Abstract
Background and aim
Despite improving the 10-year disease-free-survival, prophylactic central neck dissection (pCND) in differentiated thyroid carcinoma (DTC) should only be considered in patients with high risk factors for lymph node (LN) metastasis due to the increases in the risk of postoperative complications. Our aim was to identify the risk factors for central lymph node metastasis (CLNM) in DTC.
Method
We searched PubMed, Scopus, Web of science, Cochrane library for eligible studies from inception to November 1, 2021 and a systematic review and meta-analysis were carried out to identify the risk factors for CLNM in DTC.
Results
We included 41 studies with total of 27,741 patients in this study. The pooled results in this meta-analysis showed that these risk factors were significantly associated with CLNM: age < 45 years (odds ratio (OR) 1.64, 95% confidence interval (CI) 1.34–1.99, p < 0.00001), male sex (OR 1.73, 95% CI 1.54–1.93, p < 0.00001), multifocality (OR 1.87, 95% CI 1.59–2.19, p < 0.00001), bilateral disease (OR 1.43, 95% CI 1.15–1.78, p < 0.001), capsular invasion (OR 1.67, 95% CI 1.10–2.54, p < 0.02), lymphovascular invasion (OR 4.89, 95% CI 2.76–8.66, p < 0.00001) and extra-thyroidal extension (OR 2.43, 95% CI 1.97–3.00, p < 0.00001). In addition, young age (< 45 years), male sex, multifocality, and extra-thyroidal extension were significantly associated with large-volume CLNM in clinically N0 DTC patients. However, the presence of Hashimoto’s thyroiditis was not a predictors of large-volume CLNM.
Conclusion
Young age (< 45 years), male sex, bilateral disease, multifocality, capsular invasion, lymphovascular invasion and extra-thyroidal extension are significantly associated with CLNM and pCND would be expected to have a higher yield in patients with these risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the incidence of thyroid cancer has been shown to be increasing at the fastest rate among all malignancies globally [1, 2]. In 2020, 43,646 patients died from thyroid malignancy (27,740 females and 15,906 males) [3] and if this growth is maintained, by 2030, thyroid malignancy will be the fourth most common cancer [4].
Differentiated thyroid carcinoma (DTC), a term used to describe papillary thyroid cancer (PTC), follicular thyroid cancer (FTC) and Hürthle cell thyroid cancer (HTC), accounts for approximately 95% of all thyroid malignancies [5]. The expression ‘clinically lymph node-negative (cN0)’ is used to describe the patients without the clinical evidence of central lymph node metastasis (CLNM) on ultrasound or other imaging modalities preoperatively.
For clinically lymph node-positive patients, central neck dissection (CND) should be performed [6], while for cN0 patients, whether to perform prophylactic central neck dissection (pCND) remains controversial. Some studies have reported that bilateral prophylactic CND for staging of the neck in PTC, followed by personalized adjuvant radioiodine treatment, is associated with better 10-year disease-free-survival and loco-regional control of the disease, without increasing the risk of morbidity [7]. In contrast, other studies did not show a significant decrease in the risk of loco-regional control with pCND in patients with cN0 PTC [8]. Nevertheless, therapeutic CND based on some prognostic features associated with an increased risk of metastasis and recurrence (age, sex, multifocal disease, extra-thyroidal extension, capsular invasion and lymphovascular invasion) may be more reasonable than pCND [9,10,11,12,13]. Thus identifying risk factors of CLNM could guide surgeons to consider which cN0 thyroid malignancy patients require pCND. Our aim was to identify the risk factors for CLNM in differentiated thyroid malignancy.
Patients and methods
Search strategy
We searched PubMed, Web of science, Scopus, and the Cochrane Library for data from inception to November 1, 2021 using a combination of the following terms: “clinically node negative”, “Risk Factors” and “Thyroid Neoplasms”. All the studies were reviewed and evaluated by two authors (Hafez, L.G.& Elkomos, B.E.) according to the pre-defined eligibility criteria. We obtained full texts of the manuscripts found to be potentially eligible based on abstracts for full review.
Inclusion and exclusion criteria
The eligible studies included the following: (1) randomized controlled trials and prospective or retrospective cohort studies; (2) target population were patients with DTC; (3) studies designating to detect the risk factors for LNM for cN0 as a primary aim; (4) studies providing a sufficient description of the methods and baseline characteristics; and (5) English language studies.
The following types of studies were excluded from our study: (1) unrelated or in vitro studies; (2) reviews, case reports and case series; (3) studies designed to analyse information from the United Network for Organ Sharing database; and (4) studies included patients with thyroid cancer other than DTC (e.g., medullary thyroid carcinoma, anaplastic thyroid carcinoma, lymphoma).
Outcomes of interest
We assessed seven risk factors for LNM in CTC including age, sex, bilateral disease, multifocality, capsular invasion, lymphovascular invasion and extra-thyroidal extension as primary outcomes in this meta-analysis. In addition, our secondary outcomes were to detect the risk factors for large-volume LNM.
Quality assessment and data extraction
A modification of the Newcastle–Ottawa scale was used to assess the quality of all cohort studies included in this meta-analysis [14]. This scale includes three domains: (1) selection of study groups (four points); (2) comparability of groups (two points); and (3) ascertainment of exposure and outcomes (three points). Only studies with seven or more scores were included.
We extracted data on study characteristics (author, year of publication and country where the study was conducted), patient characteristics (age and sex), follow up, tumor characteristics (size, bilateral disease, multifocality, capsular invasion, lymphovascular invasion, extra-thyroidal extension and recurrence). The data were extracted by two authors (Hafez, L.G.& Elkomos, B.E.) independently.
Statistical analysis
This meta-analysis was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions that is recommended by the Cochrane Collaboration. [15] The pooled odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated with fixed effects models for all the risk factors. However, if there was moderate or considerable heterogeneity (I2 > 40), random effects models were used to solve the heterogeneity between studies. All calculations for the current meta-analysis were performed using the Review Manager 5.4 for Windows (Cochrane Collaboration, Oxford, United Kingdom).
Assessment of publication bias and heterogeneity
Funnel plots were generated to visually inspect the publication bias. In addition, we used the statistical methods the Begg–Mazumdar rank correlation test and the Egger regression asymmetry test for detecting funnel plot asymmetry. Statistical heterogeneity was assessed with forest plots and the inconsistency statistic (I2). An I2 value of 40% or less corresponded to low heterogeneity. Statistical significance was considered at p < 0.05.
Results
Characteristics and quality assessment of eligible studies
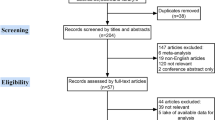
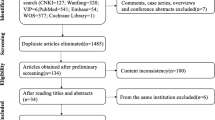
As shown in Fig. 1, 132 articles were identified from the four databases using the following search string: “clinically node negative”, “Risk Factors” and “Thyroid Neoplasms”. After proper selection according to our eligibility criteria, 41 studies with 27,741 participants were included in the meta-analysis. These trials included 38 retrospective cohort studies and 3 prospective studies. However, none of the included studies were randomized studies.
Study characteristics [author, year of publication and country where the study was conducted], patient characteristics [age and sex], follow up time, tumor characteristics [size, bilateral disease, multifocality, capsular invasion, lymphovascular invasion, extra-thyroidal extension, and recurrence] were extracted from all the included studies (ESM Table 1). The quality assessment was conducted according to a modification of the Newcastle–Ottawa scale. Most of the cohort studies included in this analysis demonstrated sufficient quality with reasonable selection criteria, comparable patient characteristics, and adequate follow-up.
Primary outcome
Age
According to 19 studies (7904 participants), the incidence of CLNM was significantly higher in the patient aged < 45 years (39.4%) than those age > 45 years (26.5%) (OR 1.64, 95% CI 1.34–1.99, p < 0.00001) (Fig. 2). A random effect was used to calculate odds ration because of high level heterogeneity (I2 = 64%, p < 0.0001).
Sex
The incidence of CLNM was stated in 26 studies (9906 participants) and showed that the metastasis was more in male patients than female patients (41.5% and 28.3%, respectively) (OR 1.73, 95% CI 1.54–1.93, p < 0.00001) (Fig. 3). A fixed effect was used due to low level of heterogeneity (I2 = 32%, p < 0.06).
Multifocality
The relation between multifocality and LNM was reported in 21 studies (8872 participants). These studies revealed that the rate of CLNM was remarkably high in multifocal tumor than in unifocal tumor (40% and 27.6%, respectively) (OR 1.87, 95% CI 1.59–2.19, p < 0.00001) (Fig. 4). A random effect was used as heterogeneity was high (I2 = 46%, p < 0.01).
Bilateral disease
Eleven studies (5723 participants) reported the effect of the presence of bilateral thyroid tumor and the rate of CLNM. According to these studies, the patients with bilateral tumors showed a significantly higher incidence of CLNM than those with unilateral tumors (36.5% and 25.6%, respectively) (OR 1.43, 95% CI 1.15–1.78, p < 0.001) (Fig. 5). The heterogeneity was high (I2 = 43%, p < 0.06), so a random effect was used.
Capsular invasion
As reported by 10 studies (3730 participants), the presence of capsular invasion was a predictor of CLNM with a rate of 29.6% versus 18.5% in the absence of capsular invasion (OR 1.67, 95% CI 1.10–2.54, p < 0.02) (Fig. 6). A random effect was used as heterogeneity was high (I2 = 65%, p < 0.003).
Lymphovascular invasion
According to the pooled ORs from 9 studies (3725 participants), CLNM was associated with the presence of LVI as the rate of CLNM was 60.3% versus 28.3% in the absence of lymphovascular invasion (OR 4.89, 95% CI 2.76–8.66, p < 0.00001) (Fig. 7). A random effect was used to solve heterogeneity (I2 = 47%, p < 0.06).
Extra-thyroidal extension
According to the pooled ORs in 16 studies (6814 participants), the incidence of recurrence after total thyroidectomy was significantly higher in the patients with extra-thyroidal extension than those without extra-thyroidal extension (52.1% and 31.1%, respectively) (OR 2.43, 95% CI 1.97–3.00, p < 0.00001) (Fig. 8). A random effect was used as heterogeneity was high (I2 = 43%, p < 0.04).
Subgroup analysis
Countries (Asia, America and Europe)
A subgroup analysis was done to detect the risk factors of CLNM in clinically node negative patients according to the country. For example, according to the Chinese studies, young age (< 45 years), male sex, bilateral disease, multifocality, capsular invasion, lymphovascular invasion and extra-thyroidal extension were significantly associated with CLNM (ESM Table 2).
Secondary outcomes
According to ATA guidelines, [6] the presence of less than five pathological LNM (< 0.2 cm in the largest dimension) are classified as low-risk disease with recurrence risk < 5%. However, the presence of more than five metastatic lymph nodes (< 3 cm in the largest dimension) are classified as intermediate-risk disease with recurrence risk > 20%. Our secondary outcome was to detect the risk factors for large-volume lymph node metastasis (more than five metastatic lymph nodes) after total thyroidectomy. The risk factors for large- and small-volume lymph node metastases were reported in two studies (1311 Participants) and the pooled results showed that male gender, multifocality, and extra-thyroidal extension are significantly associated with large-volume CLNM in patients with DTC (Table 1).
Publication bias assessment
There was no evidence of publication bias. The funnel plot analysis demonstrated a symmetrical appearance, and the p values were greater than 0.05 for all comparisons according to the Begg–Mazumdar test and Eggers test.
Discussion
The incidence of differentiated thyroid malignancy is rising worldwide [3]. According to the American Thyroid Association (ATA) guidelines, neck dissection is recommended for patients with cN1 DTC [6]. However, the sensitivity of ultrasound for detecting metastatic central neck lymph nodes preoperatively has been reported to range from 10.9 to 30%. This is because they are usually small and are obscured by the overlying thyroid gland [56,57,58]. In addition, 30–65% of patients with clinically node negative papillary thyroid microcarcinoma had CLNM (detected only on histopathology) [59, 60].
For these reasons, some studies have argued that prophylactic central neck dissection (pCND) allows staging of the cancer more accurately for evaluating the necessity of radioactive iodine therapy [61] and this will lead to a decrease in the local recurrence and improve disease-specific survival [62]. Moreover, the ATA guidelines also recommend prophylactic CND in patients with cN0 PTC, especially for advanced primary tumors (T3 or T4) [6]. On the other hand, prophylactic central neck dissection increases the risk of postoperative complications, mainly hypoparathyroidism and laryngeal nerve injury [63].
However, two factors are widely adopted and well-established. Firstly, there is an increased risk of complications in the second operation when the tumor recurs in the central compartment [64]. Secondly, lymph node metastasis has a strong association with recurrence after total thyroidectomy [65]. Therefore, some studies have recommended that prophylactic central neck dissection should only be considered in patients with high risk factors [61]. The pooled results in this meta-analysis showed that young age (< 45 years), male gender, bilateral disease, multifocality, capsular invasion, lymphovascular invasion and extra-thyroidal extension are significantly associated with CLNM in patients with clinically N0 DTC. In other words, prophylactic central neck dissection would be expected to have higher yield in patients with these risk factors.
To begin with the age, thyroid cancer rarely occurs in children under age of 15 years and the incidence of thyroid malignancy increases with age, peaking between the fifth and eighth decades [66]. However, the ATA risk stratification system does not include age as a predictor of recurrence. According to the pooled results from the included studies, the incidence of CLNM is significantly higher in the patient aged < 45 years than those aged > 45 years. Turning to the sex, despite being more common in females and this predominance has been explained by hormonal factors or biological changes that occur during pregnancy [67], this meta-analysis showed that the incidence of CLNM is higher in male sex.
According to Lu et al., multifocality may arise from intrathyroidal metastases from a single cancer cell clone or from multiple independent origins [68] and it can be seen in 18–87% of cases [69]. In this meta-analysis, it is a significant risk factor CLNM in cN0 thyroid malignancy. The Incidence of bilateral tumors is high and increases with the number of tumor foci in multifocal PTC [70, 71]. According to some studies, bilateral tumor is not more aggressive than unilateral disease with regards to histopathologic features, tumor stage and prognostic outcomes [72]. However, this meta-analysis showed that the patients with bilateral tumors have a significantly higher incidence of CLNM than those with unilateral tumors.
Tumor capsular invasion that is associated with the FTC has received more attention than in the PTC. This is because the differentiation between follicular adenomas and carcinomas depends on the demonstration of angioinvasion and/or tumor capsular invasion [73]. The presence of histologic capsule invasion is detected in approximately half of DTC, with a lower incidence in PTC (49.8%) than in FTC (73.5%) [74]. According to the pooled results of the included studies, our meta-analysis shows that the presence of capsular invasion is a predictor for CLNM.
Lymphovascular invasion in patients with thyroid cancer was described by Mete et al. by the presence of malignant cells in lymphovascular spaces or endothelial lining of lymphovascular cannels, invasion of malignant cells through a vessel wall and endothelium, or the presence of thrombus adherent to intravascular tumor [75]. The incidence of lymphovascular invasion in differentiated thyroid cancer ranges from 2 to 14% [76, 77]. Despite being a poor prognostic factor in different cancers [78, 79], lymphovascular invasion is not included into the staging systems for DTC. As reported by Pontius et al., the presence of lymphovascular invasion among patients with PTC is associated with significantly decreased survival [77]. In addition, this study showed that CLNM is significantly associated with the presence of lymphovascular invasion (60.3% versus 28.3% in the absence of lymphovascular invasion).
Extra-thyroidal extension has been defined as the growth of tumor outside the thyroid gland and into the nearby or surrounding tissues. For differentiated thyroid malignancies, the American Joint Committee on Cancer (AJCC) includes extra-thyroidal extension as part of the staging system. Stage T3 is defined as a “tumor greater than 4 cm limited to the thyroid or any tumor with minimal extra-thyroidal extension,” Stage T4a is defined as a “a tumor of any size that has grown beyond the thyroid capsule invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve,” and stage T4b is defined as a “tumor of any size that encases the carotid artery or mediastinal vessels or invades the prevertebral fascia” [80]. As mentioned before, according to the ATA guidelines [6], prophylactic CND may be performed in patients with DTC with clinically uninvolved central neck lymph nodes, for advanced primary tumors (T3 or T4). However, these guidelines state that these are weak recommendations with poor-quality evidence. However, this meta-analysis showed that the incidence of recurrence after total thyroidectomy is significantly higher in the patients with extra-thyroidal extension than those without extra-thyroidal extension. In addition, Youngwirth et al. showed that extra-thyroidal extension is associated with a dramatic decrease in survival rate for patients with DTC [81].
The highest incident cases of thyroid cancer were reported in China and reached 41,511 cases in 2017 [82]. Our subgroup analysis according to the country where the study was conducted showed that, in China, age < 45 years, male sex, bilateral disease, multifocality, capsular invasion, lymphovascular invasion and extra-thyroidal extension are associated with LNM in clinically N0 DTC patients.
The recurrence rate after total thyroidectomy in large-volume LNM (more than five metastatic lymph nodes) was higher than that in small-volume LNM (less than or equal five metastatic lymph nodes) and the recurrence-free survival of large-volume LNM was dramatically poorer than that of small-volume LNM [49]. According to the pooled results from two of the included studies, young age (< 45 years), male gender, multifocality, and extra-thyroidal extension were significantly associated with large-volume LNM in clinically N0 DTC patients. However, the presence of Hashimoto’s thyroiditis was not a predictors of large-volume LNM.
To best of our knowledge, it is the first time the risk factors for large-volume LNM have been included in a meta-analysis. In addition, all the studies that reported the risk factors for LNM in clinically node negative for DTC were included in the study.
Nevertheless, we acknowledge some limitations in our study. Firstly, all the included studies were cohort studies and no randomized controlled trials could be found. Secondly, the heterogeneity in some results could not be explained in the subgroup analysis. Finally, only two of the included studies reported the risk factors for large-volume LNM. In other words, further studies are needed to better assess this point.
Conclusion
Young age (< 45 years), male gender, bilateral disease, multifocality, capsular invasion, lymphovascular invasion and extra-thyroidal extension are associated with LNM in clinically N0 DTC patients and prophylactic central neck dissection would be expected to have higher yield in patients with these factors.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Abbreviations
- DTC:
-
Differentiated thyroid cancer
- PTC:
-
Papillary thyroid cancer
- PTMC:
-
Papillary thyroid microcarcinoma
- FTC:
-
Follicular thyroid cancer
- HTC:
-
Hürthle cell thyroid cancer
- cN0:
-
Clinically lymph node-negative
- pCND:
-
Prophylactic central neck dissection
- CLNM:
-
Central lymph node metastasis
- LNM:
-
Lymph node metastasis
- ATA:
-
American Thyroid Association
- LVI:
-
Lymphovascular invasion
References
Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM (2017) Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317(13):1338. https://doi.org/10.1001/jama.2017.2719
Morris LGT, Sikora AG, Tosteson TD, Davies L (2013) The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 23(7):885–891. https://doi.org/10.1089/thy.2013.0045
La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F et al (2015) Thyroid cancer mortality and incidence: a global overview: thyroid cancer mortality and incidence. Int J Cancer 136(9):2187–2195. https://doi.org/10.1002/ijc.29251
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74(11):2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155
Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013:965212. https://doi.org/10.1155/2013/965212
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE et al (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133. https://doi.org/10.1089/thy.2015.0020
Barczyński M, Konturek A, Stopa M, Nowak W (2013) Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg 100(3):410–418. https://doi.org/10.1002/bjs.8985
Kim SK, Woo J-W, Lee JH, Park I, Choe J-H, Kim J-H et al (2016) Prophylactic central neck dissection might not be necessary in papillary thyroid carcinoma: analysis of 11,569 cases from a single institution. J Am Coll Surg 222(5):853–864. https://doi.org/10.1016/j.jamcollsurg.2016.02.001
White ML, Gauger PG, Doherty GM (2007) Central lymph node dissection in differentiated thyroid cancer. World J Surg 31(5):895–904. https://doi.org/10.1007/s00268-006-0907-6
Clayman GL, Agarwal G, Edeiken BS, Waguespack SG, Roberts DB, Sherman SI (2011) Long-term outcome of comprehensive central compartment dissection in patients with recurrent/persistent papillary thyroid carcinoma. Thyroid 21(12):1309–1316. https://doi.org/10.1089/thy.2011.0170
Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M et al (2011) A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery 150(6):1048–1057. https://doi.org/10.1016/j.surg.2011.09.003
Sancho JJ, Lennard TWJ, Paunovic I, Triponez F, Sitges-Serra A (2014) Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 399(2):155–163. https://doi.org/10.1007/s00423-013-1152-8
Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L (2006) Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery 140(6):1000–1005. https://doi.org/10.1016/j.surg.2006.08.001. (discussion 1005–7)
Peterson J, Welch V, Losos M, Tugwell P (2011) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute, Ottawa, pp 1–12
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 10:ED000142. https://doi.org/10.1002/14651858.ED000142
Koo BS, Choi EC, Yoon Y-H, Kim D-H, Kim E-H, Lim YC (2009) Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg 249(5):840–844. https://doi.org/10.1097/SLA.0b013e3181a40919
Liang J, Li Z, Fang F, Yu T, Li S (2017) Is prophylactic central neck dissection necessary for cN0 differentiated thyroid cancer patients at initial treatment? A meta-analysis of the literature. Acta Otorhinolaryngol Ital 37(1):1–8. https://doi.org/10.14639/0392-100X-1195
Vergez S, Sarini J, Percodani J, Serrano E, Caron P (2010) Lymph node management in clinically node-negative patients with papillary thyroid carcinoma. Eur J Surg Oncol 36(8):777–782. https://doi.org/10.1016/j.ejso.2010.06.015
So YK, Son Y-I, Hong SD, Seo MY, Baek C-H, Jeong H-S et al (2010) Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery 148(3):526–531. https://doi.org/10.1016/j.surg.2010.01.003
Roh J-L, Kim J-M, Park CI (2011) Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol 18(8):2245–2250. https://doi.org/10.1245/s10434-011-1600-z
Zhou Y-L, Gao E-L, Zhang W, Yang H, Guo G-L, Zhang X-H et al (2012) Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J Surg Oncol 10(1):67. https://doi.org/10.1186/1477-7819-10-67
Caliskan M, Park JH, Jeong JS, Lee C-R, Park SK, Kang S-W et al (2012) Role of prophylactic ipsilateral central compartment lymph node dissection in papillary thyroid microcarcinoma. Endocr J 59(4):305–311. https://doi.org/10.1507/endocrj.ej11-0366
Kim B-Y, Jung C-H, Kim J-W, Lee S-W, Kim C-H, Kang S-K et al (2012) Impact of clinicopathologic factors on subclinical central lymph node metastasis in papillary thyroid microcarcinoma. Yonsei Med J 53(5):924–930. https://doi.org/10.3349/ymj.2012.53.5.924
Hartl DM, Leboulleux S, Al Ghuzlan A, Baudin E, Chami L, Schlumberger M et al (2012) Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg 255(4):777–783. https://doi.org/10.1097/SLA.0b013e31824b7b68
Lee KE, Chung IY, Kang E, Koo DH, Kim KH, Kim S-W et al (2013) Ipsilateral and contralateral central lymph node metastasis in papillary thyroid cancer: patterns and predictive factors of nodal metastasis. Head Neck 35(5):672–676. https://doi.org/10.1002/hed.23016
Wu Y, Gu J, Shang J, Wang W, Wang K (2013) Central lymph node metastasis in cN0 papillary thyroid carcinoma. J BUON 18(3):733–738
Wang W, Gu J, Shang J, Wang K (2013) Correlation analysis on central lymph node metastasis in 276 patients with cN0 papillary thyroid carcinoma. Int J Clin Exp Pathol 6(3):510–515
Miao S, Mao X, Pei R, Xiang C, Lv Y, Shi Q et al (2013) Predictive factors for different subgroups of central lymph node metastasis in unilateral papillary thyroid carcinoma. ORL J Otorhinolaryngol Relat Spec 75(5):265–273. https://doi.org/10.1159/000354267
Lang BH-H, Chan DTY, Wong KP, Wong KKC, Wan KY (2014) Predictive factors and pattern of locoregional recurrence after prophylactic central neck dissection in papillary thyroid carcinoma. Ann Surg Oncol 21(13):4181–4187. https://doi.org/10.1245/s10434-014-3872-6
Liang K, He L, Dong W, Zhang H (2014) Risk factors of central lymph node metastasis in cN0 papillary thyroid carcinoma: a study of 529 patients. Med Sci Monit 20:807–811. https://doi.org/10.12659/MSM.890182
Wang Q, Chu B, Zhu J, Zhang S, Liu Y, Zhuang M et al (2014) Clinical analysis of prophylactic central neck dissection for papillary thyroid carcinoma. Clin Transl Oncol 16(1):44–48. https://doi.org/10.1007/s12094-013-1038-9
Zhang L-Y, Liu Z-W, Liu Y-W, Gao W-S, Zheng C-J (2015) Risk factors for nodal metastasis in cN0 papillary thyroid microcarcinoma. Asian Pac J Cancer Prev 16(8):3361–3363. https://doi.org/10.7314/apjcp.2015.16.8.3361
Park KN, Kang KY, Hong HS, Jeong H-S, Lee SW (2015) Predictive value of estimated tumor volume measured by ultrasonography for occult central lymph node metastasis in papillary thyroid carcinoma. Ultrasound Med Biol 41(11):2849–2854. https://doi.org/10.1016/j.ultrasmedbio.2015.02.018
Gao Y, Qu N, Zhang L, Chen J-Y, Ji Q-H (2016) Preoperative ultrasonography and serum thyroid-stimulating hormone on predicting central lymph node metastasis in thyroid nodules as or suspicious for papillary thyroid microcarcinoma. Tumour Biol 37(6):7453–7459. https://doi.org/10.1007/s13277-015-4535-3
Yuan J, Zhao G, Du J, Chen X, Lin X, Chen Z et al (2016) To identify predictors of central lymph node metastasis in patients with clinically node-negative conventional papillary thyroid carcinoma. Int J Endocrinol 2016:6109218. https://doi.org/10.1155/2016/6109218
Xue S, Wang P, Liu J, Li R, Zhang L, Chen G (2016) Prophylactic central lymph node dissection in cN0 patients with papillary thyroid carcinoma: A retrospective study in China. Asian J Surg 39(3):131–136. https://doi.org/10.1016/j.asjsur.2015.03.015
Lin X, Chen X, Jiru Y, Du J, Zhao G, Wu Z (2016) Evaluating the influence of prophylactic central neck dissection on TNM staging and the recurrence risk stratification of cN0 differentiated thyroid carcinoma. Bull Cancer 103(6):535–540. https://doi.org/10.1016/j.bulcan.2016.04.003
Yuan J, Li J, Chen X, Lin X, Du J, Zhao G et al (2017) Identification of risk factors of central lymph node metastasis and evaluation of the effect of prophylactic central neck dissection on migration of staging and risk stratification in patients with clinically node-negative papillary thyroid microcarcinoma. Bull Cancer 104(6):516–523. https://doi.org/10.1016/j.bulcan.2017.03.005
Li M, Zhu X-Y, Lv J, Lu K, Shen M-P, Xu Z-L et al (2017) Risk factors for predicting central lymph node metastasis in papillary thyroid microcarcinoma (CN0): a study of 273 resections. Eur Rev Med Pharmacol Sci 21(17):3801–3807
Sessa L, Lombardi CP, De Crea C, Tempera SE, Bellantone R, Raffaelli M (2018) Risk factors for central neck lymph node metastases in micro- versus macro-clinically node negative papillary thyroid carcinoma. World J Surg 42(3):623–629. https://doi.org/10.1007/s00268-017-4390-z
Calò PG, Lombardi CP, Podda F, Sessa L, Santini L, Conzo G (2017) Role of prophylactic central neck dissection in clinically node-negative differentiated thyroid cancer: assessment of the risk of regional recurrence. Updates Surg 69(2):241–248. https://doi.org/10.1007/s13304-017-0438-8
An C, Zhang X, Wang S, Zhang Z, Yin Y, Xu Z et al (2017) Efficacy of superselective neck dissection in detecting metastasis in patients with cN0 papillary thyroid carcinoma at high risk of lateral neck metastasis. Med Sci Monit 23:2118–2126. https://doi.org/10.12659/msm.900273
Han Z, Xue S, Wang P, Zhang L (2018) Central lymph node metastasis as a predictor for lateral lymph node metastasis in clinically node-negative T3 and T4 papillary thyroid carcinoma. In: 2018 9th international conference on information technology in medicine and education (ITME). IEEE
Ryu YJ, Kang SJ, Cho JS, Yoon JH, Park MH (2018) Identifying risk factors of lateral lymph node recurrence in clinically node-negative papillary thyroid cancer. Medicine (Baltimore) 97(51):e13435. https://doi.org/10.1097/MD.0000000000013435
Zhang Q, Wang Z, Meng X, Duh Q-Y, Chen G (2019) Predictors for central lymph node metastases in CN0 papillary thyroid microcarcinoma (mPTC): a retrospective analysis of 1304 cases. Asian J Surg 42(4):571–576. https://doi.org/10.1016/j.asjsur.2018.08.013
Xu S-Y, Yao J-J, Zhou W, Chen L, Zhan W-W (2019) Clinical characteristics and ultrasonographic features for predicting central lymph node metastasis in clinically node-negative papillary thyroid carcinoma without capsule invasion. Head Neck 41(11):3984–3991. https://doi.org/10.1002/hed.25941
Ryu YJ, Cho JS, Park MH, Yoon JH (2019) Identifying risk factors of recurrence for clinically node negative papillary thyroid carcinoma with pathologic N1a. BMC Surg 19(1):78. https://doi.org/10.1186/s12893-019-0541-5
Ngo DQ, Ngo QX, Van Le Q (2020) Pediatric thyroid cancer: Risk factors for central lymph node metastasis in patients with cN0 papillary carcinoma. Int J Pediatr Otorhinolaryngol. 133(110000):110000. https://doi.org/10.1016/j.ijporl.2020.110000
Shen G, Ma H, Huang R, Kuang A (2020) Predicting large-volume lymph node metastasis in the clinically node-negative papillary thyroid microcarcinoma: a retrospective study: a retrospective study. Nucl Med Commun 41(1):5–10. https://doi.org/10.1097/MNM.0000000000001119
Zhang C, Li B-J, Liu Z, Wang L-L, Cheng W (2020) Predicting the factors associated with central lymph node metastasis in clinical node-negative (cN0) papillary thyroid microcarcinoma. Eur Arch Otorhinolaryngol 277(4):1191–1198. https://doi.org/10.1007/s00405-020-05787-1
Zhou B, Qin J (2021) High-risk factors for lymph node metastasis in contralateral central compartment in unilateral papillary thyroid carcinoma (cT1N0). Eur J Surg Oncol 47(4):882–887. https://doi.org/10.1016/j.ejso.2020.10.018
Feng J-W, Ye J, Wu W-X, Qu Z, Qin A-C, Jiang Y (2020) Management of cN0 papillary thyroid microcarcinoma patients according to risk-scoring model for central lymph node metastasis and predictors of recurrence. J Endocrinol Invest 43(12):1807–1817. https://doi.org/10.1007/s40618-020-01326-1
Yan Y, Wang Y, Liu N, Duan Y, Chen X, Ye B et al (2021) Predictive value of the Delphian lymph node in cervical lymph node metastasis of papillary thyroid carcinoma. Eur J Surg Oncol 47(7):1727–1733. https://doi.org/10.1016/j.ejso.2021.02.010
Zhou B, Wei L, Qin J (2021) Analyze and compare the predictors of ipsilateral central lymph node metastasis in papillary thyroid carcinoma with cT1a and cT1b stage. Asian J Surg 44(11):1357–1362. https://doi.org/10.1016/j.asjsur.2021.02.008
Huang J, Song M, Shi H, Huang Z, Wang S, Yin Y et al (2021) Predictive factor of large-volume central lymph node metastasis in clinical N0 papillary thyroid carcinoma patients underwent total thyroidectomy. Front Oncol 11:574774. https://doi.org/10.3389/fonc.2021.574774
Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K et al (2006) Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg 30(1):91–99. https://doi.org/10.1007/s00268-005-0113-y
Hwang HS, Orloff LA (2011) Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer: efficacy of preoperative Neck US. Laryngoscope 121(3):487–491. https://doi.org/10.1002/lary.21227
Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B et al (2007) Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab 92(9):3590–3594. https://doi.org/10.1210/jc.2007-0444
Lim YC, Choi EC, Yoon Y-H, Kim E-H, Koo BS (2009) Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg 96(3):253–257. https://doi.org/10.1002/bjs.6484
Wada N, Duh Q-Y, Sugino K, Iwasaki H, Kameyama K, Mimura T et al (2003) Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg 237(3):399–407. https://doi.org/10.1097/01.sla.0000055273.58908.19
Song CM, Lee DW, Ji YB, Jeong JH, Park JH, Tae K (2016) Frequency and pattern of central lymph node metastasis in papillary carcinoma of the thyroid isthmus: central lymph node metastasis of thyroid isthmus cancer. Head Neck 38(S1):E412–E416. https://doi.org/10.1002/hed.24009
Moo T-A, McGill J, Allendorf J, Lee J, Fahey T 3rd, Zarnegar R (2010) Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg 34(6):1187–1191. https://doi.org/10.1007/s00268-010-0418-3
Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K et al (2003) An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 13(4):381–387. https://doi.org/10.1089/105072503321669875
Vini L, Hyer SL, Marshall J, A’Hern R, Harmer C (2003) Long-term results in elderly patients with differentiated thyroid carcinoma. Cancer 97(11):2736–2742. https://doi.org/10.1002/cncr.11410
Wang LY, Palmer FL, Nixon IJ, Thomas D, Shah JP, Patel SG et al (2014) Central lymph node characteristics predictive of outcome in patients with differentiated thyroid cancer. Thyroid 24(12):1790–1795. https://doi.org/10.1089/thy.2014.0256
SEER cancer statistics review, 1975–2010—previous version—SEER cancer statistics review. SEER. http://seer.cancer.gov/csr/1975_2010/. Accessed 15 Jan 2022
Kravdal O, Glattre E, Haldorsen T (1991) Positive correlation between parity and incidence of thyroid cancer: new evidence based on complete Norwegian birth cohorts. Int J Cancer 49(6):831–836. https://doi.org/10.1002/ijc.2910490606
Lu Z, Sheng J, Zhang Y, Deng J, Li Y, Lu A et al (2016) Clonality analysis of multifocal papillary thyroid carcinoma by using genetic profiles: Field cancerization and MPTC. J Pathol 239(1):72–83. https://doi.org/10.1002/path.4696
Iacobone M, Jansson S, Barczyński M, Goretzki P (2014) Multifocal papillary thyroid carcinoma–a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 399(2):141–154. https://doi.org/10.1007/s00423-013-1145-7
Kim ES, Kim TY, Koh JM, Kim YI, Hong SJ, Kim WB et al (2004) Completion thyroidectomy in patients with thyroid cancer who initially underwent unilateral operation. Clin Endocrinol (Oxf). 61(1):145–148. https://doi.org/10.1111/j.1365-2265.2004.02065.x
So YK, Kim MW, Son Y-I (2015) Multifocality and bilaterality of papillary thyroid microcarcinoma. Clin Exp Otorhinolaryngol 8(2):174–178. https://doi.org/10.3342/ceo.2015.8.2.174
Polat SB, Cakir B, Evranos B, Baser H, Cuhaci N, Aydin C et al (2019) Preoperative predictors and prognosis of bilateral multifocal papillary thyroid carcinomas. Surg Oncol 28:145–149. https://doi.org/10.1016/j.suronc.2018.12.004
Delellis RA (2004) Pathology and genetics of tumours of endocrine organs. Iarc Press, Lyon
Furlan JC, Bedard YC, Rosen IB (2007) Significance of tumor capsular invasion in well-differentiated thyroid carcinomas. Am Surg 73(5):484–491. https://doi.org/10.1177/000313480707300514
Mete O, Asa SL (2011) Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol 24(12):1545–1552. https://doi.org/10.1038/modpathol.2011.119
Kim JM, Kim TY, Kim WB, Gong G, Kim SC, Hong SJ et al (2006) Lymphovascular invasion is associated with lateral cervical lymph node metastasis in papillary thyroid carcinoma. Laryngoscope 116(11):2081–2085. https://doi.org/10.1097/01.mlg.0000242118.79647.a9
Pontius LN, Youngwirth LM, Thomas SM, Scheri RP, Roman SA, Sosa JA (2016) Lymphovascular invasion is associated with survival for papillary thyroid cancer. Endocr Relat Cancer 23(7):555–562. https://doi.org/10.1530/ERC-16-0123
Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, Jegal YJ (2011) The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer 14(3):198–203. https://doi.org/10.4048/jbc.2011.14.3.198
Skancke M, Arnott SM, Amdur RL, Siegel RS, Obias VJ, Umapathi BA (2019) Lymphovascular invasion and perineural invasion negatively impact overall survival for stage II adenocarcinoma of the colon. Dis Colon Rectum 62(2):181–188. https://doi.org/10.1097/DCR.0000000000001258
Cuccurullo V, Mansi L (2011) AJCC cancer staging handbook: From the AJCC cancer staging manual (7th edition): Springer, New York 2010, ISBN: 978-0-387-88442-4. Eur J Nucl Med Mol Imaging 38(2):408–408. https://doi.org/10.1007/s00259-010-1693-9
Youngwirth LM, Adam MA, Scheri RP, Roman SA, Sosa JA (2017) Extrathyroidal extension is associated with compromised survival in patients with thyroid cancer. Thyroid 27(5):626–631. https://doi.org/10.1089/thy.2016.0132
Teodoriu L, Ungureanu MC, Leustean L, Preda C, Ciobanu D, Grierosu I et al (2021) Updated incidence of thyroid cancer in the North East region of Romania after 35 years of Chernobyl fallout. Is there a link between? Diagnostics (Basel). 11(5):907. https://doi.org/10.3390/diagnostics11050907
Acknowledgements
This research would not have been possible without the exceptional support and effort of our supervisor, Prof. Mahmoud Ahmed Mohamed El-Shafaei, and we would like to show our gratitude to Ass. Prof. Dr Hesham Mohamed Ali Omran, and Dr Ahmed Saeed Saad for sharing their pearls of wisdom.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No official funding was provided to conduct the current meta-analysis.
Author information
Authors and Affiliations
Contributions
LGH: data gathering and data extraction. BEE: data gathering, data extraction. Figures 1 and all tables. MAME-S: writing the manuscript. HMAO: helped writing the manuscript. ASS: done the statistical analysis of the meta-analysis all figures except No. 1. All authors have approved the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest for all authors.
Ethical approval and consent to participate
No ethical approval is required because we were collecting and synthesizing data from previous clinical studies.
Consent for publication
It is a meta-analysis, and no special consent required from the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hafez, L.G., Elkomos, B.E., El-Shafaei, M.A.M. et al. The risk of central nodal metastasis based on prognostic factors of the differentiated thyroid carcinoma: a systematic review and meta-analysis study. Eur Arch Otorhinolaryngol 280, 2675–2686 (2023). https://doi.org/10.1007/s00405-023-07863-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-07863-8