Abstract
Purpose
Subretinal drusenoid deposits (SDDs) are distinct extracellular alteration anterior to the retinal pigment epithelium (RPE). Given their commonly uniform phenotype, a hereditary predisposition seems likely. Hence, we aim to investigate prevalence and determinants in patients’ first-degree relatives.
Methods
We recruited SDD outpatients at their visits to our clinic and invited their relatives. We performed a full ophthalmic examination including spectral domain–optical coherence tomography (SD-OCT) and graded presence, disease stage of SDD as well as percentage of infrared (IR) en face area affected by SDD. Moreover, we performed genetic sequencing and calculated a polygenic risk score (PRS) for AMD. We conducted multivariable regression models to assess potential determinants of SDD and associations of SDD with PRS.
Results
We included 195 participants, 123 patients (mean age 81.4 ± 7.2 years) and 72 relatives (mean age 52.2 ± 14.2 years), of which 7 presented SDD, resulting in a prevalence of 9.7%. We found older age to be associated with SDD presence and area in the total cohort and a borderline association of higher body mass index (BMI) with SDD presence in the relatives. Individuals with SDD tended to have a higher PRS, which, however, was not statistically significant in the multivariable regression.
Conclusion
Our study indicates a potential hereditary aspect of SDD and confirms the strong association with age. Based on our results, relatives of SDD patients ought to be closely monitored for retinal alterations, particularly at an older age. Further longitudinal studies with larger sample size and older relatives are needed to confirm or refute our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
The outer retina is a metabolically highly active tissue with a large turnover of lipids in the visual cycle of the photoreceptors and resulting degradation products. Drusen represent focal accumulations of metabolic by-products including lipids and lipoproteins on the inner surface of Bruch’s membrane (BM) posterior to the retinal pigment epithelium (RPE) and are a hallmark age-related macular degeneration (AMD) lesion [1, 2]. In contrast, subretinal drusenoid deposits (SDDs) also known as reticular pseudodrusen are distinct extracellular alterations of the retina anterior to the RPE monolayer and can occur isolated or in relation to another retinal pathology such as AMD or rare genetic diseases such as pseudoxanthoma elasticum (PXE) [3,4,5,6,7,8].
SDDs have been linked to different functional impairments such as scotopic vision impairments, and AMD patients with SDD have been reported at higher risk for conversion to late stage atrophic AMD [3,4,5, 9]. Previous studies have reported a stronger impairment of scotopic visual function (e.g., rod intercept time, dark adaptation time) in patients with SDD compared to phenotypes presenting with predominantly drusen. All these findings point at different underlying disease pathways in presence of SDD prior leading towards outer retinal atrophy compared to eyes without SDD [10]. However, overall prevalence estimates vary significantly from 0.41 to 18.1% between studies depending on imaging modalities [11,12,13,14,15].
Previously, older age, higher body mass index (BMI), smoking, cardiovascular risk factors, and thinner choroid have been reported as determinants of SDD [4, 11, 16, 17]. Moreover, specific genetic variants in the age-related maculopathy susceptibility 2 (ARMS2), complement factor H (CFH), and hepatic triglyceride lipase (LIPC) genes have been identified as potential genetic risk factors [16, 17], while lipid-lowering drugs such as statins seem to have a protective effect [13]. While the commonly uniform phenotype suggests a common genetic component to SDD, we know very little about the heritability to date as barely any data exist on the frequency of SDD in relatives of patients with SDD. Hence, we aim to evaluate prevalence and determinants of SDD in first-degree relatives of patients with SDD and the association with AMD-linked genetic polymorphisms.
Methods
Subjects
We recruited index patients with presence of SDD (dot and/or ribbon type), potentially with a mixed phenotype with conventional drusen, from the outpatient clinic of the university eye hospital Bonn. Eligible patients were invited to approach potential first-degree relatives for inclusion. Included first-degree relatives were scheduled for a separate appointment. We acquired two informed consents (IC), one general and one for genetic testing, from all patients and relatives. The study adhered to the tenets of the Declaration of Helsinki and had local ethical committee approval.
Examination protocol
We acquired demographic characteristics and medical history data including medication, smoking habits, and history of ophthalmic and cardiovascular diseases through a standardized questionnaire. Refraction and best-corrected visual acuity (BCVA) were assessed using the NIDEK (ARK-560A, NIDEK, Japan) auto-refractometer, and intraocular pressure (IOP) was measured using a non-contact tonometer (Tomey FT-1000). BCVA and low-luminance visual acuity (LLVA) were assessed using a standardized ETDRS chart as described elsewhere [18].
Subsequently, all index patients received mydriatic eye drops (0.5% tropicamide and 2.5% phenylephrine) for their appointment in the outpatient clinic, whereas most relatives opted out of pupil dilatation at their appointment.
All participants underwent near-infrared (IR) and spectral domain–optical coherence tomography (SD-OCT) imaging of the macula in both eyes, using the SPECTRALIS OCT (Heidelberg Engineering, Germany) with a scan pattern of 121 B-scans at 30° × 25° and 25 automated real-time (ART) frames in high-resolution (HR) mode. In index patients with poor fixation due to low sharp and central vision, fewer B-scans with less ART frames and lower resolution were performed. Subsequently, all participants underwent a complete ophthalmic examination of both eyes by an ophthalmologist, including slit-lamp examination and fundoscopy. Lastly, blood samples were taken (two 7.5 ml ethylenediaminetetraacetic acid (EDTA) tubes) for genetic testing.
Image grading
Grading for SDD was performed on en face IR and SD-OCT B-scan imaging by two experienced graders (BJH, MMM) based on previously reported criteria (network of roundish irregularities, hyperreflective abnormalities/elevations anterior to the RPE, minimum five lesions) with senior arbitration in cases of doubt (RPF) [6, 19,20,21,22]. Moreover, in case of sufficient image quality, we measured the percentage of area with present SDD on the en face 30° IR image adjusted for individual image size (supplemental figure 1). In addition, we assessed the presence of retinal lesions associated with AMD such as drusen, pigmentary abnormalities, geographic atrophy (GA), and macular neovascularization (MNV) based on SD-OCT.
Statistical analysis of clinical data
We performed descriptive statistics with mean and standard deviation (SD) for normally distributed and median and interquartile-range (IQR) for non-normally distributed data and calculated the prevalence of SDD within the group of relatives. Subsequently, we conducted multivariable logistic regression models with prevalent SDD as dependent variable and controlled for potential determinants. Models were controlled for age, sex, body mass index (BMI), smoking status (never, former, current), spherical equivalent (SE), previous cataract surgery, and prevalence of hypertension and diabetes. Co-variables were chosen a priori on the basis of literature and availability [3, 12, 13]. Moreover, we performed multivariable linear regression models with the percentage of affected area as outcome controlling for the same potential confounders. All analyses were performed with the statistical software RStudio (version 4.0.2, R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/).
Genetic analyses
Samples of 189 individuals (index patients and relatives who consented to genetic testing) were genotyped on an Illumina GSA array Infinium iSelect 24×1 HTS Custom Beadship. Genotypes were then imputed using a local pipeline based on the bioinformatic tool Minimac4 and haplotypes from the 1000 Genomes Project [23]. Quality control steps were performed including the exclusion of single nucleotide polymorphisms (SNPs) that have either an imputation quality of R^2 < 0.3, a genotype rate of less than 90% or a minor allele frequency of less than 0.1% or that fail the Hardy-Weinberg equilibrium. Finally, individuals with more than 10% missing genotype data were dismissed. To derive a polygenic risk score (PRS), 69 SNPs associated with AMD were used. These include among others loci affecting pathways of inflammation as well as the lipid metabolism [24]. Overall, 54 out of 69 considered SNPs as well as 165 individuals passed the quality control. Based on those SNPs, the PRS was calculated via plink2 (http://www.cog-genomics.org/plink/2.0/, [25]) using the effect estimates derived by Han et al. [24]. Finally, the PRS was scaled via z-score within the cohort resulting in the final AMD PRS.
Statistical analysis of polygenic risk data
To investigate associations of the PRS and the AMD and SDD status of individuals, a generalized linear mixed model was fitted; AMD and SDD status was defined as binary response variable (yes/no; questionable cases were used as absent SDD) and regressed on the AMD PRS while adjusting for sex and age and considering the family ID as a random variable. As there were too few participants with SDD and without AMD (N = 7), we could not adjust our genetic SDD models for AMD status due to lack of statistical robustness.
Two further linear mixed models were fitted using the percentage of SDD in both eyes, respectively, as linear outcome variable and AMD PRS as a predictor and adjusting for sex, age, and AMD status. Family ID was again considered as a random variable. Data were available for 90 individuals for the right eye and 96 individuals for the left eye.
Lastly, SDD status of the individuals was regressed separately on each of the 54 SNPs included in the PRS via a generalized linear mixed model while controlling for sex and age. One SNP, for which the model did not converge, was discarded. Associations were checked for nominal significance (α = 0.05) as well as Bonferroni-corrected significance (α = 0.0009).
Results
We included 195 participants, 123 index patients (mean age 81.4 ± 7.2 years; 67% women) and 72 first-degree relatives (mean age 52.2 ± 14.2 years; 56% women). While one index patient had PXE, the majority (94%) had neovascular AMD, which was treated with intravitreal injections in at least one eye.
We found present SDD in 7 of 72 first-degree relatives with a mean age of 60.1 ± 7.6 years, resulting in a prevalence of approximately 9.7%. Only two of these seven relatives showed clinical signs of intermediate AMD, while most showed small preclinical changes such as druplets. All but one of these seven were former or current smokers with a mean body mass index (BMI) of 32.8 ± 9.4 as compared to relatives without SDD who had a mean age of 51.4 ± 14.5 years, mean BMI of 26.8 ± 7.0, and never smoked in 46%.
In participants with data on SDD area, the mean percentage of area affected by SDD was 68% and 66% for the right and left eye, respectively, with large differences between index patients and relatives. Further details and population characteristics can be found in Table 1.
In logistic regression of the total cohort, we found an increased odds ratio (OR) for presence of SDD with older age (per year OR 1.27, 95% confidence interval (CI) 1.17 to 1.41, P < 0.001). In a subgroup analysis of relatives, we found a trend of higher OR of SDD with higher BMI (1.16, 95%CI 1.00 to 1.45, P = 0.08). In linear regression, we found older age to be associated with a larger percentage of area affected by SDD (per year beta = 0.92, 95% CI 0.13 to 1.72, P = 0.02). We found no association with the other assessed potential determinants.
Genetic analyses
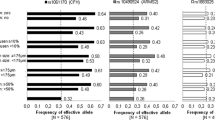
For AMD, we found a higher PRS to be borderline associated with a more likely AMD presence (per unit OR 4.99, 95%CI 0.95 to 26.23, P = 0.057) in multivariable logistic regression models. For SDD, we observed a trend of higher PRS in individuals with SDD (Fig. 1). This trend, however, was not found in the multivariable logistics regression model (OR 1.87, 95% CI 0.61 to 5.76, P = 0.27), in which only age was associated with presence of SDD. Similarly, we found only age and not the PRS to be associated with the area of SDD in the multivariable linear regression models (data not shown). We furthermore found no individual SNP to be associated with presence of SDD. Supplemental figure 2 presents a Manhattan plot for the 53 tested SNPs.
Discussion
Our study reports a prevalence estimate for SDD in first-degree relatives—mostly children—of patients with SDD and identifies age as main determinant of SDD presence. While included relatives were on average younger than index patients, those relatives presenting with SDD were older than those without. Moreover, we found an association of older age with larger retinal en face area affected by SDD and a trend of higher AMD polygenic risk score in individuals with SDD. Our study indicates a heritable trait of SDD which, however, ought to be re-evaluated in longitudinal studies with larger sample size.
First reports of SDD date back to the early 1990s [26], and we know by now that they are distinct from regular drusen and located anterior to the retinal pigment epithelium [3, 13].
Particularly, their uniform dot and ribbon-like appearance across affected patients in the different imaging modalities have given rise to various pathogenetic hypotheses, ranging from widespread inflammation [27] to dependencies on choroidal vasculature [28]. Yet, the underlying pathomechanisms still remain to be elucidated.
Our detected prevalence of 9.7% in relatives is lower than in previous studies using SD-OCT images in the general population, which is mainly explained by the much lower mean age in our cohort. The Montrachet study reported a prevalence of 18% based on SD-OCT images in a cohort with an average age of 82 years [13], and data from the Alienor study suggest a prevalence of 13% in individuals aged 77 years or older [11]. Given the age difference over 25 years, we interpret our detected prevalence to be relatively high nonetheless.
We found age to be the main driver of all detected associations in our cohort. Aging is a proxy for a range of systemic processes that can cause metabolic deterioration over the life span and potentially cause multifactorial diseases such as AMD or cardiovascular disease (CVD) [29]. To date, we do not fully understand the exact underlying pathomechanisms causing these deteriorations. As mentioned, various pathways such as the lipid metabolism or inflammation seem to play a role in AMD and potentially SDD development. The complexity of various simultaneous metabolic changes, however, makes it particularly difficult to identify specific molecular causes.
Our study confirms the previously reported association of SDD with age [13, 15], and we found that only two relatives with SDD had AMD-defining retinal lesions such as intermediate/large drusen or pigmentary abnormalities but showed small alterations such as druplets. This may indicate an independency of SDD from AMD (particularly in the early course of disease) and/or suggest that SDD could be a precursor in those at risk. Given the age difference between index patients and relatives, relatives may have been too young to present large drusen and may still develop these in the course of their lives.
We found a trend of higher BMI in relatives with SDD, which is in agreement with previous studies and may mirror the metabolic deterioration in SDD formation [4, 11, 16, 17]. The fact that we only found the effect in the younger relatives may indicate that BMI has a larger impact at a younger age when fewer other risk factors are present.
As expected, AMD patients showed a higher AMD PRS, which was only borderline statistically significant due to lack of statistical power. Moreover, individuals with SDD seemed to have a higher PRS, which, however, did not remain associated in the multivariable models likely due to lack of statistical power. We hypothesize that the entanglement between AMD and SDD may have created noise in the data and hence lowered statistical power of the AMD PRS to discriminate those with and without SDD. This is in line with previous literature that reported partly contradicting results about the association of SDD with AMD risk genes [3]. While some studies report an association with polymorphisms in the gene for CFH [30, 31] and the ARMS2 gene [32, 33], other studies could not find any association [34,35,36].
Our results support that genetic risk contributes to SDD formation. The incidence and onset of AMD in those with SDD, however, remain to be elucidated in larger longitudinal studies. Based on our data, clinical implications include recommendations to screen relatives of persons affected by both SDD and AMD. Moreover, it remains essential to inform patients and their relatives of prevention measures such as smoking cessation, regular physical activity, and an adequate diet [29, 37].
The direct clinical consequences of SDD presence for the individual still remain to be elucidated. While functional impairments such as scotopic deterioration may occur over time, it remains unclear whether these are directly caused by SDDs or whether presence of SDD indicates a more general metabolic worsening that also cause scotopic impairment.
The strengths of this study include a standardized assessment protocol for patients and relatives including interviews, a full ophthalmic examination and high-resolution retinal imaging. Examinations were conducted by trained staff, and grading for SDD was performed according to a previously described classification [3] by two graders (BJH and MMM) with senior arbitration (RPF) in case of disagreement. SDDs were semi-quantitatively graded by area of SDD in addition to the commonly used binary grading. The genetic analyses are based on state-of-the-art sequencing techniques, and we investigated all previously described loci [24]. To our knowledge, this is the first study to primarily investigate presence of SDD and genetic predisposition in close relatives of patients with SDD.
However, several limitations need to be acknowledged. Our study may underlie a selection bias. Index patients were selected based on presence of SDD at their regularly scheduled visit at the outpatient clinic, mainly for neovascular AMD. Hence, our index patients are potentially more likely to have more severe AMD phenotypes than individuals with SDD in the general population. Moreover, included relatives may be more prone to participate due to, e.g., severe ophthalmic symptoms in their related index patient. However, we think that any bias likely would lead to stronger associations as the inclusion of more severe cases may indicate a higher genetic risk of AMD and potentially SDDs and hence increased chances to detect associations. We have no information on reasons for index patients not to contact their relatives. Main reasons for contacted relatives not to participate were long-distance drives to the clinic, no intact relationships to their relative index patient, and generally no interest in participating in research.
The included relatives were on average younger than our index patients, which likely is one of the main drivers for the relatively low prevalence. At the same time, early structural changes towards SDD development in relatives might not yet be detectable with current available image resolution in IR and SD-OCT imaging leading to an underestimated prevalence of SDDs in our study cohort. In addition, inclusion of older relatives may more likely pick up those who will develop SDD and/or AMD within the later course of their life. We tried to overcome this shortcoming by including the AMD PRS which is independent of age and found that it was on average higher in index patients as compared to relatives indicating that not all relatives have similar genetic predisposition (supplemental figure 3). Yet, we lack genetic data from a control group of individuals without SDD and their respective relatives. Moreover, differences in dilation and presence of cataract between index patients and relatives may have introduced noise in our data. Lastly, the moderate sample size did not provide large statistical power for genetic analyses such as the SNP-wise modeling within the Manhattan plot leaving us presumably underpowered for genetic discoveries. Hence, we likely missed associations in the multivariable models, underscoring the need for further large-scale analyses.
In conclusion, our study indicates a hereditary predisposition for SDDs in relatives of patients with SDD and confirms the strong association with age as important non-modifiable risk factor. Based on our results, relatives of patients with SDD ought to be closely monitored for retinal alterations, particularly at an older age. Our study lays foundation for further longitudinal studies with larger sample size to evaluate the genetic predisposition of SDD and confirm and extend or refute our findings.
References
Jun S, Datta S, Wang L, Pegany R, Cano M, Handa JT (2019) The impact of lipids, lipid oxidation, and inflammation on AMD, and the potential role of miRNAs on lipid metabolism in the RPE. Exp Eye Res 181:346–355
Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS (2005) Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res 81:731–741
Spaide RF, Ooto S, Curcio CA (2018) Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol 63:782–815
Sivaprasad S, Bird A, Nitiahpapand R, Nicholson L, Hykin P, Chatziralli I (2016) Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv Ophthalmol 61:521–537
Schmitz-Valckenberg S, Steinberg JS, Fleckenstein M, Visvalingam S, Brinkmann CK, Holz FG (2010) Combined confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography imaging of reticular drusen associated with age-related macular degeneration. Ophthalmology 117:1169–1176
Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y (2010) Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 117:303–12.e1
Gliem M, Hendig D, Finger RP, Holz FG, Charbel Issa P (2015) Reticular pseudodrusen associated with a diseased bruch membrane in pseudoxanthoma elasticum. JAMA Ophthalmol 133:581–588
Wu Z, Fletcher EL, Kumar H, Greferath U, Guymer RH (2022) Reticular pseudodrusen: a critical phenotype in age-related macular degeneration. Prog Retin Eye Res 88:101017
Sassmannshausen M, Pfau M, Thiele S, Fimmers R, Steinberg JS, Fleckenstein M, Holz FG, Schmitz-Valckenberg S (2020) Longitudinal analysis of structural and functional changes in presence of reticular pseudodrusen associated with age-related macular degeneration. Invest Ophthalmol Vis Sci 61:19
Zhang Y, Wang X, Sadda SR, Clark ME, Witherspoon CD, Spaide RF, Owsley C, Curcio CA (2020) Lifecycles of individual subretinal drusenoid deposits and evolution of outer retinal atrophy in age-related macular degeneration. Ophthalmol Retina 4:274–283
Chan H, Cougnard-Grégoire A, Delyfer M-N, Combillet F, Rougier M-B, Schweitzer C, Dartigues J-F, Korobelnik J-F, Delcourt C (2016) Multimodal imaging of reticular pseudodrusen in a population-based setting: the Alienor study. Invest Ophthalmol Vis Sci 57:3058–3065
Finger RP, Chong E, McGuinness MB, Robman LD, Aung KZ, Giles G, Baird PN, Guymer RH (2016) Reticular pseudodrusen and their association with age-related macular degeneration: the Melbourne Collaborative Cohort Study. Ophthalmology 123:599–608
Gabrielle P-H, Seydou A, Arnould L, Acar N, Devilliers H, Baudin F, Ben Ghezala I, Binquet C, Bron AM, Creuzot-Garcher C (2019) Subretinal drusenoid deposits in the elderly in a population-based study (the Montrachet study). Invest Ophthalmol Vis Sci 60:4838–4848
Joachim N, Mitchell P, Rochtchina E, Tan AG, Wang JJ (2014) Incidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohort. Ophthalmology 121:917–925
Bikbov MM, Kazakbaeva GM, Rakhimova EM, Rusakova IA, Gilmanshin TR, Zainullin RM, Panda-Jonas S, Fakhretdinova AA, Tuliakova AM, Safiullina KR, Bolshakova NI, Gizzatov AV, Ponomarev IP, Jonas JB (2022) Prevalence and determinants of reticular pseudodrusen in the Russian Ural Eye and Medical Study. Acta Ophthalmol 100(8):e1701–e1707. https://doi.org/10.1111/aos.15145
Dutheil C, Le Goff M, Cougnard-Grégoire A, Gattoussi S, Korobelnik J-F, Rougier M-B, Schweitzer C, Delcourt C, Delyfer M-N (2020) Incidence and risk factors of reticular pseudodrusen using multimodal imaging. JAMA Ophthalmol 138:467–477
Rabiolo A, Sacconi R, Cicinelli MV, Querques L, Bandello F, Querques G (2017) Spotlight on reticular pseudodrusen. Clin Ophthalmol 11:1707–1718
Pondorfer SG, Heinemann M, Wintergerst MWM, Pfau M, Strömer AL, Holz FG, Finger RP (2020) Detecting vision loss in intermediate age-related macular degeneration: a comparison of visual function tests. PLoS One 15:e0231748
Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF (2010) Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology 117:1775–1781
Querques G, Querques L, Martinelli D, Massamba N, Coscas G, Soubrane G, Souied EH (2011) Pathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degeneration. Retina (Philadelphia, Pa.) 31:518–526
Spaide RF, Curcio CA (2010) Drusen characterization with multimodal imaging. Retina (Philadelphia, Pa.) 30:1441–1454
Saßmannshausen M, Behning C, Weinz J, Goerdt L, Terheyden JH, Chang P, Schmid M, Poor SH, Zakaria N, Finger RP, Holz FG, Pfau M, Schmitz-Valckenberg S, Thiele S, MACUSTAR Consortium Members (2023) Characteristics and spatial distribution of structural features in age-related macular degeneration: a MACUSTAR Study Report. Ophthalmol Retina 7(5):420–430. https://doi.org/10.1016/j.oret.2022.12.007
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR (2015) A global reference for human genetic variation. Nature 526:68–74
Han X, Gharahkhani P, Mitchell P, Liew G, Hewitt AW, MacGregor S (2020) Genome-wide meta-analysis identifies novel loci associated with age-related macular degeneration. J Hum Genet 65:657–665
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4:7
Mimoun G, Soubrane G, Coscas G (1990) Les drusen maculaires. J Fr Ophtalmol 13:511–530
Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV (2006) Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci 47:5495–5504
Grewal DS, Chou J, Rollins SD, Fawzi AA (2014) A pilot quantitative study of topographic correlation between reticular pseudodrusen and the choroidal vasculature using en face optical coherence tomography. PLoS One 9:e92841
Mauschitz MM, Finger RP (2022) Age-related macular degeneration and cardiovascular diseases: revisiting the common soil theory. Asia Pac J Ophthalmol (Phila) 11:94–99
Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BEK (2008) The epidemiology of retinal reticular drusen. Am J Ophthalmol 145:317–326
Elfandi S, Ooto S, Ueda-Arakawa N, Takahashi A, Yoshikawa M, Nakanishi H, Tamura H, Oishi A, Yamashiro K, Yoshimura N (2016) Clinical and genetic characteristics of Japanese patients with age-related macular degeneration and pseudodrusen. Ophthalmology 123:2205–2212
Smith RT, Merriam JE, Sohrab MA, Pumariega NM, Barile G, Blonska AM, Haans R, Madigan D, Allikmets R (2011) Complement factor H 402H variant and reticular macular disease. Arch Ophthalmol 129:1061–1066
Yoneyama S, Sakurada Y, Mabuchi F, Imasawa M, Sugiyama A, Kubota T, Iijima H (2014) Genetic and clinical factors associated with reticular pseudodrusen in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 252:1435–1441
Ueda-Arakawa N, Ooto S, Nakata I, Yamashiro K, Tsujikawa A, Oishi A, Yoshimura N (2013) Prevalence and genomic association of reticular pseudodrusen in age-related macular degeneration. Am J Ophthalmol 155:260–269.e2
Puche N, Blanco-Garavito R, Richard F, Leveziel N, Zerbib J, Tilleul J, Mimoun G, Querques G, Cohen SY, Souied EH (2013) Genetic and environmental factors associated with reticular pseudodrusen in age-related macular degeneration. Retina (Philadelphia, Pa.) 33:998–1004
Boddu S, Lee MD, Marsiglia M, Marmor M, Freund KB, Smith RT (2014) Risk factors associated with reticular pseudodrusen versus large soft drusen. Am J Ophthalmol 157:985–993.e2
Heesterbeek TJ, Lorés-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI (2020) Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt 40:140–170
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding for genotyping was acquired via the Australian NHRMC synergy grant (#1181010) awarded to Robyn Guymer, Alice Pébay, Erica Fletcher, Melanie Bahlo, Zhichao Wu, and Brendan Ansell. The authors would like to express their gratitude.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of the medical faculty of the University of Bonn and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors. This study was approved by the ethics committee of the medical faculty of the University of Bonn.
Conflict of interest
Matthias M. Mauschitz has received consulting fees from Biogen. Jan H. Terheyden has received support from Heidelberg Engineering, Optos, Carl Zeiss Meditec, CenterVue, Novartis, and Okko. Robert P. Finger has received grants from Novartis and Consulting fees from Bayer, Santen, Opthea, Novelion, ProQR, Oxford Innovation, Roche, Alimera, Santhera, Inositec, and Ellex. None of the aforementioned relations had any impact on this study. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mauschitz, M.M., Hochbein, B.J., Klinkhammer, H. et al. Prevalence and determinants of subretinal drusenoid deposits in patients’ first-degree relatives. Graefes Arch Clin Exp Ophthalmol 262, 53–60 (2024). https://doi.org/10.1007/s00417-023-06221-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06221-y