Abstract
The aim of our study was to establish empirically to what extent reduced glucose uptake in the precuneus, posterior cingulate and/or temporo-parietal cortex (PCTP), which is thought to indicate brain amyloidosis in patients with dementia or MCI due to Alzheimer’s Disease (AD), permits to distinguish amyloid-positive from amyloid-negative patients with non-classical AD phenotypes at the single-case level. We enrolled 127 neurodegenerative patients with cognitive impairment and a positive (n. 63) or negative (n. 64) amyloid marker (cerebrospinal fluid or amy-PET). Three rating methods of FDG-PET scan were applied: purely qualitative visual interpretation of uptake images (VIUI), and visual reading assisted by a semi-automated and semi-quantitative tool: INLAB, provided by the Italian National Research Council, or Cortex ID Suite, marketed by GE Healthcare. Fourteen scans (11.0%) patients remained unclassified by VIUI or INLAB procedures, therefore, validity values were computed on the remaining 113 cases. The three rating approaches showed good total accuracy (77–78%), good to optimal sensitivity (81–93%), but poorer specificity (62–75%). VIUI showed the highest sensitivity and the lowest specificity, and also the highest proportion of unclassified cases. Cases with asymmetric temporo-parietal hypometabolism and a progressive aphasia or corticobasal clinical profile, in particular, tended to be rated as AD-like, even if biomarkers indicated non-amyloid pathology. Our findings provide formal support to the value of PCTP hypometabolism for single-level diagnosis of amyloid pathophysiology in atypical AD, but also highlight the risk of qualitative assessment to misclassify patients with non-AD PPA or CBS underpinned by asymmetric temporo-parietal hypometabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research criteria for the diagnosis of Alzheimer’s Disease (AD), in the Mild Cognitive Impairment (MCI) or dementia stages [1,2,3], incorporate biomarkers in the diagnostic process, converging on the recommendations about the use of the so-called pathophysiological biomarkers, namely Positron Emission Tomography with ligands for cerebral amyloid deposits (amy-PET) and Aβ, Tau and phospho-Tau in cerebrospinal fluid (CSF), but diverging on the role of brain 18-fluorodeoxy-glucose PET (FDG-PET). According to the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) [2], in the absence of markers of Aβ deposits, a specific FDG-PET pattern, i.e. hypometabolism in the Precuneus, posterior Cingulate and posterior Temporo-Parietal (PCTP) regions, may be used as a marker of an underlying AD pathophysiology. On the other hand, the International Working Group (IWG) [3] classifies PCTP hypometabolism as a topographical marker, which maps spreading of neurodegeneration but lacks sufficient pathophysiological specificity.
The link between AD and PCTP hypometabolism has received robust support in the literature, crucially also from case reports and group studies including AD patients with post-mortem or in vivo positive markers of amyloidopathy [4,5,6,7,8,9,10,11,12,13,14,15,16]. These regions are highly interconnected and hyperactive hubs of the default mode network, and seem to be particularly vulnerable to Aβ aggregation due to their high metabolic demands [17]. Remarkably, PCTP hypometabolism has been demonstrated not only in the classical, amnestic, type of AD, but also in its atypical presentations, i.e. the frontal [13, 14], linguistic [13,14,15,16] and visuospatial [7, 13,14,15] variants. However, only a limited number of studies have so far assessed the validity of PCTP hypometabolism as an index of amyloidopathy at the single patient-level in samples with a neuropathological or biomarker-based diagnosis of AD [18,19,20,21,22,23]; moreover, only one study [24] considered a mixed, amnestic and atypical, pool of amy-positive (A+) and amy-negative (A − ) patients.
The current study was designed to fill this literature gap and test the validity of PCTP hypometabolism for single-subject diagnosis of Aβ-related cognitive deficits in a specific clinical scenario: diagnostic work up of referrals to a tertiary memory clinic, with a focus on atypical presentations of AD, in various disease stages. All patients had undergone amy-PET or CSF as pathophysiological biomarkers, which were used as gold standard for a clinico-biological diagnosis of AD.
Recent consensus recommendations from the European Association of Nuclear Medicine and European Academy of Neurology (EANM-EAN) [25], emphasised the usefulness of semi-automated processing to assist visual reading, thus we also compared the validity of PCTP assessed with a purely qualitative approach, consisting in the visual interpretation of uptake images (VIUI), with two automated tools that allow semi-quantification of FDG-uptake and comparison with an age-matched database of healthy controls.
Materials and methods
Participants
We reviewed medical records (incorporating neurological examination, MiniMental State Examination—MMSE—score and neuroimaging reports) of subjects referred for cognitive disturbances to the memory clinic of San Gerardo Hospital, Monza, between January 2015 and January 2020. Criteria for eligibility were the following: (1) diagnosis of dementia or MCI according to NINCDS-ADRDA criteria, or of preclinical AD defined as an asymptomatic condition, at the time of FDG-PET, with biomarker evidence of AD pathology, or converted to MCI/dementia at follow-up [1,2,3]; (2) availability of brain FDG-PET scan performed as part of the routine diagnostic work up within six months from neurological assessment; (3) availability of a physiopathological biomarker for amyloid status (amyloid-PET or Tau/Aβ ratio in CSF) performed for research purposes, or presence of a genetic mutation for a neurodegenerative disorder. Individual, syndrome-level diagnoses did not take into account the results of brain FDG-PET scan and were based on standardised criteria for amnestic AD [2], Posterior Cortical Atrophy (PCA) [26], behavioural variant Frontotemporal Dementia (bFTD) [27], Dementia with Lewy Bodies (DLB) [28], Corticobasal Syndrome (CBS) [29], Primary Progressive Aphasia (PPA) [30], or Progressive Supranuclear Palsy (PSP) [31].
Exclusion criteria were evidence of moderate-to-severe vascular burden on structural neuroimaging or history of other neurological disorders, major psychiatric diseases, brain injury, mental insufficiency, substance abuse, severe medical conditions.
All participants were unpaid volunteers and gave written informed consent for participation. The study was approved by our institution's ethics committee, Comitato Etico Brianza, and was carried out in accordance with the ethical standards of 1964 Declaration of Helsinki and its later amendments.
Acquisition and processing of FDG-PET scans
All scans were acquired with the same General Electric Discovery LS PET/CT scanner in our Nuclear Medicine Unit, within 6 months from the neurological assessment.
Patients were instructed to fast for at least 6 hours. Before the exam, they were measured blood glucose levels (whole sample’s mean levels: 105.0 ± 19.6 mg/dL), and then received an intravenous bolus of approximately 200 MBq of 18F-FDG. After lying supine in a quiet, dimly lit room for approximately 45 min, they were transferred to the scanner. First, a CT scan was performed for attenuation correction, then PET images were acquired for 15 min, with a thickness of 3.27 mm and a matrix of 128 × 128 pixels. Subsequent image reconstruction followed an ordered subset expectation maximisation (OSEM) algorithm.
Processing of PET images with INLAB
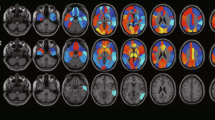
INLAB is a validated automated service for analysis of PET images [32] based on Statistical Parametric Mapping (SPM; http://www.fil.ion.ucl.ac.uk/spm) developed by the Bioimaging Lab of the Italian National Research Council (https://www.ibfm.cnr.it/), and freely available online. Individual patients’ PET scans are uploaded as DICOM files archived together in.zip format. They are reoriented along the anterior–posterior commissure, spatially normalised to an FDG-PET dementia-specific template [33], smoothed with an isotropic 3D Gaussian kernel of 8 mm FWHM, and proportionally scaled to a whole-brain mean intensity value. Pre-processed images are then tested for relative hypometabolism by comparison with a reference group of 112 healthy controls [34], using the two sample t-test design of SPM5, including age as covariate. Cluster-level significance threshold is set at p < 0.05 FWE-corrected, and only clusters with a minimum size of 100 voxels are retained. Areas of significant hypometabolism are finally displayed as areas of black and white shading on a three-orientation ‘glass brain’, as well as in a colour statistical parametric map. A table reporting set-, cluster- and voxel-level statistics and anatomical coordinates of significant clusters is also produced. All outputs can be downloaded as .pdf documents (Fig. 1).
Processing of PET images with Cortex ID Suite
Cortex ID Suite is a software package developed and marketed by GE Healthcare (Waukesha, WI, USA) that computes individual patients’ mean FDG-uptake for cerebellum and pons, also used as reference regions for scaling, and 12 supratentorial, bilateral regions of interest (ROI): lateral and mesial prefrontal cortex, anterior and posterior cingulate, sensorimotor cortex, precuneus, superior and inferior parietal lobe, lateral and mesial temporal cortex, lateral occipital cortex, and primary visual cortex. Patients’ mean values are compared with those of an inbuilt dataset of scans from 294 healthy controls. Results of this comparison are shown in a colour map projected on a 3D stereotactic surface, with manipulable colour scale, and also expressed as Z-scores displayed in a table, with a Z-score ≤ − 2.0 as cutoff for significant hypometabolism. All outputs can be downloaded as .pdf documents (Fig. 1).
Rating of PET images
PET images were all assessed by one rater, M.M., a Nuclear Medicine specialist with over 20 years of experience reading PET scans, who was blinded to clinical diagnosis and biomarker status. For the VIUI rating procedure, a second, independent, rater, S.P., a last-year resident in Nuclear Medicine, was involved, with the aim to evaluate inter-rater reliability between two assessors with different levels of expertise.
VIUI was based solely on visual inspection of qualitative images, which were displayed on a terminal on which orientation (axial, coronal, and sagittal) and colour scale could be manipulated (Fig. 1). INLAB rating was based on visual inspection of colour statistical parametric maps and glass brain images, and on semi-quantitative indices provided by SPM. Cortex ID Suite rating was based on visual inspection of 3D colour maps and on the Z-scores computed by the software.
The rater was asked to focus on PCTP regions, rate them as normal/hypometabolic/unclassifiable, and made a judgement about whether or not the observed pattern reflected a diagnosis of AD. Specifically, following criteria similar to those applied in previous studies [21, 22, 35], images consistent with AD were agreed upon a priori to show hypometabolism in the precuneus/posterior cingulate and/or temporo-parietal regions, either restricted to these areas, or clearly predominant in these areas than in the frontal, fronto-parietal or occipital regions.
The three ratings (VIUI and evaluation of INLAB and Cortex ID Suite outputs) were performed in three distinct sessions, blinded to the results of prior ratings.
Statistical analysis
Statistical analysis was performed with SPSS version 27.0 (IBM Corp., Armonk, NY, USA) or MedCalc Statistical Software version 20.027 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org; 2020).
Comparisons between A+ and A − cases were carried out with Student’s t-test for continuous variables (age, disease duration, MMSE score) and Chi-square test for categorical variables (sex), setting threshold for significance at p < 0.05.
Total accuracy, sensitivity, specificity and positive and negative predictive values (PPV, NPV) were calculated as measures of the validity of PCTP hypometabolism to classify correctly A+ and A − cases. They were computed for each of the three PET scans rating methods. Positive and negative Likelihood Ratios (+ LR, − LR) were also calculated, with the formulas: sensitivity/(100—specificity), (100—sensitivity)/specificity. A +LR value > 1 means that a positive test is more likely to occur in patients with the condition (i.e. Aβ pathology) than in those without the condition, while a − LR value < 1 means that a negative test is more likely to occur in patients without the condition than in those with the condition. Differences in accuracy between the three rating methods were tested for statistical significance by computing receiver-operating characteristic (ROC) curves and comparing the areas under the curve (AUC) using DeLong et al. methodology [36], in MedCalc.
Fleiss kappa was run to determine the degree of concordance between the three rating methods, and Cohen’s kappa was run to measure inter-rater reliability for VIUI.
Results
Characteristics of the study sample
From an initial pool of 150 patients meeting inclusion criteria, we excluded 23 cases due to the reasons detailed in Fig. 2.
The final study sample was, therefore, composed by 127 patients, whose general features are shown in Table 1. They all met criteria for MCI or dementia, except three, who were in a preclinical disease stage: all three complained of word finding difficulties at the time of FDG-PET, and later converted to PPA (n. 2) or (CBS) (n. 1). Sixty-three out of 127 (49.6%) were A+ and 64 A − (Table 1). The two groups showed overlapping age (t = 0.014, p = 0.989) and sex distribution (x2 = 0.378, p = 0.593); mean MMSE score (t = − 1.916, p = 0.058) and disease duration (t = 1.859, p = 0.066) were marginally not significant. CSF (with Tau/Aβ ratio ≥ 1.0 as cutoff for amyloid positivity [37]) was the most frequent biomarker, being available in 94/127 (74.0%) cases (40 A+ and in 54 A − ). There were only three cases of genetic mutations: one Presenilin 1 and one Presenilin 2 presenting as PCA, and one Progranulin presenting with predominant language deficits. The remaining 30 patients (19 A+ and 11 A − ) had PET with amyloid tracer as biomarker ([18F]Flutemetamol in 13 cases, [18F]Florbetapir in 12, and [18F]Florbetaben in 10, analysed qualitatively in most cases). Within the A+ group, the amnestic and PCA presentations accounted for more than half of all cases, while the most prevalent phenotypes within the A − group were CBS and PPA, followed by bFTD (overall significance for comparison of proportion of the different syndromes between the two groups: x2 = 42.372, p = 0.0001). Within each clinical syndrome, the proportion of A+ patients was as follows: 9/26 PPA (34.6%), 9/25 CBS (36.0%), 20/24 amnestic AD (83.3%), 17/18 PCA (94.4%), 4/15 bFTD (26.7%), 1/6 DLB (16.7%), 1/5 not otherwise specified syndrome (20.0%), 0/5 PSP, and 2/3 subjective complaints (66.7%).
Results of analyses on FDG-PET ratings
In 14 out of 127 patients (11.0%), PET scans could not be rated as positive or negative by VIUI (n. 9), on INLAB images (n. 1), or by both VIUI and INLAB procedures (n. 4), while Cortex ID Suite allowed to reach a clear-cut conclusion in all cases. Their main demographic, clinical and PET imaging features are reported in the Supplementary material. Most (11/14, 78.6%) were A − patients for which the two raters could not rule out significant PCTP hypometabolism, and approximately half of them showed impairment of language.
Inter-rater reliability for VIUI was calculated on all 127 cases, while validity values were calculated on the 113 patients whose scans could be rated as either normal or hypometabolic.
Inter-rater reliability for VIUI
Cohen’s k indicated high agreement between the two Nuclear Medicine physicians in defining presence/absence of PCTP hypometabolism (0.83 [95% CI 0.70 to 0.97], p = 0.000).
There were 11 discordant ratings: four scans were rated as AD-like by M.M. and as negative by S.P., three were rated as AD-like by S.P. and as negative by M.M., and four were rated as AD-like (two) or negative (two) by M.M. and as unclassifiable by S.P.
Agreement between the three rating methods
VIUI showed moderate agreement with both rating of INLAB maps and rating of Cortex ID Suite maps (Table 2). In most of the 21 discrepant cases, PCTP regions were rated as hypometabolic on VIUI and as normal in INLAB images (n. 16, 88.9%) and Cortex ID Suite images (n. 14, 66.7%).
Between the two automated methods, agreement was strong (Table 5), and the majority of the 11 discrepancies (n. 9, 81.8%) were scans rated as AD-like on Cortex ID Suite output and as normal on INLAB output.
Results of validity analysis
Total accuracy of PCTP hypometabolism in classifying A+ and A − cases was around 77% for all three rating procedures (Table 3). Sensitivity and NPV were generally high (ranging from 81 to 93% and from 79 to 89%, respectively), while specificity and PPV were generally lower (ranging from 62 to 75% and from 72 to 77%, respectively). +LR ranged from 2.4 to 3.2, and − LR from 0.25 to 0.11. VIUI showed the highest sensitivity, NPV and − LR, while rating of INLAB images showed the highest specificity, PPV and +LR.
Comparison of AUCs between the three rating methods (Fig. 3) did not show significant differences (p = 0.9277 between VIUI and INLAB, p = 0.8611 between VIUI and Cortex ID, p = 0.7182 between INLAB and Cortex ID).
Characteristics of misclassified cases
A total of 40 patients were misclassified by one or more of the three rating methods: 28 were false positive, i.e. A − cases whose PCTP regions were rated as hypometabolic, and 12 were false negative, i.e. A+ cases without evidence of significant PCTP hypometabolism.
Table 4 shows the main characteristics of the 12 false-negative cases, seven of which were false negative for two or more rating methods. These patients were overall relatively old: 11/12 (91.7%), were above 70 years of age and their mean age (74.5 years ± 3.1) was 5 years older than the entire study sample’s. They also were in an initial disease stage: eight (66.7%) showed a maximum symptoms duration of 2 years, and seven (58.3%) had an MMSE score ≥ 26/30. Finally, they showed diverse clinical presentations, but the amnestic phenotype was slightly prevalent (n. 5, 41.7%).
In all eight misclassified cases with CSF as biomarker, the Tau/Aβ ratio was well above cutoff for amyloid positivity. As to FDG-PET scans, most patients (n. 8, 66.7%) showed hypometabolism in the fronto-temporal or fronto-parietal regions.
Table 5 displays the main characteristics of the 28 cases misclassified as false positive, half of which were false positive for two or more rating methods. Fifteen (53.4%) were below 70 years of age, and 11 (39.3%) had a pre-senile onset. The great majority were in an early disease stage or were only mildly impaired (n. 20, 71.4%, had an MMSE score ≥ 24, and n. 24, 85.7%, a score ≥ 20). Two phenotypes were prevalent: 11 patients (39.3%) had CBS and 8 (28.6%) PPA; the next more frequent diagnosis was DLB (n. 4, 14.3%). Notably, in addition to the cases of progressive aphasia, six more patients, five with CBS and the patient with the Progranulin mutation, also showed remarkable language deficits. The great majority of these patients (n. 21, 75.0%) had CSF as biomarker. Only in one case (FP22) was the Tau/Aβ ratio close to the cutoff for amyloid positivity.
With regard to FDG scans, in most of these patients, hypometabolism within the PCTP system encompassed the temporo-parietal carrefour (n. 13, 46.4%) or the precuneus/posterior cingulate and the parietal cortex (n. 10, 35.7%), and was asymmetric (n. 20, 71.4%), with a left-hemisphere predominance (n. 17, 60.7%). Additional clusters of reduced FDG-uptake were reported in the frontal or fronto-temporal regions in the majority of patients (n. 19, 67.9%); only in seven cases (25.0%), no other area of hypometabolism was evident outside PCTP.
Discussion
FDG-PET neuroimaging is considered an essential part of the diagnostic algorithm for dementia [25, 38, 39]. In this study, we investigated the role of hypometabolism in PCTP areas in predicting the presence of Aβ pathology at the individual level, in patients with atypical MCI or dementia whose amyloid status was established by CSF, amy-PET, or presence of a genetic mutation. Hypometabolism was assessed through three different approaches: purely qualitative VIUI, and visual + semi-quantitative assessment based on either INLAB or Cortex ID Suite softwares. The three procedures all showed good (77–78%) total accuracy, good to optimal sensitivity (81 to 93%), but poorer specificity (62 to 75%), in agreement with the results from prior studies that converged in showing high sensitivity (85–94%) but more diverse specificity (50–83%) [18,19,20, 23, 24]. VIUI, which is clinically the most used approach, also showed a good agreement between the expert rater and the Nuclear Medicine resident, indicating that the procedure does not require extremely advanced skills.
The generally high sensitivity of PCTP hypometabolism for amyloid positivity in classical as well as atypical presentations of AD (more than 68% of A+ cases had a non-amnestic syndrome) is in agreement with prior structural and functional neuroimaging studies showing that the involvement of these regions is a common feature of AD, irrespective of the clinical phenotype [6, 7, 13,14,15,16, 40, 41], and further supports the idea that this metabolic signature of AD mirrors disease pathophysiology over and above symptoms profile. VIUI, in particular, showed higher sensitivity than INLAB and Cortex ID Suite maps and computations. This finding comes as a confirmation of past evidence suggesting that the overall superiority of semi-quantitative over qualitative reading does not relate to sensitivity, rather to specificity, and varies greatly across tools [8, 25, 42, 43]. Morbelli et al. [43] posited that trained readers might be able to detect minor but meaningful abnormalities that do not reach the threshold for significance on statistical maps. As an example, readers have the possibility to base their evaluation on inter-hemispheric asymmetries, which only few automated tools allow to compute [43].
Scrutiny of socio-demographic and clinical features of our 12 false-negative cases highlighted some relatively consistent features (e.g. they were generally old and in an early disease stage), but was not helpful in identifying clear-cut reasons for their misclassification. Certain characteristics of our 28 false-positive cases, on the other hand, provide an at least partial account for the lower specificity found for PCTP hypometabolism. The majority of these patients, whose biomarkers suggested non-amyloid pathophysiology but who were regarded as having an AD-like FDG pattern, had a diagnosis of CBS (some with predominant language disturbances) or PPA, and showed hypometabolism involving the parietal, temporo-parietal and/or temporal areas, quite often with an asymmetric distribution. In PPA, the poor specificity of quantitative and qualitative FDG-PET in predicting underlying pathology, especially in cases with asymmetric PCTP hypometabolism, had already been pointed out [6, 44, 45]. Altogether, these features suggest that the rater did not fail in judging presence or absence of significant PCTP hypometabolism, rather misinterpreted true PCTP hypometabolism as an index of amyloid pathology, while it was a correlate of the clinical syndrome. In fact, some of the symptoms typical of CBS or of (at least some subtype of) PPA, such as limb apraxia, spatial deficits, or impairment of speech, show degeneration of the parietal and temporal cortex [46,47,48,49,50,51,52].
An additional characteristic common to several false-positive cases was the presence of quite diffuse FDG abnormalities, both within and outside the PCTP system (in particular at the level of the frontal lobes). This suggests that in these cases, the areas of hypometabolism typical for AD might be involved secondarily to the spreading of neurodegeneration from the networks initially targeted by non-AD pathology. For instance, six false-positive PPAs were patients with semantic dementia in whom the typical basal temporal abnormalities appeared to extend to the posterior temporal and inferior parietal cortex.
Specificity was particularly poor for purely qualitative VIUI (the major number of—false–—positives was in fact the main reason for the relatively poor agreement between this procedure and the two semi-quantitative ratings). Interestingly, VIUI also showed the highest proportion of unclassified cases, who were for the most part A − patients in whom raters could not disambiguate doubtful PCTP abnormalities. As established by EANM-EAN Delphi round [25], in these cases, quantitative methods may be helpful in providing confirmation on the significance of such abnormalities, and increase specificity.
The main limitation of the present study resides in the absence of an autopsy confirmation of the biomarker-based diagnoses, which would have first allowed to overcome possible shortcomings of having used different types of in vivo biomarkers as gold standard, even if amy-PET and CSF are generally considered interchangeable [2, 3]. Moreover, autopsy-based diagnosis would have allowed to detect possible cases of mixed pathology, whose presence in our sample may have resulted in an underestimation of the accuracy of FDG-PET in detecting brain amyloidosis. In fact, like in previous studies [21, 22, 35], we did not consider hypometabolism in AD-typical regions as an index of brain amyloidosis when there was more prominent hypometabolism elsewhere in the brain (as may be seen in the presence of co-pathology). We believe, though, that this risk was limited: none of our 12 false-negative cases showed significant PCTP hypometabolism in addition to hypometabolism outside of PCTP, reducing the probability that they were cases of mixed pathology. A second limitation was the lack of information about apolipoprotein E4 allele status, which has been shown to be associated with an AD-like metabolic pattern unrelated to amyloid deposition [53, 54] and might, therefore, account for some of our false-positive cases. Third, we are aware that several automated methods for the assessment of FDG-PET scans are available, and that our conclusions on INLAB and Cortex ID Suite are not generalizable to all semi-quantitative techniques. Fourth, our findings were obtained in a heterogeneous population of MCI/dementia referrals to a tertiary, university-based memory clinic, and might not be readily generalizable to other clinical settings. Finally, the procedure followed in the study for VIUI does not completely overlap with the real clinical routine, whereby raters are not blinded to clinical information. This deviation from clinical practice, however, has probably led to an underestimation, and not a misleading overestimation, of performance of qualitative rating, since access to patients’ clinical records would have probably increased accuracy.
Our study overall supports the usefulness of PCTP hypometabolism in identifying biomarker-confirmed amyloidopathy at the single-patient level, but, as already emerged from previous reports [18,19,20,21, 23, 24], is sensitive more than it is specific. Importantly, this evidence was achieved in a particularly challenging scenario, since our cohort included patients in various disease stages and with classical as well as atypical variants of AD. Qualitative rating of FDG scans showed particularly high sensitivity, but lower specificity than visual rating combined with semi-quantitative data. Asymmetric, left temporo-parietal hypometabolism associated with language deficits, in particular, tended to be mistaken for a marker of amyloid pathophysiology. As recommended by Nuclear Medicine and Neurology experts [25], resorting to automated methods in such cases, or in cases with ambiguous patterns, would increase the rate of clear-cut classifications and yield the best sensitivity–specificity trade off.
Change history
28 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7(3):270–279
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7:263–269
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K et al (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 13:614–629
McGeer PL, Kamo H, Harrop R, McGeer EG, Martin WRW, Pate BD et al (1986) Comparison of PET, MRI, and CT with pathology in a proven case of Alzheimer’s disease. Neurology 36(12):1569–1574
Mielke R, Schröder R, Fink GR, Kessler J, Herholz K, Heiss WD (1996) Regional cerebral glucose metabolism and postmortem pathology in Alzheimer’s disease. Acta Neuropathol 91(2):174–179
Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC et al (2008) Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 64(4):388–401
Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O’Neil JP, Janabi M et al (2011) Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology 76(21):1789–1796
Mosconi L, McHugh PF (2011) FDG- and amyloid-PET in Alzheimer’s disease: Is the whole greater than the sum of the parts? Q J Nuclear Med Mol Imag 55:250–264
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE (1997) Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 42(1):85–94
Grothe MJ, Teipel SJ (2016) Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer’s disease correspond to dissociable functional brain networks. Hum Brain Mapp 37(1):35–53
Jeon SY, Yi D, Byun MS, Choi HJ, Kim HJ, Lee JH et al (2016) Differential patterns of regional cerebral hypometabolism according to the level of cerebral amyloid deposition in patients with amnestic mild cognitive impairment. Neurosci Lett 632:104–108
Minoshima S, Foster NL, Sima AAF, Frey KA, Albin RL, Kuhl DE (2001) Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol 50(3):358–365
Dronse J, Fliessbach K, Bischof GN, Von Reutern B, Faber J, Hammes J et al (2017) In vivo patterns of Tau pathology, Amyloid-β Burden, and neuronal dysfunction in clinical variants of Alzheimer’s Disease. J Alzheimer’s Dis 55(2):465–471
Wang Y, Shi Z, Zhang N, Cai L, Li Y, Yang H et al (2019) Spatial patterns of hypometabolism and amyloid deposition in variants of Alzheimer’s disease corresponding to brain networks: a prospective cohort study. Mol Imaging Biol 21(1):140–148
Lehmann M, Ghosh PM, Madison C, Laforce R, Corbetta-Rastelli C, Weiner MW et al (2013) Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain 136(3):844–858
Matías-Guiu JA, Cabrera-Martín MN, Moreno-Ramos T, Valles-Salgado M, Fernandez-Matarrubia M, Carreras JL et al (2015) Amyloid and FDG-PET study of logopenic primary progressive aphasia: evidence for the existence of two subtypes. J Neurol 262(6):1463–1472
Yu M, Sporns O, Saykin AJ (2021) The human connectome in Alzheimer disease—relationship to biomarkers and genetics. Nat Rev Neurol. https://doi.org/10.1038/s41582-021-00529-1
Womack KB, Diaz-Arrastia R, Aizenstein HJ, Arnold SE, Barbas NR, Boeve BF et al (2011) Temporoparietal hypometabolism in frontotemporal lobar degeneration and associated imaging diagnostic errors. Arch Neurol 68(3):329–337
Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR et al (2007) FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 130(10):2616–2635
Silverman DHS, Chen W, Czernin J, Kowell AP, Gambhir SS, Phelps ME et al (2001) Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. J Am Med Assoc 286(17):2120–2127
Jagust W, Reed B, Mungas D, Ellis W, DeCarli C (2007) What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 69(9):871–877
Cerami C, Della Rosa PA, Magnani G, Santangelo R, Marcone A, Cappa SF et al (2015) Brain metabolic maps in Mild Cognitive Impairment predict heterogeneity of progression to dementia. NeuroImage Clin 7:187–194
Sha SJ, Ghosh PM, Lee SE, Corbetta-Rastelli C, Jagust WJ, Kornak J et al (2015) Predicting amyloid status in corticobasal syndrome using modified clinical criteria, magnetic resonance imaging and fluorodeoxyglucose positron emission tomography. Alzheimer’s Res Ther. https://doi.org/10.1186/s13195-014-0093-y
Taswell C, Villemagne VL, Yates P, Shimada H, Leyton CE, Ballard KJ et al (2015) 18F-FDG PET improves diagnosis in patients with focal-onset dementias. J Nuclear Med. https://doi.org/10.2967/jnumed.115.161067
Nobili F, Arbizu J, Bouwman F, Drzezga A, Agosta F, Nestor P et al (2018) European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: delphi consensus. Eur J Neurol 25(10):1201–1217
Schott JM, Lehmann M, Primativo S, Rossor MN, Ryan NS, Shakespeare TJ et al (2017) Consensus classification of posterior cortical atrophy. Alzheimer’s Dement 13(8):870–884
Rascovsky K, Grossman M (2013) Clinical diagnostic criteria and classification controversies in frontotemporal lobar degeneration. Int Rev Psychiatry 25(2):145–158
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D et al (2017) Diagnosis and management of dementia with Lewy bodies. Neurology. https://doi.org/10.1136/jnnp-2011-300875
Mathew R, Bak TH, Hodges JR (2012) Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry 83(4):405–410
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76(11):1006–1014
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32(6):853–864
Perani D, Della Rosa PA, Cerami C, Gallivanone F, Fallanca F, Vanoli EG et al (2014) Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin 6:445–454
Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I et al (2014) A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12(4):575–593
Gallivanone F, Della Rosa PA, Perani D, Gilardi MC, Castiglioni I (2017) The impact of different 18FDG PET healthy subject scans for comparison with single patient in SPM analysis. Q J Nucl Med Mol Imaging 61(1):115–132
Perani D, Cerami C, Caminiti SP, Santangelo R, Coppi E, Ferrari L et al (2016) Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur J Nucl Med Mol Imaging 43(3):499–508
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837
Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM (2007) Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64(3):343–349
Chételat G, Arbizu J, Barthel H, Garibotto V, Law I, Morbelli S et al (2020) Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. https://doi.org/10.1016/S1474-4422(20)30314-8
Garibotto V, Herholz K, Boccardi M, Picco A, Varrone A, Nordberg A et al (2017) Clinical validity of brain fluorodeoxyglucose positron emission tomography as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol Aging. https://doi.org/10.1016/j.neurobiolaging.2016.03.033
Lehmann M, Rohrer JD, Clarkson MJ, Ridgway GR, Scahill RI, Modat M et al (2010) Reduced cortical thickness in the posterior cingulate gyrus is characteristic of both typical and atypical Alzheimer’s disease. J Alzheimer’s Dis 20(2):587–598
Whitwell JL, Jack CR, Przybelski SA, Parisi JE, Senjem ML, Boeve BF et al (2011) Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging 32(9):1531–1541
Sarikaya I, Kamel W, Ateyah K, Essa N, AlTailji S, Sarikaya A (2021) Visual versus semiquantitative analysis of F-18 fluorodeoxyglucose-positron emission tomography brain images in patients with dementia. World J Nucl Med 20(1):82
Morbelli S, Brugnolo A, Bossert I, Buschiazzo A, Frisoni GB, Galluzzi S et al (2015) Visual Versus semi-quantitative analysis of 18F-FDG-PET in Amnestic MCI: An European Alzheimer’s Disease Consortium (EADC) project. J Alzheimer’s Dis 44(3):815–826
Bouwman F, Orini S, Gandolfo F, Altomare D, Festari C, Agosta F et al (2018) Diagnostic utility of FDG-PET in the differential diagnosis between different forms of primary progressive aphasia. Eur J Nucl Med Mol Imaging 45(9):1526–1533
Nestor PJ, Balan K, Cheow HK, Fryer TD, Knibb JA, Xuereb JH et al (2007) Nuclear imaging can predict pathologic diagnosis in progressive nonfluent aphasia. Neurology 68(3):238–239
Peigneux P, Salmon E, Garraux G, Laureys S, Willems S, Dujardin K et al (2001) Neural and cognitive bases of upper limb apraxia in corticobasal degeneration. Neurology 57(7):1259–1268
Jo S, Oh JS, Cheong EN, Kim HJ, Lee S, Oh M et al (2021) FDG-PET patterns associated with ideomotor apraxia and imitation apraxia in patients with corticobasal syndrome. Park Relat Disord 88:96–101
Dodich A, Cerami C, Inguscio E, Iannaccone S, Magnani G, Marcone A et al (2019) The clinico-metabolic correlates of language impairment in corticobasal syndrome and progressive supranuclear palsy. NeuroImage Clin. https://doi.org/10.1016/j.nicl.2019.102009
Parmera JB, de Almeida IJ, de Oliveira MCB, Silagi ML, de Godoi CC, Studart-Neto A et al (2021) Metabolic and structural signatures of speech and language impairment in corticobasal syndrome: a multimodal PET/MRI study. Front Neurol. https://doi.org/10.3389/fneur.2021.702052
Utianski RL, Botha H, Martin PR, Schwarz CG, Duffy JR, Clark HM et al (2019) Clinical and neuroimaging characteristics of clinically unclassifiable primary progressive aphasia. Brain Lang. https://doi.org/10.1016/j.bandl.2019.104676
Routier A, Habert MO, Bertrand A, Kas A, Sundqvist M, Mertz J et al (2018) Structural, microstructural, and metabolic alterations in primary progressive aphasia variants. Front Neurol. https://doi.org/10.3389/fneur.2018.00766
Cerami C, Dodich A, Greco L, Iannaccone S, Magnani G, Marcone A et al (2017) The role of single-subject brain metabolic patterns in the early differential diagnosis of primary progressive aphasias and in prediction of progression to dementia. J Alzheimer’s Dis 55(1):183–197
During EH, Osorio RS, Elahi FM, Mosconi L, De Leon MJ (2011) The concept of FDG-PET endophenotype in Alzheimer’s disease. Neurol Sci 32:559–569
Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207
Acknowledgements
No acknowledgement.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. The authors did not receive support from any organisation for the submitted work.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by VI, CC, MM, SP, AF, FF and CM. The first draft of the manuscript was written by VI and CC, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study has been approved by our institution’s ethics committee, Comitato Etico Brianza, and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isella, V., Crivellaro, C., Formenti, A. et al. Validity of cingulate–precuneus–temporo-parietal hypometabolism for single-subject diagnosis of biomarker-proven atypical variants of Alzheimer’s Disease. J Neurol 269, 4440–4451 (2022). https://doi.org/10.1007/s00415-022-11086-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11086-y