Abstract
Purpose
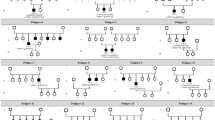
Phenotypic heterogeneity with variable severity has been reported in female carriers of retinitis pigmentosa GTPase regulator (RPGR) mutations, including a male-type phenotype. A phenomenon not fully understood is peripapillary retinal nerve fiber layer (pRNFL) thickening in male patients with RPGR-associated X-linked retinitis pigmentosa, especially in the temporal sector. We aim to describe the genetic spectrum, retinal phenotypes, and pRNFL thickness in a cohort of Caucasian RPGR-mutation heterozygotes.
Methods
A cross-sectional study was conducted at an inherited retinal degeneration (IRD) reference center in Portugal. Female patients heterozygous for clinically significant RPGR variants were identified using the IRD-PT registry. A complete ophthalmologic examination was performed, complemented by macular and peripapillary spectral domain optical coherence tomography (SD-OCT), ultra-widefield color fundus photography (UW-CFP), and ultra-widefield fundus autofluorescence (UW-FAF). The retinal phenotypes were graded according to previously described classifications. The pRNFL thickness across the superior, inferior, nasal, and temporal quadrants was compared to the Spectralis® RNFL age-adjusted reference database.
Results
Forty-eight eyes from 24 females (10 families) were included in the study. Genetic analysis yielded 8 distinct clinically significant frameshift variants in RPGR gene, 3 of which herein reported for the first time. No association was found between mutation location and best-corrected visual acuity (BCVA) or retinal phenotype. Age was associated with worse BCVA and more advanced phenotypes on SD-OCT, UW-CFP, and UW-FAF. Seven women (29.17%) presented a male-type phenotype on UW-FAF in at least one eye. An association was found between UW-FAF and pRNFL thickness in the temporal sector (p = 0.003), with the most advanced fundus autofluorescence phenotypes showing increased pRNFL thickness in this sector.
Conclusion
This study expands the genetic landscape of RPGR-associated disease by reporting 3 novel clinically significant variants. We have shown that clinically severe phenotypes are not uncommon among female carriers. Furthermore, we provide novel insights into pRNFL changes observed in RPGR heterozygotes that mimic what has been reported in male patients.



Similar content being viewed by others
Data availability
This work and its original data have not been previously published.
References
Verbakel SK, Van Huet RAC, Boon CJF, Den Hollander AI, Collin RWJ, Klaver CCW et al (2018) Non-syndromic retinitis pigmentosa. Prog Retin Eye Res 66:157–186. https://doi.org/10.1016/J.Preteyeres.2018.03.005
Nanda A, Salvetti AP, Clouston P, Downes SM, MacLaren RE (2018) Exploring the variable phenotypes of RPGR carrier females in assessing their potential for retinal gene therapy. Genes (Basel) 9(12):643. https://doi.org/10.3390/Genes9120643
De Silva SR, Arno G, Robson AG, Fakin A, Pontikos N, Mohamed MD et al (2021) The X-linked retinopathies: physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Prog Retin Eye Res 82:100898. https://doi.org/10.1016/J.Preteyeres.2020.100898
Yang J, Zhou L, Ouyang J, Xiao X, Sun W, Li S, Zhang Q (2021) Genotype-phenotype analysis of RPGR variations: reporting of 62 Chinese families and a literature review. Front Genet 12:600210. https://doi.org/10.3389/Fgene.2021.600210
Jacobson SG, Yagasaki K, Feuer WJ, Roman AJ (1989) Interocular asymmetry of visual function in heterozygotes of X-linked retinitis pigmentosa. Exp Eye Res 48(5):679–691. https://doi.org/10.1016/0014-4835(89)90009-2
Comander J, Weigel-Difranco C, Sandberg MA, Berson EL (2015) Visual function in carriers of X-linked retinitis pigmentosa. Ophthalmology 122(9):1899–1906. https://doi.org/10.1016/J.Ophtha.2015.05.039
Fahim AT, Daiger SP (2016) The role of X-chromosome inactivation in retinal development and disease. Adv Exp Med Biol 854:325–331. https://doi.org/10.1007/978-3-319-17121-0_43
Fahim AT, Sullivan LS, Bowne SJ, Jones KD, Wheaton DKH, Khan NW et al (2020) X-chromosome inactivation is a biomarker of clinical severity in female carriers of RPGR-associated X-linked retinitis pigmentosa. Ophthalmol Retina 4(5):510–520. https://doi.org/10.1016/J.Oret.2019.11.010
Salvetti AP, Nanda A, MacLaren RE (2021) RPGR-related X-linked retinitis pigmentosa carriers with a severe “male pattern”. Ophthalmologica 244(1):60–67. https://doi.org/10.1159/000503687
Kilgore DA, Kilgore TA, Sukpraprut-Braaten S, Schaefer GB, Uwaydat SH (2021) Multimodal imaging of an RPGR carrier female. Ophthalmic Genet 42(3):312–316. https://doi.org/10.1080/13816810.2021.1881981
Talib M, Van Schooneveld MJ, Van Cauwenbergh C, Wijnholds J, Ten Brink JB, Florijn RJ et al (2018) The spectrum of structural and functional abnormalities in female carriers of pathogenic variants in the RPGR gene. Invest Ophthalmol Vis Sci 59(10):4123–4133. https://doi.org/10.1167/Iovs.17-23453
Grover S, Fishman GA, Anderson RJ, Lindeman M (2000) A longitudinal study of visual function in carriers of X-linked recessive retinitis pigmentosa. Ophthalmology 107(2):386–396. https://doi.org/10.1016/S0161-6420(99)00045-7
Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Roman AJ, Windsor EA et al (2007) Inner retinal abnormalities in X-linked retinitis pigmentosa with RPGR mutations. Invest Ophthalmol Vis Sci 48(10):4759–4765. https://doi.org/10.1167/Iovs.07-0453
Birtel TH, Birtel J, Hess K, Clemens AC, Lindner M, Herrmann P et al (2021) Analysis of imaging biomarkers and retinal nerve fiber layer thickness in RPGR-associated retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 259(12):3597–3604. https://doi.org/10.1007/S00417-021-05233-W
Gao H, Huang X, He J, Zou T, Chen X, Xu H (2021) The roles of microglia in neural remodeling during retinal degeneration. Histol Histopathol 37:18384. https://doi.org/10.14670/Hh-18-384
Marques JP, Carvalho AL, Henriques J, Murta JN, Saraiva J, Silva R (2020) Design, development and deployment of a web-based interoperable registry for inherited retinal dystrophies in Portugal: the IRD-PT. Orphanet J Rare Dis 15(1):304. https://doi.org/10.1186/S13023-020-01591-6
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. https://doi.org/10.1038/Gim.2015.30
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174 https://www.Ncbi.Nlm.Nih.Gov/Pubmed/843571
Andreasson S, Breuer DK, Eksandh L, Ponjavic V, Frennesson C, Hiriyanna S et al (2003) Clinical studies of X-linked retinitis pigmentosa in three Swedish families with newly identified mutations in the RP2 And RPGR-ORF15 genes. Ophthalmic Genet 24(4):215–223. https://doi.org/10.1076/Opge.24.4.215.17228
Li HP, Yuan SQ, Wang XG, Sheng XL, Li XR (2020) Myopia with X-linked retinitis pigmentosa results from a novel gross deletion of RPGR gene. Int J Ophthalmol 13(8):1306–1311. https://doi.org/10.18240/Ijo.2020.08.18
Al-Maskari A, O’Grady A, Pal B, Mckibbin M (2009) Phenotypic progression in X-linked retinitis pigmentosa secondary to a novel mutation in the RPGR gene. Eye (Lond) 23(3):519–521. https://doi.org/10.1038/Eye.2008.427
Hendriks M, Verhoeven VJM, Buitendijk GHS, Polling JR, Meester-Smoor MA, Hofman A et al (2017) Development of refractive errors-qhat can we learn from inherited retinal dystrophies? Am J Ophthalmol 182:81–89. https://doi.org/10.1016/J.Ajo.2017.07.008
Sanchez Tocino H, Diez Montero C, Villanueva Gomez A, Lobo Valentin R, Montero-Moreno JA (2019) Phenotypic high myopia in X-linked retinitis pigmentosa secondary to a novel mutation in the RPGR gene. Ophthalmic Genet 40(2):170–176. https://doi.org/10.1080/13816810.2019.1605385
Ogino K, Oishi M, Oishi A, Morooka S, Sugahara M, Gotoh N et al (2015) Radial fundus autofluorescence in the periphery in patients with X-linked retinitis pigmentosa. Clin Ophthalmol 9:1467–1474. https://doi.org/10.2147/Opth.S89371
Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC (2016) Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 51:1–40. https://doi.org/10.1016/J.Preteyeres.2015.06.003
Rattner A, Nathans J (2005) The genomic response to retinal disease and injury: evidence for endothelin signaling from photoreceptors to glia. J Neurosci 25(18):4540–4549. https://doi.org/10.1523/Jneurosci.0492-05.2005
Martinez-Fernandez De La Camara C, Nanda A, Salvetti AP, Fischer MD, MacLaren RE (2018) Gene therapy for the treatment of X-linked retinitis pigmentosa. Expert Opin Orphan Drugs 6(3):167–177. https://doi.org/10.1080/21678707.2018.1444476
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Human Research Ethics Committee (HREC) of Centro Hospitalar e Universitário de Coimbra (CHUC) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

Supplemental Fig. 1
Top Graphical representation depicting the positive correlation between logMAR best-corrected visual acuity (BCVA) and age (R2 = 0.123, p = 0.016). Bottom Graphical representation depicting the negative correlation between logMAR BCVA and spherical equivalent (R2 = 0.535, p < 0.001). (PNG 79 kb)
ESM 1
(DOCX 14 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marques, J.P., Pinheiro, R., Carvalho, A.L. et al. Genetic spectrum, retinal phenotype, and peripapillary RNFL thickness in RPGR heterozygotes. Graefes Arch Clin Exp Ophthalmol 261, 867–878 (2023). https://doi.org/10.1007/s00417-022-05809-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05809-0