Abstract
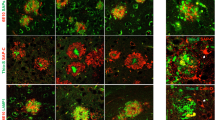
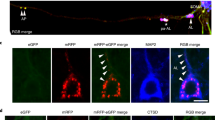
Double-labeling immunofluorescence and confocal microscopy have been used to learn about the local relationship between amyloid, mitochondria, and cytochrome c oxidase (COX) in dystrophic neurites of senile plaques in the frontal cortex in Alzheimer's disease (AD). Dystrophic neurites surrounding amyloid plaques are filled with mitochondrial porin-immunoreactive structures. In contrast with tangle-bearing and non-tangle-bearing neurons, which express mitochondrial porin and COX subunit 4, porin-immunoreactive neurites of senile plaques lack COX subunit 4. Parallel western blot studies in mitochondria-enriched fractions of the frontal cortex in the same cases disclosed reduced expression levels of COX, but not of prohibitin, in AD stages VB/C of Braak. Co-localization of porin and lysosomal associated protein 1, as revealed by double-labeling immunofluorescence and confocal microscopy, suggests that mitochondria may be engulfed by lysosomes in dystrophic neurites. These findings support a local link between amyloid deposition, abnormal mitochondria and impaired respiratory chain function (resulting from decrease of COX expression) in dystrophic neurites of senile plaques in AD.




Similar content being viewed by others
References
Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG (2003) Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol 161:41–54
Barrachina M, Maes T, Buesa C, Ferrer I (2006) Up-regulation of lysosome associated-membrane protein 1 (Lamp-1) in Alzheimer’s disease. Neuropathol Appl Neurobiol 32:505–516
Braak H, Braak E, Peter J, Morrison JH (1999) Temporal sequence of Alzheimer’s disease related pathology. In: Peters A, Morrison JH (eds) Cerebral cortex, vol 14, neurodegenerative and age-related changes in structure and function of cerebral cortex. Kluwer Academic/Plenum Publishers, Dordrecht, pp 475–512
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Brion JP, Couck AM, Bruce M, Anderton B, Flament-Durand J (1991) Synaptophysin and chromogranin a immunoreactivities in seniole plaques of Alzheimer’s disease. Brain Res 539:143–150
Butterfield DA, Perluigi M, Sultana R (2006) Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol 545:39–50
Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA (2002) Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem 80:91–100
Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD (2005) Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J 19:2040–2041
Castellani R, Hirai K, Aliev G, Drew KL, Nunomura A, Takeda A, Cash AD, Obrenovich ME, Perry G, Smith MA (2002) Role of mitochondrial dysfunction in Alzheimer’s disease. J Neurosci Res 70:357–360
Cataldo AM, Paskevich PA, Kominami E, Nixon RA (1991) Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc Natl Acad Sci USA 88:10998–11002
Cataldo AM, Barnett JL, Berman SA, Li J, Quarless S, Bursztajn S, Lippa, Nixon RA (1995) Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: evidence for early up-regulation of the endosomal-lysosomal system. Neuron 14:671–680
Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA (2005) Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1–42. J Neurosci 25:672–679
Duyckaerts C, Dickson DW (2003) Neuropathology of Alzheimer’s disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press, Basel, pp 47–68
Ferrer I, E Martí A Tortosa J Blasi (1998) Dystrophic neurites of senile plaques are defective in proteins involved in exocytosis and neurotransmission. J Neuropathol Exp Neurol 57:218–225
Fiala JC (2007) Mechanisms of amyloid plaque pathogenesis. Acta Neuropathol 114:551–571
Fiala JC, Feinberg M, Peters A, Barbas H (2007) Mitochondrial degeneration in dystrophic neurites of senile plaques may lead to extracellular deposition of fine filaments. Brain Struct Funct 212:195–207
Gillardon F, Rist W, Kussmaul L, Vogel J, Berg M, Danzer K, Kraut N, Hengerer B (2007) Proteomic and functional alterations in brain mitochondria from Tg2576 mice occur before amyloid plaque deposition. Proteomics 7:605–616
Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M (2004) Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. J Biol Chem 279:51654–51660
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017–3023
Horie H, Takenaka T, Kaiho M (1983) Effects of disruption of microtubules on translocation of particles and morphology in tissue cultured neurites. Brain Res 288:85–93
Kidd M (1964) Alzheimer’s disease: an electron microscopical study. Brain 87:307–320
Kim HS, Lee JH, Lee JP, Kim EM, Chang KA, Park CH, Jeong SJ, Wittendorp MC, Seo JH, Choi SH, Suh YH (2002) Amyloid beta peptide induces cytochrome C release from isolated mitochondria. NeuroReport 13:1989–1993
Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN (1992) Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem 59:776–779
Luse SA, Smith KR Jr (1964) The ultrastructure of senile plaques. Am J Pathol 44:553–563
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 304:448–452
Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy H (2006) Mitochondria are a direct site of accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15:1437–1449
Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G (2007) Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol 66:525–532
Muñoz D (1991) Chromogranin A-like immunoreactive neurites are major constituents of senile plaques. Lab Invest 1991 64:826–832
Mutisya EM, Bowling AC, Beal MF (1994) Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem 63:2179–2184
Nixon RA, Cataldo AM, Mathews PM (2000) The endosomal–lysosomal system of neurons in Alzheimer’s disease pathogenesis: a review. Neurochem Res 9–10:1161–1172
Onyango I, Khan S, Miller B, Swerdlow R, Trimmer P, Bennett P (2006) Mitochondrial genomic contribution to mitochondrial dysfunction in Alzheimer’s disease. J Alzheimers Dis 9:183–193
Pamplona R, Dalfó E, Ayala V, Bellmunt J, Prat J, Ferrer I, Portero M (2005) Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. J Biol Chem 280:21522–21530
Pilling AD, Horiuchi D, Lively CM, Saxton WM (2006) Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell 17:2057–2068
Terry RD, Gonatas NK, Weiss M (1964) Ultrastuctural studies in Alzheimer’s presenile dementia. Am J Pathol 44:269–297
Tsukita S, Ishikawa H (1980) The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol 84:513–530
Wang J, Xiong S, Xie C, Marbesbery WR, Lovell MA (2005) Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem 93:953–962
Wang X, Su B, Perry G, Smith MA, Zhu X (2007) Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer’s disease. Free Radic Biol Med 43:1569–1573
Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, Hirai K, Smith MA (2006) Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimer’s Dis 9:147–153
Zhu X, Su B, Wang X, Smith MA, Perry G (2007) Causes of oxidative stress in Alzheimer’s disease. Cell Mol Sci 64:2202–2210
Acknowledgments
This work was funded by grant PI05/1570 and supported by the European Commission under the Sixth Framework Programme (BrainNet Europe II, LSHM-CT-2004-503039). We wish to thank T Yohannan for editorial advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Gracia, E., Torrejón-Escribano, B. & Ferrer, I. Dystrophic neurites of senile plaques in Alzheimer’s disease are deficient in cytochrome c oxidase. Acta Neuropathol 116, 261–268 (2008). https://doi.org/10.1007/s00401-008-0370-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0370-6