Abstract
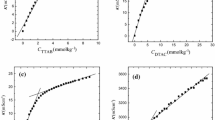
The micellar properties of tetradecyltrimethylammonium nitrate (C14TANO3) in aqueous solutions in the temperature range of 10 to 35 °C and in aqueous solutions of benzyl alcohol (BzOH) at 25 °C were studied conductometrically. The specific conductivity data served for the evaluation of critical micelle concentration, cmc, and the degree of ionization of the micelles, β, of the surfactant. From the temperature dependence of the cmc the thermodynamic parameters for micellization of C14TANO3 were calculated by applying Muller′s modified equation. BzOH was found to affect strongly the cmc and β values of the surfactant. The plot of the cmc/cmco ratio (where cmco is for pure water) as a function of BzOH molality, exhibits a characteristic break, which was attributed to the commencement of self-association of BzOH in aqueous solution at a molality of ca. 0.05. By applying the theoretical treatment suggested by Motomura for binary surfactant systems, the molar fraction of BzOH in the micelles at cmc, was estimated as a function of molality of the alcohol. C14TANO3 appears to be slightly more hydrophobic compared to the corresponding bromide.








Similar content being viewed by others
References
Samis CS, Hartley GS (1938) Trans Faraday Soc 34:1288
Ford WP, Ottewill RH, Parreira HC (1966) J Colloid Interface Sci 21:522
Emerson MF, Holtzer EW (1967) J Phys Chem 71:1898
Underwood AL, Anacker EW (1987) J Colloid Interface Sci 117:242
Czapkiewicz J, Czapkiewicz-Tutaj B (1980) J Chem Soc Faraday Trans I 76(8):1663
Larsen JW, Magid LJ (1974) J Am Chem Soc 96:5774
Paredes S, Tribout M, Sepúlveda L (1984) J Phys Chem 88:1871
Sepúlveda L, Cortés J (1985) J Phys Chem 89:5322
Ohlendorf D, Interthal W, Hoffmann H (1986) Rheol Acta 25:468
Soltero JFA, Puig JE, Manero O, Schulz PC (1995) Langmuir 11:33337
Evans DF, Mitchell DJ, Ninham BW (1986) J Phys Chem 90:2817
Chen V, Evans DF, Ninham BW (1987) 91:1823
Nydén M, Söderman O (1995) Langmuir 11:1537
Claesson P, Ninham BW (1992 Langmuir 8:1406
Brady JE, Evans DF, Warr GG, Grieser F, Ninham BW (1986) J Phys Chem 90:1853
Czapkiewicz J, Palma B, unpublished results
Soldi V, Keiper J, Romsted LS, Cuccovia IM, Chaimovich H (2000) Langmuir 16:59
González-Pérez A, Del Castillo JL, Czapkiewicz J, Rodríguez JR (2002) Colloid Polym Sci 280:503
González-Pérez A, Czapkiewicz J, Del Castillo JL, Rodríguez JR (2003) J Colloid Interface Sci 262:525
Hoffmann H, Ulbright W (1977) Z Phys Chem NF 106:167
Shinoda K, Hutchinson E (1962) J Phys Chem 66:577
Evans DF, Wightman PJ (1982) J.Colloid Interface Sci 86:515
Aguiar J, Molina-Bolivar JA, Peula-Garcia JM, Carnero Ruiz C (2002) J Colloid Interface Sci. 255:382
Oremusová J, Greksaková O (1999) Tenside Surf Det 36:322
Zielinski R, Ikeda S, Nomura H, Kato S (1989) J Colloid Interface Sci 129:175
Gaillon L, Hamidi M, Leliévre J, Gaboriaud R (1997) J Chim Phys 94:707
Zana R (1980) J Colloid Interface Sci 78:331
Rodríguez J, González-Pérez A, Del Castillo JL, Czapkiewicz J (2002) J Colloid Interface Sci 250:438
Muller N (1993) Langmuir 9:96
Kresheck GC (2001) J Phys Chem B 105:4380
Shugihara G, Nakano TY, Sulthana SB, Rakshit AK (2001) J Oleo Sci 50:29
Chen LY, Lin SY, Huang CC (1998) J Phys Chem B 102:4350
Motomura K, Yamanaka M, Aratono M (1984) Colloid Polym Sci 262:948
Pons R, Bury R, Erra P, Treiner C (1991) Colloid Polym Sci 269:62
Acknowledgements
This work was supported by the Xunta de Galicia, (Project PGIDIT03PXIB20601PR). A. González-Pérez is grateful to the University of Santiago de Compostela for his postdoctoral grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Pérez, A., Czapkiewicz, J., Del Castillo, J.L. et al. Micellar properties of tetradecyltrimethylammonium nitrate in aqueous solutions at various temperatures and in water-benzyl alcohol mixtures at 25 °C. Colloid Polym Sci 282, 1359–1364 (2004). https://doi.org/10.1007/s00396-004-1054-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1054-y