Abstract
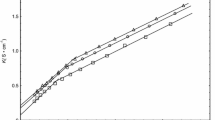
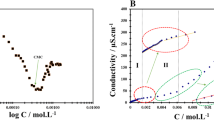
The effect of co-solvent N-methylacetamide (NMA) (0.035, 0.046, 0.127, and 0.258 mol kg−1) on the micellization behaviour of anionic surfactant sodium dodecylsulphate (SDS) (3.21–10.35 mmol kg−1) and cationic surfactant cetyltrimethylammonium bromide (CTAB) (0.19–3.72 mmol kg−1) in aqueous solution was explored by employing conductivity measurements at different temperatures (298.15–313.15 K). The critical micelle concentration (CMC) values for SDS and CTAB in aqueous solutions of NMA were determined from the conductivity versus surfactant concentration plots. The variations in the CMC values of SDS with NMA concentration are in striking contrast to those observed in the case of CTAB. The various relevant thermodynamic parameters of micellization, viz. standard enthalpy change, ΔH om , standard entropy change, ΔS om , and standard Gibbs free energy change, ΔG om , were determined using the temperature variation of the CMC values and counterion binding. The results not only relate these thermodynamic parameters to the consequences of intermolecular interactions but are also able to differentiate between SDS–water–NMA and CTAB–water–NMA systems in terms of contributions from head groups as well as alkyl chains of surfactants.




Similar content being viewed by others
References
Lindman B, Wennerstrom H (1980) Amphiphile aggregation in aqueous solution topics in current chemistry, vol 87. Springer, New York, pp 1–83
Bakshi MS (1996) Micelle formation by sodium dodecyl sulphate in water-additive systems. Bull Chem Soc Jpn 69:2723–2729
Pan A, Naskar B, Prameela GKS, Kumar BVNP, Aswal VK, Bhattacharya SC, Mandal AB, Moulik SP (2014) Micellization and related behavior of sodium dodecylsulfate in mixed binary solvent media of tetrahydrofuran (Tf) and formamide (Fa) with water: a detailed physicochemical investigation. Soft Matter 10:5682–5694
Eaglund D, Armitage G, Franks F (eds) (1967) Physico-chemical processes in mixed aqueous solvents. Elsevier, New York
Tokuhiro T, Ionescu LG, Mittal KL (eds) (1979) Solution chemistry of surfactants, vol 1. Plenum, New York, pp 497–506
Jiao J, Zhang Y, Fang L, Yua L, Sun L, Wang R, Cheng N (2013) Electrolyte effect on the aggregation behavior of 1-butyl-3-methylimidazolium dodecylsulfate in aqueous solution. J Colloid Interf Sci 402:139–145
Kresheck GC, Franks F (eds) (1978) Water, a comprehensive treatise, vol 4. Plenm, New York, Chap. 2
Emerson MF, Holtzer A (1967) The hydrophobic bond in micellar systems. Effects of various additives on the stability of micelles of sodium dodecyl sulfate and of n-dodecyltrimethylammonium bromide. J Phys Chem 71:3320–3330
Lin IJ, Metzer A (1971) Effect of dissolved paraffinic gases on the surface tension and critical micelle concentration (CMC) of aqueous solutions of dodecylamine hydrochloride (DACl). J Phys Chem 75:3000–3004
Chauhan MS, Sharma K, Kumar G, Chauhan S (2003) A conductometric study of dimethyl sulfoxide effect on micellization of sodium dodecylsulfate in dilute aqueous electrolyte solutions. Colloids Surf A 221:135–140
Graciani MM, Munoz M, Rodriguez A, Moya ML (2005) Water–N,N-dimethylformamide alkyltrimethylammonium bromide micellar solutions: thermodynamic, structural, and kinetic studies. Langmuir 21:3303–3310
Pan A, Naskar B, Prameela GKS, Kumar BVNP, Mandal AB, Bhattacharya SC, Moulik SP (2012) Amphiphile behavior in mixed solvent media I: self-aggregation and ion association of sodium dodecylsulfate in 1,4-dioxane–water and methanol–water media. Langmuir 28:13830–13843
Singh HN, Saleem SM, Singh RP, Birdi KS (1980) Micelle formation of ionic surfactants in polar non-aqueous solvents. J Phys Chem 84:2191–2199
Bakshi MS (1993) Micelle formation by anionic and cationic surfactants in binary aqueous solvents. J Chem Soc Faraday Trans 89:4323–4326
Moulik SP, Paul BK (1998) Structure, dynamic and transport properties of microemulsions. Adv Colloid Interf Sci 78:99–195
Uchegbu IF, Vyas SP (1998) Nonionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm 172:33–70
Bakshi MS, Kaur G, Thakur P, Banipal TS, Possmayer F, Petersen NO (2007) Surfactant selective synthesis of gold nanowires by using a DPPC-surfactant mixture as a capping agent at ambient conditions. J Phys Chem C 111:5932–5940
Khullar P, Singh V, Mahal A, Dave PN, Thakur S, Kaur G, Singh J, Kamboj SS, Bakshi MS (2012) Bovine serum albumin bioconjugated gold nanoparticles: synthesis, hemolysis, and cytotoxicity toward cancer cell lines. J Phys Chem C 116:8834–8843
Klotz IM, Franzen JS (1962) Hydrogen bonds between model peptides in solution. J Am Chem Soc 84:3461–3466
Chauhan S, Kumar K, Singh K, Jyoti J (2014) Volumetric, compressibility, and surface tension studies on micellization behavior of SDS in aqueous medium: effect of sugars. J Surfactant Deterg 17:169–175
Chauhan S, Chauhan MS, Sharma P, Rana DS (2013) Thermodynamics and micellization of cetyltrimethyl ammonium bromide in the presence of lysozyme. Fluid Phase Equlibria 187:1–6
Kumar K, Patial BS, Chauhan S (2015) Conductivity and fluorescence studies on the micellization properties of sodium cholate and sodium deoxycholate in aqueous medium at different temperatures: effect of selected amino acids. J Chem Thermodyn 82:25–32
Kaushal D, Rana DS, Chauhan S (2013) Effect of furosemide on denaturation of lysozyme in the presence of ionic surfactant at different temperatures. Fluid Phase Equilibr 360:239–247
Ali A, Ansari NA (2010) Studies on the effect of amino acids/peptide on the micellization of SDS at different temperature. J Surfactant Deterg 13:441–449
Roy JC, Islam MN, Aktaruzzaman G (2014) The effect of NaCl on the Krafft temperature and related behavior of cetyltrimethylammonium bromide in aqueous solution. J Surfactant Deterg 17:231–242
Ali A, Nabi F, Malik NA, Tasneem S, Uzair S (2014) Study of micellization of sodium dodecyl sulfate in non-aqueous media containing lauric acid and dimethylsulfoxide. J Surfactant Deterg 17:151–160
Hoque MA, Khan MA, Hossain MD (2013) Interaction of cefalexin monohydrate with cetyldimethylethylammonium bromide. J Chem Thermodyn 60:71–75
Mehta SK, Bhasin KK, Chauhan R, Dham S (2005) Effect of temperature on critical micelle concentration and thermodynamic behavior of dodecyldimethylethylammonium bromide and dodecyltrimethylammonium chloride in aqueous media. Colloids Surf A 255:153–157
Ray GB, Ghosh S, Moulik SP (2009) Physicochemical studies on the interfacial and bulk behavior of sodium N-dodecanoyl sarcosinate (SDDS). J Surfactant Deterg 12:131–143
Hoque MA, Hossain MD, Khan MA (2013) Interaction of cephalosporin drugs with dodecyltrimethylammonium bromide. J Chem Thermodyn 63:135–141
Chauhan S, Kaur M, Kumar K, Chauhan MS (2014) Study of the effect of electrolyte and temperature on the critical micelle concentration of dodecyltrimethylammonium bromide in aqueous medium. J Chem Thermodyn 78:175–181
Mahajana RK, Kaur R, Aswal VK (2013) Effect of urea on the aggregation behavior of gemini surfactants and their mixed micelles with Pluronic L64. Colloids Surf A 419:61–66
Gonzalez-Perez A, Del Castillo JL, Czapkiewicz J, Rodriguez JR (2004) Thermodynamics of micellization of decyldimethylbenzylammonium bromide in aqueous solution. Colloids Surf A 232:183–189
Mehta SK, Chaudhary S, Bhasin KK, Kumar R, Aratono M (2007) Conductometric and spectroscopic studies of sodium dodecyl sulfate in aqueous media in the presence of organic chalcogen. Colloids Surf A 304:88–95
Chauhan S, Singh R, Sharma K, Kumar K (2014) Interaction study of anionic surfactant with aqueous non-ionic polymers from conductivity, density and speed of sound measurements. J Surfactant Deterg. doi:10.1007/s11743-014-1613-2
Das D, Ismail K (2008) Aggregation and adsorption properties of sodium dodecyl sulfate in water-acetamide mixtures. J Colloid Interf Sci 327:198–203
Helgeson ME, Hodgdon TK, Kaler EW, Wagner NJ (2010) A systematic study of equilibrium structure, thermodynamics, and rheology of aqueous CTAB/NaNO3 wormlike micelles. J Colloid Interf Sci 349:1–12
Nusselder JJH, Engberts JBFN (1992) Toward a better understanding of the driving force for micelle formation and micellar growth. J Colloid Interf Sci 148:353–361
Kang KH, Kim HU, Lim KH (2001) Effect of temperature on critical micelle concentration and thermodynamic potentials of micellization of anionic ammonium dodecyl sulfate and cationic octadecyl trimethyl ammonium chloride. Colloids Surf A 189:113–121
Acknowledgments
S. Chauhan thanks UGC for the financial assistance under the project (F. No. 42-249/2013/SR). K. Kumar and D. S. Rana thank UGC, New Delhi for the Financial Assistance under UGC BSR fellowship (F. No. 7–75/2007/BSR) and Dr. D. S. Kothari, Post Doctorate Fellowship, respectively.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Chauhan, S., Kumar, K., Rana, D.S. et al. A Comparative Study on the Aggregation and Thermodynamic Properties of Anionic Sodium Dodecylsulphate and Cationic Cetyltrimethylammonium Bromide in Aqueous Medium: Effect of the Co-solvent N-Methylacetamide . J Surfact Deterg 19, 193–200 (2016). https://doi.org/10.1007/s11743-015-1748-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1748-9