Abstract
Previous studies in animal models showed that exercise-induced metabolites accumulation may sensitize the mechanoreflex-induced response. The aim of this study was to assess whether the magnitude of the central hemodynamic and ventilatory adjustments evoked by isolated stimulation of the mechanoreceptors in humans are influenced by the prior accumulation of metabolic byproducts in the muscle. 10 males and 10 females performed two exercise bouts consisting of 5-min of intermittent isometric knee-extensions performed 10% above the previously determined critical force. Post-exercise, the subjects recovered for 5 min either with a suprasystolic circulatory occlusion applied to the exercised quadriceps (PECO) or under freely-perfused conditions (CON). Afterwards, 1-min of continuous passive leg movement was performed. Central hemodynamics, pulmonary data, and electromyography from exercising/passively-moved leg were recorded throughout the trial. Root mean square of successive differences (RMSSD, index of vagal tone) was also calculated. Δpeak responses of heart rate (ΔHR) and ventilation (\(\Delta \dot{V}_{{\text{E}}}\)) to passive leg movement were higher in PECO compared to CON (ΔHR: 6 ± 5 vs 2 ± 4 bpm, p = 0.01; 3.9 ± 3.4 vs 1.9 ± 1.7 L min−1, p = 0.02). Δpeak of mean arterial pressure (ΔMAP) was significantly different between conditions (5 ± 3 vs − 3 ± 3 mmHg, p < 0.01). Changes in RMSSD with passive leg movement were different between PECO and CON (p < 0.01), with a decrease only in the former (39 ± 18 to 32 ± 15 ms, p = 0.04). No difference was found in all the other measured variables between conditions (p > 0.05). These findings suggest that mechanoreflex-mediated increases in HR and \(\dot{V}_{{\text{E}}}\) are sensitized by metabolites accumulation. These responses were not influenced by biological sex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular and ventilatory adjustments to physical exercise are achieved through the cooperation of different mechanisms. The most important are a feedforward efferent mechanism (i.e., central command), the arterial baroreflex, and the exercise pressor reflex, which is a negative-loop feedback mechanism originating from the working muscle, entailing a mechanosensitive and a metabosensitive branch (Fisher et al. 2015). This feedback from the working muscles is sent to the central nervous system through group III/IV afferent fibers, which convey information about the mechanical distortion of their receptive fields (group III), and metabolic changes happening in the intramuscular milieu (group IV) (Fisher et al. 2015). While the involvement of group IV fibers on hemodynamic regulation is well established (Boushel 2010), the role of mechanosensitive afferent fibers is more difficult to demonstrate. In the last decade, strong evidence for an important role of these fibers in the hemodynamic regulation came using intrathecal fentanyl injection (a potent opioid receptors agonist), which partially blocks afferent feedback to the central nervous system. Indeed, blocking afferent feedback resulted in a substantial decrease in the chronotropic response that typically accompanies passive leg movement (Trinity et al. 2010). Furthermore, using the same pharmacological approach in healthy volunteers (Amann et al. 2010) and a spinal cord injury model, in which afferent feedback is intrinsically interrupted (Venturelli et al. 2012), it has been shown that muscle afferent fibers play a pivotal role also in the ventilatory adjustments to exercise (Amann et al. 2010) and passive movement (Venturelli et al. 2012), respectively.
Classical studies carried out in animal models indicate that metabolic changes in the exercising muscle may sensitize the response to mechanical stress (Rotto et al. 1990; Rotto and Kaufmann 1988). In humans, this issue is more controversial. A previous study utilizing passive stretch of the wrist during post-exercise ischemia, found an increase in the blood pressure response and sympathetic activation (Cui et al. 2008). Similarly, other investigators found a vagally-mediated transient increase in heart rate (HR) when static stretch of the calf muscles was superimposed to circulatory occlusion of the limb (Drew et al. 2008a, b). However, Fisher et al. showed that HR response to static calf stretch was not different after exercise bouts carried out at different intensities, and therefore likely different levels of metabolites accumulation (Fisher et al. 2005). Moreover, since different subsets of group III/IV muscle afferents respond to different stimuli, there is evidence that mechanically-sensitive fibers may be preferentially activated during movement compared to static stretch (Hayes et al. 2005). Therefore, static stretch and dynamic movements may yield different outcomes in terms of autonomic control.
Recently, a study found that when passive cycling was coupled with circulatory occlusion of the lower limbs, chronotropic and ventilatory responses were augmented (Lis et al. 2020). However, as highlighted by Fernandes and Vianna (2020), the accumulation of metabolic byproducts and the presence of electromyographic recordings are of paramount importance to parse out alternative explanations when studying the interaction between metabo- and mechanoreflex.
The aim of this study was to evaluate mechanoreflex-induced central hemodynamic and ventilatory responses when a passive, mechanical stimulation was superimposed over circulatory occlusion compared to when recovery is allowed to take place under freely perfused conditions. Also, our secondary objective was to assess whether a sex-specific response in these assessments was present. Our hypothesis was that mechanoreflex-induced responses would be higher when exercise-induced metabolites were trapped in the muscle by circulatory occlusion and that this outcome would not be different between sexes.
Methods
Subjects and ethical approval
Twenty young healthy subjects were recruited for this study (10 males and 10 females, age: 26 ± 3 vs 22 ± 3 years, height: 176 ± 7 vs 163 ± 6 cm, and weight: 76 ± 9 vs 56 ± 7 kg). All subjects were non-smokers and none of them was taking medications as determined by a health questionnaire. They were instructed to report to the lab after having refrained from alcohol and caffeine (≥ 12 h), food (≥ 2 h), and physical exercise (≥ 24 h). The subjects were tested in the same temperature-controlled room (22–24 °C). Moreover, the last experimental session for females was performed during the early follicular phase (days 1–5 from the self-reported menstruation onset) to lessen potential effects of estrogen hormones on hemodynamic responses (Wenner and Stachenfeld 2020). No female participant reported to being using contraceptives at the time of the study. Leg dominance was established based on the self-reported foot used to kick a ball. All subjects but two reported being right-leg dominant. Written informed consent was obtained from each participant after a detailed verbal and written explanation of the experimental procedures. The study complied with the Declaration of Helsinki and was approved by the local ethical committee of the University of Verona (IRB #30444).
Experimental design and procedures
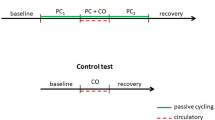
The subjects were asked to report to the lab on three different occasions. On the first experimental day, the subjects were familiarized with the study procedures and with the performance of isometric knee-extensors maximal voluntary contractions (MVC). The instructions for the performance of MVCs were to push “as hard and as fast as possible”, to reach a force plateau in the shortest time possible. A schematic representation of the study protocol is reported in Fig. 1.
Schematic representation of the study protocol. After the familiarization session (not shown) two sessions were carried out in separate days (A and B). Critical force (dashed line) in panel A was calculated as the mean force expressed in the last six contractions (dashed square). In the second session, post-exercise circulatory occlusion (PECO) or freely perfused (CON) conditions were performed in a counterbalanced manner with a ~ 30-min rest in between. Exercise intensity was set at 10% above critical force (dashed line). MVC maximal voluntary contraction; PLM passive leg movement
On the second experimental day, the subjects performed an all-out isometric knee-extensor test, in order to estimate critical force employing a 60% duty cycle (contraction/relaxation: 3 s/2 s) (Burnley 2009). The 5 min all-out test consisted of 60 knee-extension MVCs using the same duty cycle abovementioned. During the test, participants were verbally encouraged by the members of the research team to ensure maximal effort. The subjects were seated with a 90° knee flexion on a custom-built chair, with their ankle linked to the force transducer and a steel bar through a noncompliant strap, which was placed 2-cm above the lateral malleolus of the ankle. Extraneous movement of the upper body was avoided by two-crossed belts over the chest while the hips were stabilized by an additional belt. An audio recording signaled the start and stop of each contraction and the subjects were able to see the force feedback on a wall projected ~ 3 m in front of them. No information was given to the subjects concerning the time elapsed or remaining. Critical force was calculated as the average of the mean force exerted during the last six contractions (Burnley 2009).
On the third and last experimental session, participants were instrumented and allowed to rest in the sitting position for 15 min. After this resting period, a 1-min baseline measurement was collected before the subject’s lower leg was passively moved for 1 min at 1 Hz. Afterwards, they were asked to perform two identical exercise bouts in which the recovery was done either without (CON) or with post-exercise circulatory occlusion (PECO). Circulatory occlusion was obtained by inflating a tourniquet cuff (DTC-506. Daesung Maref, South Korea) around the proximal part of the exercising thigh at a suprasystolic pressure (300 mmHg) with a custom-made rapid cuff occlusion system (< 0.5 s to full occlusion). These two bouts were interspersed by a 30 min rest and were carried out in a counterbalanced manner to minimize any eventual carry-over effect. Each bout started with a 5-min baseline period. Then, a MVC was performed to normalize EMG signals. After an adequate pause (~ 1 min) allowing the hemodynamic values to return to baseline, 5 min of rhythmic isometric knee-extension (3 s on/2 s off) were performed at an intensity that was 10% above critical force, which was determined in the previous experimental visit. This intensity has been chosen because it has been shown to be adequate to induce significant metabolic perturbation in the exercising muscle (Jones et al. 2008; Burnley et al. 2012). At the end of the exercise, the subjects started the 5-min recovery period either with or without PECO. Finally, the lower leg of the subjects was again passively moved for 1 min at a frequency of 1 Hz. All passive movement procedures were performed by the same member of the research team, moving the subjects’ lower leg through the range of motion defined by 90 and 180° knee joint angles (where the fully extended knee joint is defined as 180°).
Central hemodynamics and pulmonary data
Blood pressure, cardiac output (CO), and stroke volume (SV) were measured on a beat-by-beat basis using a finger photoplethysmography device (Finapres model 2300; Ohmeda, Englewood, CO, USA). The left hand was held on a custom-made support, at the level of the heart and a photoplethysmographic cuff was placed on the fourth finger. A software extension (Non-Invasive Cardiac Output, ADInstruments, Australia) was added to the LabChart 8 software to apply the Modelflow algorithm to the raw beat-by-beat data from the non-invasive blood pressure measurement device. Mean arterial pressure (MAP) was calculated as diastolic blood pressure + 1/3 (systolic − diastolic blood pressure). HR was calculated beat-by-beat from the electrocardiographic signal collected with a dual bioamplifier (ML135, ADInstruments, Australia) at 2 kHz.
Pulmonary gas exchange (\(\dot{V}{\text{O}}_{{2}}\) and \(\dot{V}{\text{CO}}_{{2}}\)) minute ventilation (\(\dot{V}_{{\text{E}}}\)), breathing frequency (fB) and tidal volume (VT) were measured breath-by-breath with a metabolic cart (Quark b2, Cosmed, Italy). Before each session, after an appropriate warm-up, the gas analyzer and the turbine flowmeter were calibrated according to the instructions of the manufacturer.
Isometric force, surface electromyography, and rating of perceived exertion
Isometric force was measured by a force transducer (model UU2; DaCell, Korea) previously calibrated, connected to a custom-made chair through a noncompliant strap placed around the subjects’ ankle. The output from the force transducer was amplified and filtered with a 20 Hz low-cut filter and recorded at a sampling rate of 2 kHz.
Vastus lateralis electromyography (EMG) was continuously recorded with a dual bioamplifier (ML135, ADInstruments, Australia). Two surface Ag/AgCl electrodes (PG10C; Fiab, Vicchio, Florence, Italy) were attached to the skin with a 20-mm inter-electrode distance. The electrodes were placed longitudinally, in line with the underlying muscle fibers arrangement, at two-thirds of the distance between the anterior iliac spine and the lateral part of the patella (Hermens et al. 2000). Before the application of the electrodes, the skin was shaved, abraded with sandpaper, and finally cleaned with an alcohol swab in order to minimize skin impedance. The raw EMG signal was amplified and digitized online at a 2 kHz sampling frequency. Acquisition of the EMG data was done using a computer-based data acquisition and analysis system (hardware: PowerLab 16/30; ML880, ADInstruments, Bellavista, Australia and software: LabChart 8, ADInstruments, Bellavista, Australia). Finally, rating of perceived exertion (RPE) was obtained at every minute using the 6–20 Borg scale (Borg 1975).
Data analysis
All central hemodynamics and breath-by-breath data were linearly interpolated to 1-s intervals and time-aligned to the onset of passive leg movement. Successively, data from the 30 s before passive leg movement was averaged and represented baseline values. Pulmonary data was checked visually for eventual aberrant breaths. When an aberrant breath was found, it was eliminated from the analysis and data from the two adjacent breaths were linearly interpolated. All hemodynamics and pulmonary data were smoothed using a 3-s rolling average. Statistical analysis for the passive leg movement-induced responses was performed on the Δpeak values from baseline (30 s before the onset of passive leg movement).
Root mean square of successive differences (RMSSD) was calculated from the R–R intervals of the electrocardiogram during the 60-s period preceding the onset of the passive movement and the first 15 s afterwards. This time point was chosen because changes in vagal tone may be expected here based on previous literature (Drew et al. 2008a). RMSSD is a recommended time-domain measure of short-term HR variability and it is sensitive to changes in vagal tone (Task-Force 1996) and relatively free of respiratory influences (Hill and Siebenbrock 2009). RMSSD data from one subject was removed from the analysis because it was > 5 SD compared to the average data.
EMG data were analyzed with an in-house built MATLAB routine (MATLAB 2020b, Mathworks, USA). The raw EMG signal was bandpass filtered (10–500 Hz) with a fourth order, zero-phase, Butterworth filter and full-wave rectified. For the exercise EMG, a 500 ms baseline was detected between contractions, and onsets were set when the signal rose by > 3SD from baseline values. The same algorithm was applied to find contraction offset. For each muscle contraction, the root mean square (RMS) was calculated and normalized by the highest 500 ms EMGRMS obtained during a MVC performed before exercise (Laginestra et al. 2022). Moreover, the EMGRMS of the 30 s period preceding passive leg movement, and the 60 s of passive leg movement were calculated to ensure that the subjects were not voluntarily contracting the muscles of the passively moved limb.
Statistical analysis
Two-tailed independent sample t-tests were employed for baseline MVC, critical force, and exercise intensity between females and males. Paired samples t-tests were used between rest and passive leg movement within each condition for EMG measurements. Also, a three-way (condition × time × sex) ANOVA for repeated measures was performed to find eventual differences between conditions during the three phases of the protocol for all the cardiorespiratory variables (rest–exercise–recovery) and for RMSSD (baseline–movement). Successively, a two-way (condition × sex) ANOVA for repeated measures was performed to employed to find differences in the Δpeak responses to passive leg movement between PECO and CON. If significant interactions were found, pairwise differences were identified using Bonferroni post-hoc test correction for multiple comparisons when appropriate. Statistical analysis was performed with IBM SPSS Statistics 24 (IBM Corp©, 2016) and figures were made with GraphPad Prism 8.0 (GraphPad Software, Inc., 2012). Significance level was set at α < 0.05 and effect sizes were reported by calculating Cohen’s d and partial eta squared (ηp2). Data are expressed as mean ± SD unless otherwise stated.
Results
Maximal voluntary contraction, critical force, and exercise intensity
In our participants, MVC was 595 ± 158 N (females: 473 ± 81 N; males: 718 ± 113 N, t18 = −5.6, p < 0.01, d = 2.49). Critical force was 195 ± 57 N (females: 159 ± 22 N; males: 230 ± 59 N, t18 = −3.6, p < 0.01, d = 1.59), which was equivalent to 33 ± 8% MVC (females: 34 ± 7%; males: 32 ± 9%, t18 = 0.5, p = 0.17, d = 0.25). The exercise intensity used for PECO and CON was 214 ± 62 N (females: 175 ± 24 N; males: 253 ± 65 N, t18 = −3.6, p < 0.01, d = 1.59). which was equivalent to 37 ± 9% MVC (females: 38 ± 8%; males: 36 ± 10%, t18 = 0.8, p = 0.62, d = 0.22).
Central hemodynamics and root mean square of successive differences
Rest, exercise, and recovery data for the two conditions are presented in Table 1. All variables were similar between sexes and conditions at rest and during exercise (all p > 0.05). During recovery after exercise, HR, SV, and CO returned to baseline in both conditions, while MAP remained elevated during PECO.
Δpeak values for central hemodynamics variables during passive leg movement are presented in Fig. 2. There was no statistically significant difference in any cardiovascular variable between Δpeak responses observed in CON and the passive leg movement performed at the beginning of the session (all p > 0.05). No significant sex effect (F1,18 = 1.1, p = 0.31, ηp2 = 0.06) nor condition × sex interaction was found in HR (F1,18 = 1.7, p = 0.21, ηp2 = 0.09). However, a significant difference in HR behavior was found between conditions (F1,18 = 11.5, p < 0.01, ηp2 = 0.39) whereby HR increased by ~ 3% in CON (75 ± 11 to 78 ± 12 bpm) and ~ 8% in PECO (77 ± 14 to 83 ± 17 bpm). Furthermore, the change in SV was similar between conditions (CON: 69 ± 18 to 77 ± 19 mL, PECO: 67 ± 15 to 74 ± 16 mL, F1,18 = 0.5, p = 0.48, ηp2 = 0.03) and between sexes (sex: F1,18 = 1.1, p = 0.31, ηp2 = 0.06; condition × sex interaction: F1,18 = 0.3, p = 0.60, ηp2 = 0.02). However, together these adjustments did not translate into a different increase in CO (CON: 5.08 ± 0.98 to 5.54 ± 1.07 L·min−1, PECO: 5.05 ± 1.00 to 5.60 ± 1.07 L·min−1, F1,18 = 0.7, p = 0.40, ηp2 = 0.04) nor between sexes (sex: F1,18 = 1.0, p = 0.76, ηp2 = 0.01; condition × sex interaction: F1,18 = 0.2, p = 0.66, ηp2 = 0.01). A divergent response in MAP (F1,18 = 50.8, p < 0.01, ηp2 = 0.74) was found during passive leg movement in CON (92 ± 7 to 89 ± 9 mmHg) and in PECO (103 ± 8 to 108 ± 9 mmHg). No sex difference was found in MAP (sex: F1,18 = 0.1, p = 0.75, ηp2 = 0.01; condition × sex interaction: F1,18 = 0.2, p = 0.65, ηp2 = 0.01) (Fig. 2).
Passive leg movement-induced peak changes in central hemodynamics variables with post-exercise circulatory occlusion (PECO) or freely perfused recovery (CON). *Significantly different than PECO. A HR heart rate; B SV stroke volume; C CO cardiac output; D MAP mean arterial pressure. Statistical significance was set at p <0.05. Number of participants (n) = 20
No significant three-way interaction was found for RMSSD (condition × time × sex: F1,17 = 1.0, p = 0.33, ηp2 = 0.06). On the same note, no significant condition × sex (F1,17 = 1.8, p = 0.20, ηp2 = 0.01) or time × sex (F1,17 = 3.3, p = 0.09, ηp2 = 0.16) interactions were detected. On the other hand, a significant condition × time interaction was found in RMSSD (F1,17 = 13.2, p < 0.01, ηp2 = 0.44, Fig. 3). Follow-up pairwise comparison showed that RMSSD during the first 15 s of passive leg movement was different than baseline in PECO only (39 ± 18 to 32 ± 15 ms, p = 0.04, d = 0.42).
Pulmonary variables
Rest, exercise, and recovery data for the variables of interest are presented in Table 1.
Furthermore, Δpeak values for ventilatory variables in response to passive leg movement are presented in Fig. 4. There was no statistically significant difference in any ventilatory variable between Δpeak responses observed in CON and the passive leg movement performed at the beginning of the session (all p > 0.05). During passive leg movement, Δpeak for \(\dot{V}_{{\text{E}}}\) increased by ~ 40% in PECO (11.4 ± 4.8 to 15.3 ± 5.9 L·min−1) and only ~ 20% in CON (10.4 ± 2.5 to 12.3 ± 2.9 L·min−1) with this difference being statistically significant between conditions (condition: F1,18 = 5.6, p = 0.03, ηp2 = 0.24). No sex (F1,18 = 1.1, p = 0.31, ηp2 = 0.06), nor condition × sex interaction was found (F1,18 = 0.0, p = 0.99, ηp2 = 0.00). Concurrently, the increase in VT was not significantly different (F1,18 = 2.4, p = 0.15, ηp2 = 0.12) between CON (0.72 ± 0.28 to 0.77 ± 0.29 L) and PECO (0.87 ± 0.40 to 1.06 ± 0.52 L) nor between sexes (sex: F1,18 = 1.2, p = 0.28, ηp2 = 0.06; condition × sex: F1,18 = 2.0, p = 0.17, ηp2 = 0.10). Finally, also Δpeak response in fB was not different (F1,18 = 1.6, p = 0.23, ηp2 = 0.08) between CON (16.4 ± 5.2 to 21.2 ± 5.2 breaths·min−1) and PECO (14.8 ± 5.2 to 21.2 ± 5.3 breaths·min−1) and between sexes (sex: F1,18 = 1.3, p = 0.28, ηp2 = 0.07; condition × sex: F1,18 = 0.6, p = 0.45, ηp2 = 0.03).
Passive leg movement-induced peak changes in in ventilatory variables during passive leg movement with post-exercise circulatory occlusion (PECO) or freely perfused recovery (CON). *Significantly different than PECO. A \(\dot{V}_{{\text{E}}}\): minute ventilation; B fB: breathing frequency; C VT: tidal volume. Statistical significance was set at p < 0.05. Number of participants (n) = 20
Electromyography and rate of perceived exertion
EMG activity during passive leg movement was not different from the preceding resting period in both conditions CON (2.40 ± 1.90% to 2.40 ± 1.91%, p = 0.93, d = 0.24) or PECO (2.35 ± 1.89% to 2.37 ± 1.87%, p = 0.30, d = 0.24). EMGRMS and RPE during exercise are presented in Fig. 5. EMGRMS increased over time in both conditions (pooled values from min 1 to min 5: 39.6 ± 13.0% to 45.0 ± 20.5%, p < 0.01, ηp2 = 0.18), with no difference between CON and PECO (p = 0.97, ηp2 = 0.01). Also, RPE demonstrated the same behavior by increasing over time (pooled values from min 1 to min 5: 9 ± 2 to 13 ± 2, p < 0.01, ηp2 = 0.75) with no difference between conditions (p = 0.33, ηp2 = 0.06).
A Electromyography and B rating of perceived exertion (RPE) during intermittent isometric knee-extensions performed 10% above critical force. Since no differences were found between conditions, data were pooled to display the effect of time. *Significantly different than the previous time point. Statistical significance was set at p 0.05. Data are presented as mean ± SD. Number of participants (n) = 20
Discussion
The primary objective of this investigation was to study whether the central hemodynamic and ventilatory adjustments to passive leg movement (i.e., mechanoreflex) interact with the effects caused by intramuscular metabolites accumulation (i.e., metaboreflex). The main findings of this study were that chronotropic (ΔHR) and ventilatory (\(\Delta \dot{V}_{{\text{E}}}\)) responses to passive leg movement were higher in both sexes when the previously exercised muscle was maintained ischemic compared to a situation in which the muscle was freely perfused. These higher responses were accompanied by a larger decrease in the vagal tone at the onset of the passive movement. This finding supports the concept that the cardiorespiratory response to mechanical stimulation, is potentiated when metabolites are accumulated in the muscle and suggest that this phenomenon is mediated by a transient decrease in the parasympathetic drive to the heart, and likely an increase in the overall sympathetic activation.
The effects of combined metabo- and mechanoreflex activation on central hemodynamics
Whether an interaction between the mechanical and metabolic branches of muscle afferents exists in humans is still controversial with studies demonstrating an influence (Cui et al. 2008; Lis et al. 2020; Bell and White 2005; Nishiyasu et al. 2006) and others demonstrating no influence (Fisher et al. 2005; Venturelli et al. 2017). In humans, isolated muscle mechanoreceptors stimulation through passive stretch (Gladwell et al. 2005; Gladwell and Coote 2002), and dynamic movement (Venturelli et al. 2017; McDaniel et al. 2010) has been shown to be able to evoke a transient HR and blood pressure response (Cui et al. 2006). Given the rapidity of this response, and the fact that infusion of anticholinergic drugs (i.e., glycopyrrolate) abolishes it, this chronotropic adjustment is attributed to an effect on vagal withdrawal (Gladwell et al. 2005).
Our results showed a transient but significantly higher increase in HR response (~ 8% vs. ~ 3%) to passive leg movement when ischemia was maintained on the moved leg after exercise. However, this difference was too small to result in a higher CO response compared to CON, limiting its functional importance in this model. Importantly, the results from EMG data suggest that there was no involvement of central command during the passive leg movement and indicate that the observed effect is due to a reflex mechanism. Interestingly, we also observed a significant decrease in RMSSD only in PECO in response to passive leg movement (Fig. 3). This result agrees with the results of a previous study in which static stretch superimposed on PECO transiently decreased RMSSD (Drew et al. 2008a) and supports the idea that the mechanism between the mechano- and metaboreflex interaction, may be represented by a transient decrease of the vagal tone during the first seconds after movement onset. Therefore, the sensitization of group III fibers, together with the sympathoexcitation due to higher group IV afferents firing, may be a sufficient stimulus to temporarily increase HR and MAP. In fact, previous studies showed how this interaction may lower baroreflex sensitivity and, therefore, its ability to control changes in HR (Drew 2017; Drew et al. 2008a). The observation that HR was not different between PECO and CON during recovery (Table 1) agrees with previous studies showing that PECO alone does not cause enough sympathoexcitation to override the parasympathetic reactivation happening with the cessation of central command (Nishiyasu et al. 1994; Iellamo et al. 1999). Finally, a recent study by Peçanha and colleagues showed that activation of the mechanoreflex during post-exercise plays a role in attenuating heart rate recovery, highlighting the importance of these mechano-sensitive fibers on the cardiac parasympathetic branches of the autonomic nervous system (Pecanha et al. 2021).
The effects of combined metabo- and mechanoreflex activation on ventilatory drive
The role of muscle afferents stimulation on ventilatory responses is not univocal with previous studies using various approaches, yielding inconsistent results, also dependent on the modality to stimulate group III/IV afferents. For example, passive calf stretch leads to a non-significant increase in \(\dot{V}_{{\text{E}}}\) (Bruce and White 2012), while bilateral passive leg movement has been found to provoke a ~ 6 L·min−1 increase in \(\dot{V}_{{\text{E}}}\) (Bell and Duffin 2006). A significant increase in ventilation was also found when external pulsed muscle compressions were superimposed on rhythmic exercise (Nishiyasu et al. 2006). However, while the use of large muscle masses may be more appropriate because of the evidence that the magnitude of afferent feedback is related to the size of the involved muscle mass (Iwamoto and Botterman 1985) it also makes the contribution of central command harder to parse out, given the possible involvement of postural, stabilizing muscles, not directly involved in the passive movement.
In this study, we found that passive movement of a single leg with PECO was accompanied by an increase in peak \(\Delta \dot{V}_{{\text{E}}}\) that was almost twofold the one observed during CON. The fact that neither ΔVT nor ΔfB were significantly different between PECO and CON may suggest that neither factor alone is responsible for the increased response in \(\dot{V}_{{\text{E}}}\) in this model, but that may be the result of changes in both variables, which are known to be regulated following distinct inputs (Forster et al. 2012). This larger increase in \(\dot{V}_{{\text{E}}}\) during the combined activation of metabolically and mechanically sensitive afferents, may signify that the effects of the two pathways are interactive. In fact, while it is well accepted that post-exercise ischemia is unable to sustain ventilatory drive in and of itself (Bruce et al. 2019), evidence from recent studies suggests that inputs from different regulatory mechanisms (e.g. central command, chemoreflexes) need to act synergistically in order to increase the ventilatory responses to muscle afferents stimulation (Silva et al. 2018; Wan et al. 2020; Lam et al. 2019). In an elegantly designed study, Lam and colleagues recently showed that when PECO was superimposed to the previously exercised contralateral leg, the ventilatory response to a successive exercise bout (i.e., involving central command) was accentuated, with the “excess” response attributable to enhanced afferent firing (Lam et al. 2019). Accordingly, a study from Silva et al., demonstrated that when the subjects were breathing a hypoxic mixture, the ventilatory response to passive leg movement was amplified compared to the passive movement alone, demonstrating an interaction when the chemoreflex and the mechanoreflex were stimulated together (Silva et al. 2018).
The mechanism behind the increase in ventilation in our study is not clear. However, since ventilation is not controlled by parasympathetic innervation, we speculate that our findings may be the result of a concomitant sympathetic activation caused by the stimulation of group III afferents (Victor et al. 1989).In support of this idea, a previous study from our group demonstrated how passive sympathoexcitatory maneuvers (e.g., static stretching) are able to restrain the peripheral hyperemic response to a passively moved, remote limb, likely through sympathetic vasoconstriction (Zambolin et al. 2022).
Absence of sex-related differences in mechanoreflex-induced central responses
In the present study, an exploratory analysis was conducted in order to assess whether any of the central responses to the mechano-metaboreflex interaction, presented sex-related differences. Our findings did not uncover any sex-specific responses in cardiac or ventilatory responses, indicating that the males and females similarly respond to the interaction of the two branches of the group III/IV muscle afferent pathway. To our knowledge, this is the first study to provide empirical evidence about this issue. Even though the literature is scarce, a previous study found that the isolated mechanoreflex activation through passive leg movement resulted in a blunted central hemodynamic response (i.e., lower HR) in healthy young females compared to their male counterparts (Ives et al. 2013). On the other hand, similar to the present study, a recent investigation from Wan and colleagues (Wan et al. 2022), found that when passive leg movement was performed in isolation, HR response was similar between sexes. Interestingly however, biological sex had an important effect when the mechanoreflex acted in concert with the chemoreflex, highlighting, once again, the importance of studying the interaction between reflexes instead of their isolated effects. On this note, further research is needed to evaluate how biological sex and reflexes interact in the neural control of autonomic adjustments. Finally, although the sample size employed in the present study is relatively close to the ones used in previous investigations addressing similar experimental questions (Ives et al. 2013; Wan et al. 2022), future studies should consider increasing the number of participants for each sex to ensure adequate statistical power.
Experimental considerations
In the present study, the activation of the metaboreflex was performed by inflating a cuff at suprasystolic pressure (standardized at 300 mmHg for all participants). It is important to point out that this procedure is usually associated with significant pain. Since noxious stimulation is also effective in activating group IV afferents (Pollak et al. 2014) our results are likely representing the response of metabo- and nociceptors.
It may be argued that performing passive leg movement after 5 min of recovery from exercise in CON may have an effect on the observed responses for two reasons: (1) 5 min may not be enough to washout all the metabolic byproducts of exercise, and (2) given the proximity of the passive movement to exercise, this may be influenced by other factors such as arousal, defined as an increase in brain activity independent of motor command (Bell and Duffin 2004; Venturelli et al. 2012). To verify this proposition, we compared the responses observed in CON with the ones obtained during the passive leg movement performed at the beginning of each experimental session (Fig. 1, panel B). Since there were no differences in any of the studied variables between the two trials, these results support the idea that full recovery was achieved and that the observed mechanoreflex-induced responses were likely not influenced by arousal.
In this study, we decided to use the critical intensity model to establish the exercise intensity for our protocol, instead of basing it on a fixed %MVC. To the best of our knowledge, this approach is novel in the study of autonomic adjustments to metabolic and mechanical stimulation. Our choice stems from the observation that metabolites accumulation depends on the metabolic domain in which exercise is performed (Jones et al. 2008). In fact, given the high inter-subjects variability in the levels of %MVC at which critical intensity is located (Kellawan and Tschakovsky 2014; Burnley 2009), a fixed %MVC would likely create very different metabolic perturbations, which could be extremely high for a subject or extremely low for another (Kent-Braun et al. 1993). Accordingly, a study using 31P magnetic resonance spectroscopy demonstrated how the kinetics of changes in the intramuscular metabolic milieu differ when exercise is performed above, or below critical intensity (Jones et al. 2008). Also, it is becoming increasingly recognized that absolute muscle strength influences the pressor response to exercise (Lee et al. 2021; Notay et al. 2018), which may also partly be explained by this proposition. In fact, the levels of blood flow occlusion caused by higher absolute forces would, in turn, cause longer time under ischemia and different rates of metabolites accumulation. Therefore, we believe that the approach used in our study is more suitable to compare physiological responses that are highly dependent on metabolites accumulation, and we advocate for future studies to apply this concept to further investigate the role of intensity domains on autonomic adjustments to exercise, especially when between-subjects designs are employed.
Conclusions
In conclusion, in this study we have shown that mechanoreflex-induced cardiac and ventilatory responses to passive leg movement are sensitized by the metabolic conditions of the muscle in young adults, independently from biological sex. Our data suggest that a transient decrease in vagal tone and a likely concurrent increase in sympathetic activation mediate the increase in chronotropic and ventilatory responses when passive leg movement is superimposed on metaboreflex activation.
Data availability
The datasets generated in the current study are available from the corresponding author upon reasonable request.
Change history
03 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00421-023-05203-6
References
Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2010) Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109(4):966–976. https://doi.org/10.1152/japplphysiol.00462.2010
Bell HJ, Duffin J (2004) Respiratory response to passive limb movement is suppressed by a cognitive task. J Appl Physiol (1985) 97(6):2112–2120. https://doi.org/10.1152/japplphysiol.00302.2004
Bell HJ, Duffin J (2006) Rapid increases in ventilation accompany the transition from passive to active movement. Respir Physiol Neurobiol 152(2):128–142. https://doi.org/10.1016/j.resp.2005.07.008
Bell MP, White MJ (2005) Cardiovascular responses to external compression of human calf muscle vary during graded metaboreflex stimulation. Exp Physiol 90(3):383–391. https://doi.org/10.1113/expphysiol.2004.029140
Borg G (1975) Simple rating methods for estimation of perceived exertion. In: Borg G (ed) Physical work and effort. Pergamon, New York, pp 39–46
Boushel R (2010) Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199(4):367–383. https://doi.org/10.1111/j.1748-1716.2010.02133.x
Bruce RM, White MJ (2012) Muscle afferent activation causes ventilatory and cardiovascular responses during concurrent hypercapnia in humans. Exp Physiol 97(2):208–218. https://doi.org/10.1113/expphysiol.2011.061606
Bruce RM, Jolley C, White MJ (2019) The control of the exercise hyperpnoea: contributions from thin fibre skeletal muscle afferents. Exp Physiol. https://doi.org/10.1113/EP087649
Burnley M (2009) Estimation of critical torque using intermittent isometric maximal voluntary contractions of the quadriceps in humans. J Appl Physiol (1985) 106(3):975–983. https://doi.org/10.1152/japplphysiol.91474.2008
Burnley M, Vanhatalo A, Jones AM (2012) Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol (1985) 113(2):215–223. https://doi.org/10.1152/japplphysiol.00022.2012
Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI (2006) Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol 576(Pt 2):625–634. https://doi.org/10.1113/jphysiol.2006.116640
Cui J, Mascarenhas V, Moradkhan R, Blaha C, Sinoway LI (2008) Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am J Physiol Regul Integr Comp Physiol 294(2):R458–R466. https://doi.org/10.1152/ajpregu.00475.2007
Drew RC (2017) Baroreflex and neurovascular responses to skeletal muscle mechanoreflex activation in humans: an exercise in integrative physiology. Am J Physiol Regul Integr Comp Physiol 313(6):R654–R659. https://doi.org/10.1152/ajpregu.00242.2017
Drew RC, Bell MP, White MJ (2008a) Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol (1985) 104(3):716–723. https://doi.org/10.1152/japplphysiol.00956.2007
Drew RC, McIntyre DB, Ring C, White MJ (2008b) Local metabolite accumulation augments passive muscle stretch-induced modulation of carotid-cardiac but not carotid-vasomotor baroreflex sensitivity in man. Exp Physiol 93(9):1044–1057. https://doi.org/10.1113/expphysiol.2008.042234
Fernandes IA, Vianna LC (2020) Passive cycling with concomitant circulatory occlusion for testing interactions between the exercise pressor reflex afferent pathways: (re)naissance or deja vu? Clin Auton Res 30(6):589–590. https://doi.org/10.1007/s10286-020-00726-w
Fisher JP, Bell MP, White MJ (2005) Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol 90(5):773–781. https://doi.org/10.1113/expphysiol.2005.030577
Fisher JP, Young CN, Fadel PJ (2015) Autonomic adjustments to exercise in humans. Compr Physiol 5(2):475–512. https://doi.org/10.1002/cphy.c140022
Forster HV, Haouzi P, Dempsey JA (2012) Control of breathing during exercise. Compr Physiol 2(1):743–777. https://doi.org/10.1002/cphy.c100045
Gladwell VF, Coote JH (2002) Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol 540(Pt 3):1095–1102. https://doi.org/10.1113/jphysiol.2001.013486
Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH (2005) The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol 567(Pt 2):713–721. https://doi.org/10.1113/jphysiol.2005.089243
Hayes SG, Kindig AE, Kaufman MP (2005) Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol (1985) 99(5):1891–1896. https://doi.org/10.1152/japplphysiol.00629.2005
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10(5):361–374. https://doi.org/10.1016/s1050-6411(00)00027-4
Hill LK, Siebenbrock A (2009) Are all measures created equal? Heart rate variability and respiration—biomed 2009. Biomed Sci Instrum 45:71–76
Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM (1999) Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation 100(1):27–32. https://doi.org/10.1161/01.cir.100.1.27
Ives SJ, McDaniel J, Witman MA, Richardson RS (2013) Passive limb movement: evidence of mechanoreflex sex specificity. Am J Physiol Heart Circ Physiol 304(1):H154–H161. https://doi.org/10.1152/ajpheart.00532.2012
Iwamoto GA, Botterman BR (1985) Peripheral factors influencing expression of pressor reflex evoked by muscular contraction. J Appl Physiol 58(5):1676–1682. https://doi.org/10.1152/jappl.1985.58.5.1676
Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC (2008) Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol 294(2):R585–R593. https://doi.org/10.1152/ajpregu.00731.2007
Kellawan JM, Tschakovsky ME (2014) The single-bout forearm critical force test: a new method to establish forearm aerobic metabolic exercise intensity and capacity. PLoS ONE. https://doi.org/10.1371/journal.pone.0093481
Kent-Braun JA, Miller RG, Weiner MG (1993) Phases of metabolism during progressive exercise to fatigue in human skeletal muscle. J Appl Physiol (1985) 75(2):573–580. https://doi.org/10.1152/jappl.1993.75.2.573
Laginestra FG, Cavicchia A, Vanegas-Lopez JE, Barbi C, Martignon C, Giuriato G, Pedrinolla A, Amann M, Hureau TJ, Venturelli M (2022) Prior involvement of central motor drive does not impact performance and neuromuscular fatigue in a subsequent endurance task. Med Sci Sports Exerc. https://doi.org/10.1249/mss.0000000000002965
Lam E, Greenhough E, Nazari P, White MJ, Bruce RM (2019) Muscle metaboreflex activation increases ventilation and heart rate during dynamic exercise in humans. Exp Physiol. https://doi.org/10.1113/EP087726
Lee JB, Notay K, Seed JD, Nardone M, Omazic LJ, Millar PJ (2021) Sex differences in muscle metaboreflex activation following static handgrip exercise. Med Sci Sports Exerc. https://doi.org/10.1249/mss.0000000000002747
Lis A, Lopusiewicz W, Piepoli MF, Ponikowska B, Paleczny B (2020) Passive bilateral leg cycling with concomitant regional circulatory occlusion for testing mechanoreflex-metaboreflex interactions in humans. Clin Auton Res 30(6):549–556. https://doi.org/10.1007/s10286-020-00717-x
McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS (2010) Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol (1985) 108(1):76–84. https://doi.org/10.1152/japplphysiol.00895.2009
Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N (1994) Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol (1985) 77(6):2778–2783. https://doi.org/10.1152/jappl.1994.77.6.2778
Nishiyasu T, Maekawa T, Sone R, Tan N, Kondo N (2006) Effects of rhythmic muscle compression on cardiovascular responses and muscle oxygenation at rest and during dynamic exercise. Exp Physiol 91(1):103–109. https://doi.org/10.1113/expphysiol.2005.032052
Notay K, Lee JB, Incognito AV, Seed JD, Arthurs AA, Millar PJ (2018) Muscle strength influences pressor responses to static handgrip in men and women. Med Sci Sports Exerc 50(4):778–784. https://doi.org/10.1249/MSS.0000000000001485
Pecanha T, de Brito LC, Fecchio RY, de Sousa PN, Silva ND, Couto PG, de Abreu AP, da Silva GV, Mion D, Low DA, de Moraes Forjaz CL (2021) Activation of mechanoreflex, but not central command, delays heart rate recovery after exercise in healthy men. Int J Sports Med 42(7):602–609. https://doi.org/10.1055/a-1297-4475
Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M, Light AR (2014) Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99(2):368–380. https://doi.org/10.1113/expphysiol.2013.075812
Rotto DM, Kaufman MP (1988) Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985) 64(6):2306–2313. https://doi.org/10.1152/jappl.1988.64.6.2306
Rotto DM, Schultz HD, Longhurst JC, Kaufman MP (1990) Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol (1985) 68(3):861–867. https://doi.org/10.1152/jappl.1990.68.3.861
Silva TM, Aranda LC, Paula-Ribeiro M, Oliveira DM, Medeiros WM, Vianna LC, Nery LE, Silva BM (2018) Hyperadditive ventilatory response arising from interaction between the carotid chemoreflex and the muscle mechanoreflex in healthy humans. J Appl Physiol (1985) 125(1):215–225. https://doi.org/10.1152/japplphysiol.00009.2018
Task-Force (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93(5):1043–1065
Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O’Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS (2010) Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299(5):H1693–H1700. https://doi.org/10.1152/ajpheart.00482.2010
Venturelli M, Amann M, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS (2012) Central and peripheral hemodynamic responses to passive limb movement: the role of arousal. Am J Physiol Heart Circ Physiol 302(1):H333–H339. https://doi.org/10.1152/ajpheart.00851.2011
Venturelli M, Ce E, Limonta E, Bisconti AV, Devoto M, Rampichini S, Esposito F (2017) Central and peripheral responses to static and dynamic stretch of skeletal muscle: mechano- and metaboreflex implications. J Appl Physiol (1985) 122(1):112–120. https://doi.org/10.1152/japplphysiol.00721.2016
Victor RG, Rotto DM, Pryor SL, Kaufman MP (1989) Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res 64(3):592–599. https://doi.org/10.1161/01.RES.64.3.592
Wan HY, Weavil JC, Thurston TS, Georgescu VP, Bledsoe AD, Jessop JE, Buys MJ, Richardson RS, Amann M (2020) The muscle reflex and chemoreflex interaction: ventilatory implications for the exercising human. J Appl Physiol (1985) 129(4):691–700. https://doi.org/10.1152/japplphysiol.00449.2020
Wan HY, Weavil JC, Thurston TS, Georgescu VP, Morrissey CK, Amann M (2022) On the haemodynamic consequence of the chemoreflex and muscle mechanoreflex interaction in women and men: two tales, one story. J Physiol 600(16):3671–3688. https://doi.org/10.1113/JP283051
Wenner MM, Stachenfeld NS (2020) Point: investigators should control for menstrual cycle phase when performing studies of vascular control that include women. J Appl Physiol 129(5):1114–1116. https://doi.org/10.1152/japplphysiol.00443.2020
Zambolin F, Giuriato G, Laginestra FG, Ottaviani MM, Favaretto T, Calabria E, Duro-Ocana P, Bagley L, Faisal A, Peçanha T, McPhee JS, Venturelli M (2022) Effects of nociceptive and mechanosensitive afferents sensitization on central and peripheral hemodynamics following exercise-induced muscle damage. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00302.2022
Acknowledgements
The authors would like to thank the subjects who took part in this study. We also thank Dr. Francesco Pascucci for the help in the development of the MATLAB codes used for data analysis. This work was partially supported by the Italian Ministry of Research and University (MIUR—Rome, Italy) 5-yr special funding (https://www.miur.gov.it/dipartimenti-di-eccellenza).
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
FGL and MV conceived and designed research. FGL, TF, and AC, performed the experiments and analyzed data. FGL drafted the manuscript. All authors interpreted the findings, read, edited, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Communicated by I. Mark Olfert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Figure 3 has been updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laginestra, F.G., Favaretto, T., Giuriato, G. et al. Concurrent metaboreflex activation increases chronotropic and ventilatory responses to passive leg movement without sex-related differences. Eur J Appl Physiol 123, 1751–1762 (2023). https://doi.org/10.1007/s00421-023-05186-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05186-4