Abstract
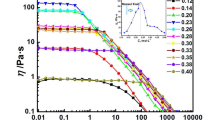
The influence of replacing water with ethanol, propanol, and butanol in alcohol–water mixtures on the rheological properties of wormlike micelles (WLMs) consisting of sodium salicylate (NaSal) and cetyltrimethylammonium bromide (CTAB) is investigated at 25 °C. At maximum viscosity of 100 mM CTAB and 60 mM NaSal, the alcohols at low concentrations of up to 8% ethanol, 3% propanol, and 2% butanol do not affect the WLM structure as indicated by the normal modulus values of the solutions, but they decrease the structural relaxation times and their viscosities. This behavior is explained by the fact that as alcohol chain length increases, it has lower miscibility in water, and hence, it can interact to a higher extent with WLMs even at lower contents. We hypothesize that alcohols adsorbed at the WLM surface make it more hydrophilic. The contacts between WLMs are therefore lost, the viscosity decreases, and the structural relaxation times become faster. At suitable contents, alcohol molecules can penetrate WLMs and damage their structure. For solutions with minimum viscosity, higher alcohol contents were required to destroy WLMs, and replacing water with ethanol leads to viscosity increase, followed by a gradual decrease at higher ethanol contents. Propanol and butanol drastically changed the WLM viscoelastic properties. Generally, as the alcohol chain length increases, WLMs become more pronounced at lower alcohol contents.
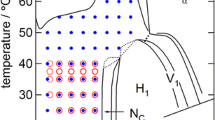
Graphical abstract
Alcohols, at relatively low amounts, do not affect the WLM structure but lead to vanishing of the entanglement between WLMs. Butanol, due to its longer chain compared to ethanol and propanol, destroys WLMs at lower contents. Generally, as alcohol chain length increases, the damage of entanglement between WLMs becomes more pronounced at lower alcohol contents.














Similar content being viewed by others
References
Abdel-Rahem R (2008) Adv Coll Interface Sci 141:24
Lu H, Yang L, Wang B, Huang Z (2016) J Dispers Sci Technol 37:159
Abdel-Rahem R, Hoffmann H (2007) J Colloid Interface Sci 312:146
Liu L, Zheng C, Lu H (2017) J Dispers Sci Technol 38:1824
Abdel-Rahem R, Hoffmann H (2006) Rhelogica Acta 45:781
Sato T, Acharya D, Kaneko M, Aramaki K, Singh Y, Ishitobi M, Kunieda H (2007) J Dispers Sci Technol 27:611
Abdel-Rahem R (2007) Tenside Surfactant Deterg 44:168
Wan T, Wang D, Hu J, Zhou Z, Zhang H, Bu D (2013) J Dispers Sci Technol 35:7
Abdel-Rahem R (2005) Tenside Surfactant Deterg 42:95
Wang F, Zhang Z, Wei Y, Zhou T, Wang X, Zhang G (2020) J Dispers Sci Technol, published online
Wang Z, Wang S, Xu L, Dou Y, Su X (2020) J Dispers Sci Technol 41:639
Wang Z, Wang S, Jing Z, Luo X (2015) J Dispers Sci Technol 37:442
Abdel-Rahem R, Gradzielski M, Hoffmann H (2005) J of Colloid Interface Sci 288:570
Abdel-Rahem R (2014) J Surfactants Deterg 17:353
Abdel-Rahem R, Reger M, Hloucha M, Hoffmann H (2014) J Dispers Sci Technol 35:64
Abdel-Rahem R (2003) Phase behavior and structural transitions in the mixtures of cationic surfactants and hydrophobic counterions, Ph.D. thesis, Bayreuth University
Yang J (2002) Curr Opin Colloid Interface Sci 7:276
Hoffmann H, Abdel-Rahem R (2010) Colloid Polym Sci 288:603
Das N, Cao H, Kaiser H, Warren G, Gladden J, Sokol P (2012) Langmuir 28:11962
Geng F, Yu L, Cao Q, Li Z, Zheng L, Xiao J, Chen H, Cao Z (2007) J Dispers Sci Technol 30:92
Yang J, Yang Z, Lu Y, Chen J, Qin W (2013) J Dispers Sci Technol 34:1124
Abdel-Rahem R (2011) J Dispers Sci Technol 32:784
Abdel-Rahem R (2012) Colloid Polym Sci 290:907
Zana R (1995) Advances in Colloid and Interface Science, 57:l
Bakshi M (1993) J Chem Soc Faraday Trans 89:4323
Sinha S, Tikariha D, Lakra J, Tiwari A, Saha S, Ghosh K (2015) J Surfactants Deterg 18:629
Walker L (2001) Curr Opin Colloid Interface Sci 6:451
Turner M, Cates M (1991) Langmuir 7:1590
Turner M, Marques C, Cates M (1993) Langmuir 9:695
Agrawal N, Yue X, Raghavan S (2020) Langmuir 36:6370
Thurn H, Hoffmann H (2019) Langmuir 35:12192
Liberatore M, Nettesheim F, Vasquez P, Helgeson M, Wagner N, Kaler E, Cook L, Porcar L, Hu Y (2009) J Rheol 53:441
Clausen T, Vinson P, Minter J, Davis H, Talmon Y, Miller W (1992) J Phys Chem 96:474
Rehage H, Hoffmann H (1988) J Phys Chem 92:4712
Berret J (2006) Rheology of wormlike micelles: equilibrium properties and shear banding transitions. Molecular Gels, Springer, Dordrecht
Jagannathan N, Venkateswaran K, Herring F, Patey G, Walker D (1987) J Phys Chem 91:4553
Szajdzinska-Pietek E, Maldonado R, Kevan L, Jones R (1986) J Colloid Interface Sci 110:514
Hayter B, Hayoun M, Zemb T (1984) Colloid Polym Sci 262:798
Dean JA (1987) Handbook of Organic Chemistry (2nd ed.) New York: McGraw-Hill Book Co
Agrawal N, Yue X, Feng Y, Raghavan S (2019) Langmuir 35:12782
Birdi KS, Backlund S, Sorensen K, Krag T, Dalsager S (1978) J Colloid Interface Sci 66:118
Dannhauser W, Cole RH (1955) J Chem Phys 23:1762
Shukla A, Rehage H (2008) Langmuir 24:8507
Geng F, Yu L, Cao Q, Li Z, Zheng L, Xiao J, Chen H, Cao Z (2009) J Dispers Sci Technol 30:92
Jiang H, Beaucage G, Vogtt K, Weaver M (2005) J of Colloid Interface Sci 509:25
Funding
The Faculty of Scientific Research and Higher Studies at the University of Petra provided financial support (Projects No. 2/1/2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Rahem, R.A., Al-Remawi, M., Daraosheh, A.Q. et al. Rheological behavior of wormlike micelles (WLMs) in alcohol/water mixed solvent: influence of alcohol chain length. Colloid Polym Sci 299, 1337–1351 (2021). https://doi.org/10.1007/s00396-021-04852-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-021-04852-3