Abstract
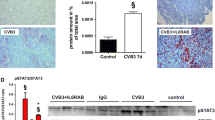
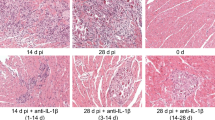
Enteroviruses, such as coxsackieviruses of group B (CVB), are able to induce a chronic inflammation of the myocardium, which may finally lead to the loss of functional tissue, remodeling processes and the development of fibrosis, thus affecting the proper contractile function of the heart. In other fibrotic diseases like scleroderma, the prostacyclin agonist iloprost was found to inhibit the extracellular signal-regulated kinase (ERK, p44/42 MAPK), a mitogen-activated protein kinase, and consecutively, the expression of the profibrotic cytokine connective tissue growth factor (CTGF), thereby preventing the development of fibrosis. As CTGF was found to mediate fibrosis in chronic CVB3 myocarditis as well, we evaluated whether the in vivo application of iloprost is capable to reduce the development of ERK/CTGF-mediated fibrosis in enteroviral myocarditis. Unexpectedly, the application of iloprost resulted in a prolonged myocardial inflammation and an aggravated fibrosis and failed to reduce activation of ERK and expression of CTGF at later stages of the disease. In addition, viral replication was found to be increased in iloprost-treated mice. Notably, the expression of cardiac inducible nitric oxide synthase (iNOS), which is known to aggravate myocardial damage in CVB3-infected mice, was strongly enhanced by iloprost. Using cultivated bone marrow macrophages (BMM), we confirmed these results, proving that iloprost potentiates the expression of iNOS mRNA and protein in CVB3-infected and IFN-gamma stimulated BMM. In conclusion, these results suggest a critical reflection of the clinical use of iloprost, especially in patients possibly suffering from an enteroviral myocarditis.







Similar content being viewed by others
References
Badesch DB, McLaughlin VV, Delcroix M, Vizza CD, Olschewski H, Sitbon O, Barst RJ (2004) Prostanoid therapy for pulmonary arterial hypertension. J Am Coll Cardiol 43(12 Suppl S):56S–61S. doi:10.1016/j.jacc.2004.02.036
Bapat S, Verkleij A, Post JA (2001) Peroxynitrite activates mitogen-activated protein kinase (MAPK) via a MEK-independent pathway: a role for protein kinase C. FEBS Lett 499:21–26. doi:10.1016/S0014-5793(01)02511-X
Bevan AL, Zhang H, Li Y, Archard LC (2001) Nitric oxide and coxsackievirus B3 myocarditis: differential expression of inducible nitric oxide synthase in mouse heart after infection with virulent or attenuated virus. J Med Virol 64:175–182. doi:10.1002/jmv.1033
Cao W, Xie YH, Li XQ, Zhang XK, Chen YT, Kang R, Chen X, Miao S, Wang SW (2011) Burn-induced apoptosis of cardiomyocytes is survivin dependent and regulated by PI3K/Akt, p38 MAPK and ERK pathways. Basic Res Cardiol 106:1207–1220. doi:10.1007/s00395-011-0199-3
Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH (2000) CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 32:1805–1819. doi:10.1006/jmcc.2000.1215
Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA (2009) Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf) 195:321–338. doi:10.1111/j.1748-1716.2008.01936.x
Dhillon AS, Meikle S, Yazici Z, Eulitz M, Kolch W (2002) Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J 21:64–71. doi:10.1093/emboj/21.1.64
Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W (2002) Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol 22:3237–3246. doi:10.1128/MCB.22.10.3237-3246.2002
Dumaz N, Marais R (2005) Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft für Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J 272:3491–3504. doi:10.1111/j.1742-4658.2005.04763.x
Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR (1999) Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J 13:1774–1786
Flesch IEA, Kaufmann SHE (1991) Mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone-marrow-macrophages: role of reactive nitrogen intermediates. Infect Immun 159:3213–3218
Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR (1996) Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol 107:404–411
Grotendorst GR, Duncan MR (2005) Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J 19:729–738. doi:10.1096/fj.04-3217com
Haywood GA, Tsao PS, von der Leyen HE, Mann MJ, Keeling PJ, Trindade PT, Lewis NP, Byrne CD, Rickenbacher PR, Bishopric NH, Cooke JP, McKenna WJ, Fowler MB (1996) Expression of inducible nitric oxide synthase in human heart failure. Circulation 93:1087–1094. doi:10.1161/01.CIR.93.6.1087
Heinzel FR, Gres P, Boengler K, Duschin A, Konietzka I, Rassaf T, Snedovskaya J, Meyer S, Skyschally A, Kelm M, Heusch G, Schulz R (2008) Inducible nitric oxide synthase expression and cardiomyocyte dysfunction during sustained moderate ischemia in pigs. Circ Res 103:1120–1127. doi:10.1161/CIRCRESAHA.108.186015
Huber M, Selinka HC, Kandolf R (1997) Tyrosine phosphorylation events during coxsackievirus B3 replication. J Virol 71:595–600
Huber M, Watson KA, Selinka HC, Carthy CM, Klingel K, McManus BM, Kandolf R (1999) Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J Virol 73:3587–3594
Ihm SH, Chang K, Kim HY, Baek SH, Youn HJ, Seung KB, Kim JH (2010) Peroxisome proliferator-activated receptor-gamma activation attenuates cardiac fibrosis in type 2 diabetic rats: the effect of rosiglitazone on myocardial expression of receptor for advanced glycation end products and of connective tissue growth factor. Basic Res Cardiol 105:399–407. doi:10.1007/s00395-009-0071-x
Javelaud D, Mauviel A (2005) Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-β: implications for carcinogenesis. Oncogene 24:5742–5750. doi:10.1038/sj.onc.1208928
Kandolf R, Hofschneider PH (1985) Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci USA 82:4818–4822
Kim KS, Hufnagel G, Chapman N, Tracy S (2001) The group B coxsackieviruses and myocarditis. Rev Med Virol 11:355–368. doi:10.1002/rmv.326
Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S (1997) Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol 122:217–224. doi:10.1038/sj.bjp.0701367
Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R (1992) Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci USA 89:314–318
Klingel K, Sauter M, Bock CT, Szalay G, Schnorr JJ, Kandolf R (2004) Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol 193:101–107. doi:10.1007/s00430-003-0190-1
Kooy NW, Lewis SJ, Royall J, Ye YZ, Kelly D, Beckman JS (1997) Extensive tyrosine nitration in human myocardial inflammation: evidence for the presence of peroxynitrite. Crit Care Med 25:812–819
Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M (2002) Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem 277:7913–7919. doi:10.1074/jbc.M108733200
Kuo CH, Ko YC, Yang SN, Chu YT, Wang WL, Huang SK, Chen HN, Wei WJ, Jong YJ, Hung CH (2011) Effects of PGI2 analogues on Th1- and Th2-related chemokines in monocytes via epigenetic regulation. J Mol Med 89:29–41. doi:10.1007/s00109-010-0694-2
Lancel S, Tissier S, Mordon S, Marechal X, Depontieu F, Scherpereel A, Chopin C, Neviere R (2004) Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. Am Coll Cardiol 42:2348–2358. doi:10.1016/j.jacc.2004.01.047
Lang C, Sauter M, Szalay G, Racchi G, Grassi G, Rainaldi G, Mercatanti A, Lang F, Kandolf R, Klingel K (2008) Connective tissue growth factor: a crucial cytokine-mediating cardiac fibrosis in ongoing enterovirus myocarditis. J Mol Med 86:49–60. doi:10.1007/s00109-007-0249-3
Leask A, Holmes A, Black CM, Abraham DJ (2003) Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-β2 in fibroblasts. J Biol Chem 278:13008–13015. doi:10.1074/jbc.M210366200
Leivonen SK, Häkkinen L, Liu D, Kähäri VM (2005) Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-β-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol 124:1162–1169. doi:10.1111/j.0022-202X.2005.23750.x
Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L (2006) Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med 41:886–895. doi:10.1016/j.freeradbiomed.2006.04.034
Liaudet L, Vassalli G, Pacher P (2009) Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci 14:4809–4814. doi:10.2741/3569
Luo H, Yanagawa B, Zhang J, Luo Z, Zhang M, Esfandiarei M, Carthy C, Wilson JE, Yang D, McManus BM (2002) Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J Virol 76:3365–3373. doi:10.1128/JVI.76.7.3365-3373.2002
Mason CS, Springer CJ, Cooper RG, Superti-Furga G, Marshall CJ, Marais R (1999) Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J 18:2137–2148. doi:10.1093/emboj/18.8.2137
Michel MC, Li Y, Heusch G (2001) Mitogen-activated protein kinases in the heart. Naunyn Schmiedebergs Arch Pharmacol 363:245–266. doi:10.1007/s002100000363
Miller CL, Cai Y, Oikawa M, Thomas T, Dostmann WR, Zaccolo M, Fujiwara K, Yan C (2011) Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res Cardiol 106:1023–1039. doi:10.1007/s00395-011-0228-2
Nijhuis M, van Maarseveen N, Schuurman R, Verkuijlen S, de Vos M, Hendriksen K, van Loon AM (2002) Rapid and sensitive routine detection of all members of the genus enterovirus in different clinical specimens by real-time PCR. J Clin Microbiol 40:3666–3670
Opavsky MA, Martino T, Rabinovitch M, Penninger J, Richardson C, Petric M, Trinidad C, Butcher L, Chan J, Liu PP (2002) Enhanced ERK 1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J Clin Invest 109:1561–1569. doi:10.1172/JCI13971
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424. doi:10.1152/physrev.00029.2006
Pesse B, Levrand S, Feihl F, Waeber B, Gavillet B, Pacher P, Liaudet L (2005) Peroxynitrite activates ERK via Raf-1 and MEK, independently from EGF receptor and p21Ras in H9C2 cardiomyocytes. J Mol Cell Cardiol 38:765–775. doi:10.1016/j.yjmcc.2005.02.020
Plum J, Huang C, Grabensee B, Schrör K, Meyer-Kirchrath J (2002) Prostacyclin enhances the expression of LPS/INF-γ-induced nitric oxide synthase in human monocytes. Nephron 91:391–398
Rutschow S, Li J, Schultheiss HP, Pauschinger M (2006) Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc Res 69:646–656. doi:10.1016/j.cardiores.2005.12.009
Shimojo T, Hiroe M, Ishiyama S, Ito H, Nishikawa T, Marumo F (1999) Nitric oxide induces apoptotic death of cardiomyocytes via a cyclic-GMP-dependent pathway. Exp Cell Res 247:38–47. doi:10.1006/excr.1998.4310
Snook JH, Li J, Helmke BP, Guilford WH (2008) Peroxynitrite inhibits myofibrillar protein function in an in vitro assay of motility. Free Rad Biol Med 44:14–23. doi:10.1016/j.freeradbiomed.2007.09.004
Stratton R, Shiwen X, Martini G, Holmes A, Leask A, Haberberger T, Martin GR, Black CM, Abraham D (2001) Iloprost suppresses connective tissue growth factor production in fibroblasts and in the skin of scleroderma patients. J Clin Invest 108:241–250. doi:10.1172/JCI12020
Stratton R, Rajkumar V, Ponticos M, Nichols B, Shiwen X, Black CM, Abraham DJ, Leask A (2002) Prostacyclin derivatives prevent the fibrotic response to TGF-β by inhibiting the Ras/MEK/ERK pathway. FASEB J 16:1949–1951. doi:10.1096/fj.02-0204fje
Szalay G, Sauter M, Hald J, Weinzierl A, Kandolf R, Klingel K (2006) Sustained nitric oxide synthesis contributes to immunopathology in ongoing myocarditis attributable to interleukin-10 disorders. Am J Pathol 169:2085–2093. doi:10.2353/ajpath.2006.060350
Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Li JJ, Yao R, Liao MY, Tu X, Liao YH, Cheng X (2012) Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol 107:232. doi:10.1007/s00395-011-0232-6
Wenzel S, Henning K, Habbig A, Forst S, Schreckenberg R, Heger J, Maxeiner H, Schlüter KD (2010) TGF-beta1 improves cardiac performance via up-regulation of laminin receptor 37/67 in adult ventricular cardiomyocytes. Basic Res Cardiol 105:621–629. doi:10.1007/s00395-010-0108-1
Xu Z, Desai M, Philip J, Sivsubramanian N, Bowles NE, Vallejo JG (2011) Conditional transgenic expression of TIR-domain-containing adaptor-inducing interferon-β (TRIF) in the adult mouse heart is protective in acute viral myocarditis. Basic Res Cardiol 106:1159–1171. doi:10.1007/s00395-011-0226-4
Zandman-Goddard G, Tweezer-Zaks N, Shoenfeld Y (2005) New therapeutic strategies for systemic sclerosis—a critical analysis of the literature. Clin Dev Immunol 12:165–173. doi:10.1080/17402520500233437
Zaragoza C, Ocampo C, Saura M, Leppo M, Wie XQ, Quick R, Moncada S, Liew FY, Lowenstein CJ (1998) The role of inducible nitric oxide synthase in the host response to coxsackievirus myocarditis. Proc Natl Acad Sci USA 95:2469–2474
Zell R, Markgraf R, Schmidtke M, Görlach M, Stelzner A, Henke A, Sigusch HH, Glück B (2003) Nitric oxide donors inhibit the coxsackievirus B3 proteinases 2A and 3C in vitro, virus production in cells, and signs of myocarditis in virus-infected mice. Med Microbiol Immunol 193:91–100. doi:10.1007/s00430-003-0198-6
Zhang C (2008) The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol 103:398–406. doi:10.1007/s00395-008-0733-0
Zhang P, Wang YZ, Kagan E, Bonner JC (2000) Peroxynitrite targets the epidermal growth factor receptor, Raf-1, and MEK independently to activate MAPK. J Biol Chem 275:22479–22486. doi:10.1074/jbc.M910425199
Zhu Y, Liu Y, Zhou W, Xiang R, Jiang L, Huang K, Xiao Y, Guo Z, Gao J (2010) A prostacyclin analogue, iloprost, protects from bleomycin-induced pulmonary fibrosis in mice. Respir Res 11:34. doi:10.1186/1465-9921-11-34
Acknowledgments
We thank Sandra Bundschuh for excellent technical assistance. This work was supported by the DFG (SFB-TR19 TP Z4 to KK), and the BMBF (01EZ0817 to KK and RK) and the Interfakultäres Zentrum für Klinische Forschung (IZKF), University Hospital Tuebingen, Germany (to SG and KK).
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gruhle, S., Sauter, M., Szalay, G. et al. The prostacyclin agonist iloprost aggravates fibrosis and enhances viral replication in enteroviral myocarditis by modulation of ERK signaling and increase of iNOS expression. Basic Res Cardiol 107, 287 (2012). https://doi.org/10.1007/s00395-012-0287-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0287-z