled dietary nitrate supplementation increases human skeletal muscle nitrate concentration and improves muscle torque production. Acta Physiol 237:13924. https://doi.org/10.1111/apha.13924
Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, MacAllister R, Hobbs AJ, Webb AJ, Ahluwalia A (2010) Inorganic nitrate supplementation lowers blood pressure in humans. Hypertension 56:274–281. https://doi.org/10.1161/HYPERTENSIONAHA.110.153536
Kapil V, Khambata RS, Jones DA, Rathod K, Primus C, Massimo G, Fukuto JM, Ahluwalia A (2020) The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol Rev 72:692–766. https://doi.org/10.1124/pr.120.019240
Kario K (2010) Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 56:765–773. https://doi.org/10.1161/HYPERTENSIONAHA.110.157149
Kim DJK, Roe CA, Somani YB, Moore DJ, Barrett MA, Flanagan M, Kim-Shapiro DB, Basu S, Muller MD, Proctor DN (2019) Effects of acute dietary nitrate supplementation on aortic blood pressures and pulse wave characteristics in post-menopausal women. Nitric Oxide 85:10–16. https://doi.org/10.1016/j.niox.2019.01.008
Knepper MA, Kwon TH, Nielsen S (2015) Molecular physiology of water balance. N Engl J Med 372:1349–1358. https://doi.org/10.1056/NEJMra1404726
Kukadia S, Dehbi HM, Tillin T, Coady E, Chaturvedi N, Hughes AD (2019) A double-blind placebo-controlled crossover study of the effect of beetroot juice containing dietary nitrate on aortic and brachial blood pressure over 24 h. Front Physiol 10:47. https://doi.org/10.3389/fphys.2019.00047
Kwon A, Kim GH, Kim MS (2022) Clinical implications of central blood pressure measured by radial tonometry and automated office blood pressure measured using automatic devices in cardiovascular diseases. Front Cardiovasc Med 9:906021. https://doi.org/10.3389/fcvm.2022.906021
Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E (2006) Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med 355:2792–2793. https://doi.org/10.1056/nejmc062800
Lericollais R, Gauthier A, Bessot N, Sesboüé B, Davenne D (2009) Time-of-day effects on fatigue during a sustained anaerobic test in well-trained cyclists. Chronobiol Int 8:1622–1635. https://doi.org/10.3109/07420520903534492
Li D, Nishi SK, Jovanovski E, Zurbau A, Komishon A, Mejia SB, Khan TA, Sievenpiper JL, Milicic D, Jenkins A, Vuksan V (2020) Repeated administration of inorganic nitrate on blood pressure and arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. J Hypertens 38:2122–2140. https://doi.org/10.1097/HJH.0000000000002524
Liu AH, Bondonno CP, Croft KD, Puddey IB, Woodman RJ, Rich L, Ward NC, Vita JA, Hodgson JM (2013) Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide 35:123–130. https://doi.org/10.1016/j.niox.2013.10.001
Lundberg JO (2012) Nitrate transport in salivary glands with implications for NO homeostasis. Proc Natl Acad Sci USA 33:13144–13145. https://doi.org/10.1073/pnas.1210412109
Mackenzie IS, Rogers A, Poulter NR, Williams B, Brown MJ, Webb DJ, Ford I, Rorie DA, Guthrie G, Grieve JWK, Pigazzani F, Rothwell PM, Young R, McConnachie A, Struthers AD, Lang CC, MacDonald TM (2022) Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet 400:1417–1425. https://doi.org/10.1016/S0140-6736(22)01786-X
Martin A, Carpentier A, Guissard N, Van Hoecke J, Duchateau J (1999) Effect of time of day on force variation in a human muscle. Muscle Nerve 10:1380–1387. https://doi.org/10.1002/(SICI)1097-4598(199910)22:10%3c1380::AID-MUS7%3e3.0.CO;2-U
McDonagh S, Wylie L, Winyard P, Vanhatalo A, Jones A (2015) The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int J Sports Med 36:1177–1185. https://doi.org/10.1055/s-0035-1554700
McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB (2014) Central blood pressure: current evidence and clinical importance. Eur Heart J 35:1719–1725. https://doi.org/10.1093/eurheartj/eht565
McKay AKA, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, Sheppard J, Burke LM (2022) Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform 17:317–331. https://doi.org/10.1123/ijspp.2021-0451
Mills CE, Govoni V, Faconti L, Casagrande M, Morant SV, Crickmore H, Iqbal F, Maskell P, Masani A, Nanino E, Webb AJ, Cruickshank JK (2020) A randomised, factorial trial to reduce arterial stiffness independently of blood pressure: proof of concept? The VaSera trial testing dietary nitrate and spironolactone. Br J Clin Pharmacol 86:891–902. https://doi.org/10.1111/bcp.14194
Minshull C, James L (2013) The effects of hypohydration and fatigue on neuromuscular activation performance. Appl Physiol Nutr Metab 38:21–26. https://doi.org/10.1139/apnm-2012-0189
Pekas EJ, Wooden TK, Yadav SK, Park SY (2021) Body mass-normalized moderate dose of dietary nitrate intake improves endothelial function and walking capacity in patients with peripheral artery disease. Am J Physiol-Regul, Integ Comp Physiol 321:162–173. https://doi.org/10.1152/ajpregu.00121.2021
Pinheiro LC, Amaral JH, Ferreira GC, Portella RL, Ceron CS, Montenegro MF, Toledo JC, Tanus-Santos JE (2015) Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radic Biol Med 87:252–262. https://doi.org/10.1016/j.freeradbiomed.2015.06.038
Rammos C, Totzeck M, Deenen R, Köhrer K, Kelm M, Rassaf T, Hendgen-Cotta UB (2015) Dietary nitrate is a modifier of vascular gene expression in old male mice. Oxid Med Cell Longev 2015:1–12. https://doi.org/10.1155/2015/658264
Ringqvist Å, Caidahl K, Petersson A-S, Wennmalm A (2000) Diurnal variation of flow-mediated vasodilation in healthy premenopausal women. Heart Circ Physiol 276:2720–2725
Rowland SN, Chessor R, French G, Robinson GP, O’Donnell E, James LJ, Bailey SJ (2021) Oral nitrate reduction is not impaired after training in chlorinated swimming pool water in elite swimmers. Appl Physiol Nutr Metab 46:86–89. https://doi.org/10.1139/apnm-2020-0357
Sawka SNS, Burke LM, Eichner ER, Maughan RJ, Montain SJ (2007) Exercise and fluid replacement. Med Sci Sports Exerc 39:377–390. https://doi.org/10.1249/mss.0b013e31802ca597
Scheer FAJL, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA (2010) Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci USA 107:20541–20546. https://doi.org/10.1073/pnas.1006749107
Senefeld JW, Wiggins CC, Regimbal RJ, Dominelli PB, Baker SE, Joyner MJ (2020) Ergogenic effect of nitrate supplementation: a systematic review and meta-analysis. Med Sci Sports Exerc 10:2250–2261. https://doi.org/10.1249/MSS.0000000000002363
Shepherd AI, Wilkerson DP, Dobson L, Kelly J, Winyard PG, Jones AM, Benjamin N, Shore AC, Gilchrist M (2015) The effect of dietary nitrate supplementation on the oxygen cost of cycling, walking performance and resting blood pressure in individuals with chronic obstructive pulmonary disease: a double blind placebo controlled, randomised control trial. Nitric Oxide 48:31–37. https://doi.org/10.1016/j.niox.2015.01.002
Sheppard JP, Hodgkinson J, Riley R, Martin U, Bayliss S, McManus RJ (2015) Prognostic significance of the morning blood pressure surge in clinical practice: a systematic review. Am J Hypertens 1:30–41. https://doi.org/10.1093/ajh/hpu104
Ship JA, Fischer DJ (1997) The relationship between dehydration and parotid salivary gland function in young and older healthy adults. J Gerontol A Biol Sci Med Sci 52A:M310–M319. https://doi.org/10.1093/gerona/52A.5.M310
Siervo M, Lara J, Ogbonmwan I, Mathers JC (2013) Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr 143:818–826. https://doi.org/10.3945/jn.112.170233
Smolensky MH, Hermida RC, Portaluppi F (2017) Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med Rev 33:4–16. https://doi.org/10.1016/j.smrv.2016.02.003
Souissi N, Gauthier A, Sesboüé B, Larue J, Davenne D (2004) Circadian rhythms in two types of anaerobic cycle leg exercise: force-velocity and 30-s wingate tests. Int J Sports Med 1:14–19. https://doi.org/10.1055/s-2003-45226
Tangphao O, Chalon S, Coulston AM, Moreno H Jr, Chan JR, Cooke JP, Hoffman BB, Blaschke TF (1999) L-arginine and nitric oxide-related compounds in plasma: comparison of normal and arginine-free diets in a 24-h crossover study. Vasc Med 4(1):27–32
Vitale JA, Weydahl A (2017) Chronotype, physical activity, and sport performance: a systematic review. Sports Med 47:1859–1868. https://doi.org/10.1007/s40279-017-0741-z
Walker MA, Bailey TG, McIlvenna L, Allen JD, Green DJ, Askew CD (2019) Acute dietary nitrate supplementation improves flow mediated dilatation of the superficial femoral artery in healthy older males. Nutrients 11:954. https://doi.org/10.3390/nu11050954
Watanabe S, Dawes C (1988) A comparison of the effects of tasting and chewing foods on the flow rate of whole saliva in man. Arch Oral Biol 33:761–764. https://doi.org/10.1016/0003-9969(88)90010-6
Williams B, Lacy PS, Baschiera F, Brunel P, Düsing R (2013) Novel description of the 24-hour circadian rhythms of brachial versus central aortic blood pressure and the impact of blood pressure treatment in a randomized controlled clinical trial: the ambulatory central aortic pressure (AMCAP) study. Hypertension 61:1168–1176. https://doi.org/10.1161/HYPERTENSIONAHA.111.00763
Woessner M, Smoliga JM, Tarzia B, Stabler T, Van Bruggen M, Allen JD (2016) A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide 54:1–7. https://doi.org/10.1016/j.niox.2016.01.002
Wojciechowska W, Staessen JA, Nawrot T, Cwynar M, Seidlerova J, Stolarz K, Gasowski J, Ticha M, Richart T, Thijs L, Grodzicki T, Kawecka-Jaszcz K, Filipovsky J (2006) Reference values in white europeans for the arterial pulse wave recorded by means of the sphygmocor device. Hypertens Res 29:475–483. https://doi.org/10.1291/hypres.29.475
Wylie LK, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM (2013) Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol 115:325–336. https://doi.org/10.1152/japplphysiol.00372.2013
Wylie LJ, Park JW, Vanhatalo A, Kadach S, Black MI, Stoyanov Z, Schechter AN, Jones AM, Piknova B (2019) Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. J Physiol 597:5565–5576. https://doi.org/10.1113/JP278076
Zoughaib WS, Hoffman RL, Yates BA, Moorthi RN, Lim K, Coggan AR (2023) Short-term beetroot juice supplementation improves muscle speed and power but does not reduce blood pressure or oxidative stress in 65–79 y old men and women. Nitric Oxide 23:00049–00056. https://doi.org/10.1016/j.niox.2023.05.005
Funding
This research was supported by the NIHR Leicester Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
SR and SB conceived and designed the study. SR undertook data collection. SR and SB analyzed the data. SR wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Communicated by Michalis G Nikolaidis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rowland, S.N., James, L.J., O’Donnell, E. et al. Influence of acute dietary nitrate supplementation timing on nitrate metabolism, central and peripheral blood pressure and exercise tolerance in young men. Eur J Appl Physiol 124, 1381–1396 (2024). https://doi.org/10.1007/s00421-023-05369-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05369-z