Abstract
Purpose
Probiotics or synbiotics consumption have been suggested to reduce the risk of cardiovascular disease (CVD) through a decline in inflammation and oxidative stress, however, the results from studies are conflicting. This study filled this knowledge gap by evaluating randomized controlled trials (RCTs) investigating probiotics or synbiotics intake on adipokines, inflammation, and oxidative stress in patients with prediabetes and type-2 diabetes mellitus (T2DM).
Methods
We systematically did search up to March 2022 in PubMed/Medline, Scopus, ISI Web of Science, and Cochrane library. A random-effect model was applied to estimate the weighted mean difference (WMD) and 95% confidence interval (95% CI) for each outcome.
Results
A total of 32 RCTs were included in the meta-analysis. This intervention led to a significant decrease in levels of C-reactive protein (CRP) (WMD − 0.62 mg/l; 95% CI − 0.80, − 0.44; p < 0.001), tumor necrosis factor-α (TNF-α) (WMD − 0.27 pg/ml; 95% CI − 0.44, − 0.10; p = 0.002) and malondialdehyde (MDA) (WMD − 0.51 µmol/l; 95% CI − 0.73, − 0.30; p < 0.001), and also a significant increase in levels of glutathione (GSH) (WMD 69.80 µmol/l; 95% CI 33.65, 105.95; p < 0.001), total antioxidant capacity (TAC) (WMD 73.59 mmol/l; 95% CI 33.24, 113.95; p < 0.001) and nitric oxide (NO) (WMD 7.49 µmol/l; 95% CI 3.12, 11.86; p = 0.001), without significant alterations in interleukin-6 (IL-6) and adipokines levels.
Conclusion
A consumption of probiotics or synbiotics could be a useful intervention to improve cardiometabolic outcomes through a reduced inflammation and oxidative stress in patients with prediabetes and T2DM.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a global health concern with a high financial and social burden on the health care system. According to the International Diabetes Federation (IDF), T2DM now affects over 10% of the adult population, and it is projected to rise to 578 million by 2030 and 783.2 million by 2045 [1, 2]. It is reported that 5–10% of those with prediabetes develop T2DM each year [3].
It is evident that inflammation and oxidative stress are prevalent in diabetes and are key factors contributing to the progression of T2DM and diabetes complications [4]. This is partially due to enhanced intestinal permeability, which has been reported in patients with T2DM [5]. Alterations in intestinal permeability can result in higher lipopolysaccharide (LPS) concentrations in the peripheral circulation, consequently increasing inflammation and oxidative stress [4, 6, 7]. In addition, metabolic dysfunction in T2DM can result in the production of large amounts of reactive oxygen species (ROS) in mitochondria [6]. Moreover, adipokines such as adiponectin and leptin play a crucial role in regulating glucose metabolism [2]. Studies on gut microbiota demonstrated an association between gut dysbiosis (the imbalance of microbes in the gut) and several chronic diseases, including obesity, inflammatory diseases, and T2DM, and its potential role in shaping host pathophysiology responses [8,9,10,11]. Gut microbiota modulation enhances insulin and adiponectin expression and decreases low-grade inflammation in T2DM [12]. The concept of regulation of the gut microbiota with probiotics, prebiotics, and synbiotics is therefore a promising approach in the management of T2DM.
Probiotics are characterized as “living microorganisms that exert beneficial effects on the health status of host” [13]. Probiotics efficiently improve the integrity of the intestinal barrier, inhibit the release of pro-inflammatory cytokines, alter oxidative stress markers and alleviate symptoms of T2DM [14, 15]. Available data regarding the effects of probiotics supplementation on inflammatory and oxidative stress biomarkers are inconsistent with some studies indicating an inverse relation [16,17,18] and other not showing any relationship [15, 19, 20]. Additionally, some studies have also shown improvement in serum adipokines levels following probiotics supplementation [21], whereas other studies reported no effect [2, 3].
Synbiotics represent a combination of probiotics and prebiotics (as non-digestible food ingredients), acting synergically [22]. The beneficial effects on metabolic profiles of synbiotic administration have previously reported in patients with T2DM [23]. Despite more research into the effects of synbiotics on cardiovascular outcomes, most studies showed notable discrepancies in the current evidence. Previous evidences have proposed that the intake of synbiotics reduces inflammatory markers and oxidative stress [23,24,25], whereas others have found no effect on inflammatory biomarkers after the administration of synbiotics [26, 27].
We aimed to conduct the current comprehensive systematic review and meta-analysis of published randomized controlled trials (RCT)s to investigate the effects of probiotics or synbiotics consumption on inflammatory and oxidative stress biomarkers and serum adipokines concentration among patients with prediabetes and T2DM. To our knowledge, this is the first GRADE-assessed systematic review, meta-analysis, and meta-regression assessing a large number of subjects across different countries on the impact of probiotics or synbiotics supplementation on these biomarkers in patients with prediabetes and T2DM.
Materials and methods
This systematic review was conducted and reported in accordance with the 2021 updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [28].
Data sources and search strategies
A comprehensive literature search was performed independently by two investigators (K.N. and S.S.), applying the online databases including PubMed/MEDLINE, ISI Web of Science, Scopus, and Cochrane library, without specific time frames and language restriction, up to March 2022. The purpose of our search was to identify clinical trials studying the effects of probiotics or synbiotics on inflammatory, and oxidative stress biomarkers, adipokines and leptin, among patients with prediabetes or T2DM. We used the following MeSH and non-MeSH terms in our search strategy to identify potentially relevant studies: ((Probiotics OR probiotic OR Synbiotics OR synbiotic OR Lactobacillus OR Bifidobacterium) AND (Intervention OR “controlled trial” OR random OR randomized OR placebo OR randomly OR “clinical trial” OR Trial OR “randomized clinical trial” OR RCT OR trial OR trials “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR parallel OR “parallel study” OR “parallel trial”) AND (“diabetes” OR “type 2 diabetes mellitus” OR “T2DM” OR “type 2 diabetes” OR “T2D” OR “prediabetes”)) (see Supplementary Table 1 for search terms used across the various databases). Reference lists of the applicable research were manually screened to prevent any publications from being missed. Unpublished and/or non-human studies, as well as gray literature, were not included. After combining search results from different databases, duplicates were removed. EndNote X21 was used to manage the records. In addition, we conducted a manual search of studies fulfilling the eligibility criteria (i.e., searching the reference lists and citations).
Eligibility criteria
All the eligible studies that were included in our analysis in accordance with the PICOS strategy as follows: (1) Population: individuals older than 18 years and with physician’s diagnosis of prediabetes or T2DM; (2) Intervention: consumption of probiotics and synbiotics (of any form, such as tablet, capsule, powder, honey, milk, yogurt and bread) in terms of dose and frequency; (3) Comparators: comparison with placebo, usual care, or any pharmacological or non-pharmacological intervention(s); (4) Outcome: those which reported mean changes and their standard deviations (SDs) of inflammatory biomarkers including tumor necrosis factor- α (TNF-α), c-reactive protein (CRP), interlukin-6 (IL-6), adipocytokines (adiponectin and leptin), and serum biomarkers of oxidative stress including glutathione (GSH), malondialdehyde (MDA), total antioxidant capacity (TAC), and nitric oxide (NO) over the length of the study for both probiotic or synbiotic and control groups or reported the required data for calculation of the related effect sizes; and (5) Study design: having a parallel or cross-over design in a RCT setting (Table 1). If more than one article was published for one dataset, the more complete one was included. Clinical trials with an additional intervention group were considered as two separate studies.
The studies were unable to be considered if they: (1) had an open clinical trial design, (2) reported outcomes that were not been clearly declared, (3) designed as an experimental study, (4) had a non-experimental (case series, case studies, case–control, cross-sectional, cohort and other retrospective studies) design, and (5) were carried out on pregnant women, and children or adolescents.
Study selection
Two researchers (K.N. and S.S.) independently assessed titles and abstracts, as well as the full-text review process for articles retrieved using the search technique, and any discrepancies about inclusion and exclusion of studies were resolved by consensus. Inclusion and exclusion criteria were developed based on a systematic process that considered the context, population, and evaluated the exposures and outcomes of the studies.
Data extraction
A standardized, pre-piloted form (Excel) was used to extract data from the included studies. The parameters that were extracted were as follows: (a) name of the first author; (b) publication year; (c) individuals’ characteristics (mean age and sex); (d) the design of the study; (e) sample size (intervention and control groups); (f) type of probiotic and synbiotic administered; (g) dosage of probiotic and synbiotic; (h) length of intervention; (i) mean changes and their SDs of all the mentioned biomarkers throughout the trial for the intervention and control groups; (j) and the confounding variables adjusted in the analyses. If the reported units for each outcomes were less common, they were converted to the most commonly used unit. Any discrepancies and disagreements about the data extraction were determined by consensus or discussion with a third researcher (O.A.).
Risk of bias assessment
The methodological quality of each included clinical trial was assessed using the Cochrane quality assessment tool on a domain-based evaluation in this meta-analysis [29]. This tool contained seven domains including random sequence generation, allocation concealment, reporting bias, other source of bias, blinding (participants and personnel), blinding (outcome assessment), and incomplete outcome data. Each domain was given a “high risk” rating if the study comprised methodological defects that may have an effect on its findings, a “low risk” rating if there was no defect for that domain and an “unclear risk” rating if the information was not enough to determine the impact. If the study was “low risk” in all areas, it was considered a high-quality study with an absolutely low risk of bias. The risk of bias was assessed independently by two reviewers (Supplementary Table 2). The overall fact of evidence across the studies was sorted in accordance with the GRADE guidelines (Grading of Recommendations Assessment, Development, and Evaluation) Working Group. The quality of evidence may be categorized into four classifications in accordance with the corresponding evaluation criteria: high, moderate, low, and very low [30].
Data synthesis and analysis
In the probiotic/synbiotic and control groups, for each variable mean changes and their SDs were applied to acquire the overall related effect sizes. If no mean changes were reported, they were calculated by taking into account the changes in the concentration of each variable during the trial.
By applying the method of the previous study [31], interquartile ranges (IQRs), 95% confidence intervals (CIs), and standard errors (SEs) were converted to SDs. We also used a random-effects model that took into account variations between studies to get the overall effect sizes. I2 statistic and Cochrane’s Q test was applied for heterogeneity determination. I2 value > 50% or P < 0.05 for the Q-test was characterized as significant heterogeneity between studies [32, 33]. Subgroup analyses were conducted to find probable sources of heterogeneity based on the predefined variables such as intervention length (≥ 12 vs. < 12 weeks), intervention type (probiotic vs. synbiotic), participants’ health status (subjects with prediabetes vs. T2DM), baseline serum levels of CRP (≥ 3 vs. < 3 mg/l), TNF-α, IL-6, adiponectin, leptin, GSH, MDA, TAC, and NO. Also, we enforced the meta-regression to differentiate the confounders and linear relations among the effect size and sample size, duration of intervention, and intervention dosage. We used sensitivity analysis to determine whether the overall effect size depended upon a specific study. Therefore, we excluded studies one by one to determine the overall effect without that study [34]. The possibility of publication bias was investigated by the formal test of Begg, Egger regression and visual inspection of funnel plot [35]. The meta-analysis was conducted using the STATA® version 14.0 (StataCorp, College Station, Lakeway, TX, USA). p value < 0.05 was considered a significant level.
Results
Study selection
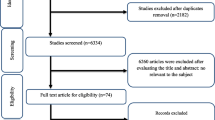
The literature search and screening process performed on this systematic review is indicated in Fig. 1. A total of 546 RCTs were retrieved from the searches. Of these, 264 duplicates were removed, leaving 282 records to be screened for eligibility by title and abstract. After excluding 220 articles, 62 papers were confirmed to assess in full text. Finally, 32 studies (39 effect sizes) with 2074 subjects measuring cardiovascular outcomes were included in this review.
Characteristics of the included studies
Table 2 is the summary of the general characteristics of the included investigations. In the current meta-analysis, 2074 participants were included (control = 956; case = 1118). Studies were published between 2012 and 2021 and were performed in Asia (n = 26) [2, 15, 20, 21, 23,24,25,26,27, 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], Europe (n = 3) [12, 19, 53], Africa (n = 1) [54], Oceania (n = 1) [3], and America (n = 1) [55]. All subjects were patients with T2DM [2, 12, 15, 19,20,21, 23,24,25,26,27, 37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55], except for two RCTs that studied individuals with prediabetes [3, 36]. Moreover, probiotic and synbiotic supplementation was used in 25 [2, 3, 12, 15, 19,20,21, 36,37,38,39,40,41,42,43,44,45,46,47, 49, 50, 52,53,54,55] and 7 RCTs [23,24,25,26,27, 48, 51], respectively. All RCTs were parallel design and their duration of supplementation ranged from 4 and 24 weeks and the sample sizes ranged from 22 to 136 participants. Also, participants’ baseline BMI ranged from 22.4 and 35.6 kg/m2 and ages from 46.4 to 66 years.
Effects of probiotic and synbiotic intake on inflammatory biomarkers
Twenty-six RCTs with 31 effect sizes evaluated CRPs as an outcome measure (intervention samples = 945/control samples = 824). Probiotic and synbiotic supplementation resulted in a reduction in CRPs (WMD − 0.62 mg/l; 95% CI − 0.80, − 0.44; p < 0.001) compared to placebo group and a large between-study heterogeneity was observed (I2 = 82.7%, p < 0.001) (Fig. 2a). According to the subgroup analyses, probiotic and synbiotic supplementation significantly decreased CRP in all subgroups except in studies among patients with normal baseline BMI (18.5–24.9 kg/m2) and individuals with prediabetes (Table 3), showing more potent effects in patients with T2DM (p < 0.001) compared to those with prediabetes (p = 0.07) and individuals with overweight (p < 0.001) and obesity (p = 0.001).
Forest plot of a random-effects meta-analysis of the effect of probiotic and synbiotic supplementation on a CRP; b TNF-α; c GSH d MDA e TAC f NO in individuals with T2DM. CI confidence interval, WMD weighted mean differences, CRP c-reactive Protein, TNF-α tumor necrosis factor-α, GSH glutathione, MDA malondialdehyde, TAC total antioxidant capacity, NO nitric oxide, T2DM type 2 diabetes mellitus
Pooled effect sizes from 10 RCTs with 12 effect sizes (intervention samples = 299/control samples = 264) showed that TNF-α concentrations reduced (WMD − 0.48 pg/ml; 95% CI − 0.81, − 0.15; p = 0.004) following probiotic and synbiotic supplementation compared to placebo consumption (Fig. 2b) with a considerable between-study heterogeneity (I2 = 84.8%, p < 0.001). In subgroup analyses, TNF-α reduction was associated with probiotic and synbiotic supplementation regardless of the length of the trial. TNF-α levels were only reduced in individuals with T2DM (p = 0.002), individuals with overweight (BMI = 25–29.9 kg/m2) (p = 0.006), and when probiotic was supplemented (p = 0.006) (Table 3).
Based on combining 13 effect sizes including 367 intervention samples and 328 control samples, a significant heterogeneity was seen for serum IL-6 levels (I2 = 52.3%, p = 0.014). However, we observed that probiotic and synbiotic supplementation did not significantly affect IL-6 levels (WMD = − 0.12 pg/ml; 95% CI − 0.40, 0.16, p = 0.391) (Supplementary Fig. 1a). Subgroup analyses revealed that IL-6 levels were significantly reduced in synbiotic supplementation (p = 0.03), but not in probiotic intake (p = 0.7). There was no difference in IL-6 based on the health status of individuals, study duration, and baseline BMI (Table 3).
Effects of probiotic and synbiotic intake on serum adipokines
Five and four RCTs with six and five effect sizes were investigated adiponectin (intervention samples = 137/control samples = 121) and leptin (intervention samples = 93/control samples = 74), respectively. There was no effect of probiotic and synbiotic supplementation on serum adiponectin (WMD = 0.66; 95% CI − 0.44, 1.77, p = 0.240) (Supplementary Fig. 1b) and leptin levels (WMD = − 2.29; 95% CI − 5.73, 1.15, p = 0.192) (Supplementary Fig. 1c). Significant heterogeneity between studies for both adiponectin (I2 = 59.4%, p = 0.031) and leptin (I2 = 82.0%, p < 0.001) was observed. However, subgroup analysis for adiponectin and leptin for baseline values, length of follow-up, health status of participants, type of supplementation, and baseline BMI for all subgroups was not possible due to a low number of studies (Table 3).
Effects of probiotic and synbiotic intake on oxidative stress
As indicated in Fig. 2c, pooled data from 12 RCTs with 13 effect sizes (intervention samples = 398/control samples = 371) showed that GSH concentrations were increased with probiotic and synbiotic supplementation compared to placebo (WMD 69.80 µmol/l; 95% CI 33.65, 105.95, p < 0.001), with a considerable between-study heterogeneity (I2 = 80.5%, p < 0.001). Data from subgroup analyses showed that probiotic/synbiotic administration were associated with increased GSH irrespective of the trial duration and baseline BMI values. In addition, GSH was only reduced with supplementation with probiotic and with any duration (Table 3).
Overall, probiotic and synbiotic supplementation decreased MDA concentrations (WMD − 0.51 µmol/l; 95% CI − 0.73, − 0.30; p < 0.001) (Fig. 2d), with a large heterogeneity seen between studies (I2 = 84.4%, p < 0.001) in a meta-analysis of 11 RCTs with 12 effect sizes (intervention samples = 330/control samples = 305). The findings from the subgroup analyses showed that probiotic and synbiotic intake reduced MDA regardless of the type of intervention and baseline BMI values, but only in participants who consumed probiotic and synbiotic for twelve or more weeks probiotic and synbiotic compared to controls (Table 3).
Meta-analysis of TAC combined data from 11 studies with 12 effect sizes (intervention samples = 342/control samples = 315). Overall, probiotic and synbiotic intake increased in TAC (WMD 73.59 mmol/l; 95% CI 33.24, 113.95, p < 0.001) (Fig. 2e). In subgroup analyses, elevated amount of TAC following the probiotic/synbiotic supplementation was irrespective of the baseline value for BMI. Additionally, the effects were stronger with longer duration (≥ 12 weeks (p < 0.001)) and with probiotics (p = 0.003) (Table 3).
The overall findings from 8 trials with 9 effect sizes (intervention samples = 264/control samples = 237) revealed that intervention with probiotic or synbiotic significantly increased NO levels (WMD 7.49 µmol/l; 95% CI 3.12, 11.86; p = 0.001) (Fig. 2f) with a significant between-study heterogeneity (I2 = 93.7%, p < 0.001). In subgroup analyses, NO levels were elevated regardless of the length of trial but only increased in those probiotic supplementation and individuals with obesity (Table 3).
Publication bias
We evaluated Egger’s regression test and found that there was a significant publication bias for CRP (p = 0.025), TNF-α (p = 0.034), NO (p = 0.024), and TAC (p = 0.009). However, no evidence of publication bias was observed for reports evaluating the influences of probiotic or synbiotic supplementation on IL-6 (p = 0.653), leptin (p = 0.369), adiponectin (p = 0.281), GSH (p = 0.141), and MDA (p = 0.619). Furthermore, there was no publication bias for CRP (p = 0.973), IL-6 (p = 0.640), TNF-α (p = 0.350), leptin (p = 0.221), adiponectin (p = 0.707), GSH (p = 0.127), NO (p = 0.251) and MDA (p = 0.945) according to Begg’s test. Publication bias was confirmed only for TAC (p = 0.016) based on Begg’s test. The funnel plots also proved these findings (Supplementary Fig. 2).
Meta-regression analysis
The analysis was carried out to assess the correlation among intervention duration (weeks) of probiotic or synbiotic supplementation and CRP, IL-6, TNF-α, TAC, GSH, NO, MDA, leptin and adiponectin levels. Based on the analysis, the associations between absolute changes in these factors and the duration of the intervention were not linear (Supplementary Fig. 3).
Grading of evidence
An evaluation of the quality of evidence using the GRADE approach is presented in Table 4. Low quality of evidence was detected for CRP, TNF-α, GSH, MDA, and NO for a very serious inconsistency (I2 = 82.7%, I2 = 88.5%, I2 = 80.5%, I2 = 84.4%, and I2 = 93.7% for heterogeneity, respectively), whereas the low quality of evidence for IL-6 and adiponectin was due to serious inconsistency (I2 = 52.3% and I2 = 59.4% for heterogeneity, respectively) and serious imprecision (wide CI). However, the evidence relating to leptin and TAC was downgraded to very low quality, because of the very serious inconsistency (I2 = 82.0% and I2 = 83.1% for heterogeneity, respectively) and serious imprecision (wide CI) for leptin and serious publication bias (p = 0.016) for TAC.
Sensitivity analysis
This analysis for CRP, TNF-α, IL-6, adiponectin, leptin, GSH, MDA, TAC, and NO did not indicate evidence of sensitivity.
Discussion
In the present meta-analysis, we evaluated the effectiveness of probiotics or synbiotics on inflammatory and oxidative stress biomarkers in patients with prediabetes and T2DM. We have demonstrated that the consumption of probiotics or synbiotics is associated with reductions in inflammatory status as measured by decreased levels of CRP, and TNF-α, without any significant changes in IL-6. Regarding oxidative stress, there was a significant decrease in MDA and an increase in TAC, GSH, and NO levels. However, probiotic or synbiotic administration did not alter leptin or adiponectin levels in individuals with prediabetes and T2DM. This suggests that the supplementation of probiotics or synbiotics could be a useful intervention to improve cardiometabolic outcomes in patients with prediabetes and T2DM.
There were five recent meta-analyses on the effects of probiotic or synbiotic supplementation on inflammatory and oxidative stress biomarkers. These focused mainly on patients with diabetic nephropathy [16, 56] or investigated the effects of only probiotic supplementation [16,17,18, 57, 58]. In addition, two meta-analyses investigated the effectiveness of probiotics or synbiotics supplementation on various outcomes. The endpoints for the first one were CRP, TAC, MDA, NO, and GSH among individuals with T2DM [56], while the studied outcomes for the other one were TNF-α, CRP, IL-6, and NO among subjects with diabetes [59]. In other words, they included fewer RCTs and limited indicators of inflammation and oxidative stress. The current meta-analysis is also the first GRADE-assessed study summarizing publications on the effects of probiotics or synbiotics supplementation on biomarkers of inflammation, oxidative stress, and circulating adipokines levels in individuals with prediabetes and T2DM. Our subgroup analysis based on study duration indicated that both short (< 12 weeks) and long-term (≥ 12 weeks) supplementation led to a significant improvement in CRP, TNF-α, GSH, and NO following the interventions. However, probiotics or synbiotics had more favorable effects on MDA and TAC when interventions were longer than 12 weeks. Moreover, the modulating effects of probiotics or synbiotics on inflammation (CRP and TNF-α levels) were more pronounced in patients with T2DM compared to individuals with prediabetes. This suggests that the effects of probiotics or synbiotics are more pronounced in patients with heightened inflammation. Probiotic or synbiotic products also showed favorable anti-oxidative effects in individuals with T2DM and prediabetes in overweight and obese populations by a significant decrease in MDA and an increase in TAC and GSH concentrations. However, a decline in NO level was more significant in patients with obesity than overweight patients or those with normal baseline BMI. Additionally, probiotics or synbiotics did not improve inflammatory status (CRP and TNF-α) in individuals with normal body weight, suggesting that probiotics or synbiotics consumption may not be beneficial in patients with normal body weight and/or BMI.
Observational studies provided further evidence on the link between inflammation and T2DM. A meta-analysis by Wang et al. showed that elevated levels of pro-inflammatory markers, including IL-6 and CRP, are significantly associated with an increased risk of T2DM [60]. Moreover, previous studies showed the positive effects of probiotics or synbiotics in improving glycemic profile and control (HBA1c) in patients with T2DM [61]. This was confirmed by a meta-analysis of 18 RCTs in patients with T2DM that showed that probiotics improved glycemic profile by reducing glucose, insulin, and HbA1c [62]. These findings suggest that probiotics or synbiotics reduce chronic low-grade inflammation associated with T2DM, which may result in a lower risk of diabetes complications [63, 64].
However, several meta-analyses have been performed on the effects of probiotics or synbiotics on biomarkers of oxidative stress. Pourrajab et al. reported that probiotic or synbiotic supplementation could significantly increase serum TAC, GSH, and NO and reduce MDA levels in adults [65]. Likewise, Hemati et al. also showed that probiotic supplementation improved antioxidant resistance and increase antioxidant enzymes in the body by increasing TAC, GSH, SOD, and NO and decreasing MDA in various populations [66]. Similar findings have been reported in other meta-analyses [67, 68].
The findings of the previous systematic reviews and meta-analyses (with smaller sample sizes) showed that probiotics or synbiotics supplementation might help improve biomarkers of oxidative stress by decreasing MDA and increasing TAC, GSH, and NO and alleviate inflammation through a decline in CRP and TNF-α with no change in IL-6 levels [56, 59]. Our findings were similar to the previous meta-analyses, which underlined the favorable effects of probiotics or synbiotics consumption on inflammatory and oxidative stress biomarkers.
The mechanisms underlying the modulation effects of probiotics or synbiotics on inflammation and oxidative stress remain largely unclear. However, we postulate four possible explanations for the relationship between probiotics or synbiotics and inflammation and oxidative stress. First, intestinal microorganisms produce short-chain fatty acids (SCFAs) [69]. The production of SCFAs can decrease the enzymatic synthesis of CRP in the liver [70]. Second, reports have shown that hyperglycemia stimulates the nuclear factor kappa B (NF-kB) pathway. The suppression of NF-kB pathway results in decreased levels of pro-inflammatory cytokine IL-6 [71]. IL-6 induces CRP gene expression and inhibits the NF-kB/IL-6 pathway which results in decreased CRP [72, 73]. Previous studies showed that probiotics or synbiotics might have hypoglycemic properties [74]; therefore, probiotic or synbiotic supplementation might modulate inflammation and oxidative stress by controlling blood glucose. Third, dyslipidemia in patients with T2DM and prediabetes is closely linked to inflammation and oxidative stress [75]. Since the lipid profile-improving influences of probiotic or synbiotic supplementation have been well documented [36, 55, 76,77,78], these supplements may reduce the biomarkers of inflammation and oxidative stress. Fourth, the antioxidative effects of probiotics or synbiotics have been reported [79]. It is well-known that antioxidants can modulate oxidative stress and inflammation [80]. While these findings are attention grabbing, further research is still needed to verify and define the possible mechanisms related to the effects of probiotics or synbiotics on oxidative stress and inflammation in patients with T2DM and prediabetes.
Strengths and limitations
This meta-analysis appears to contain many strengths and some limitations. The high number of studies and high overall sample size is the main strength of this study. Moreover, we analyzed a wide range of inflammatory and oxidative stress biomarkers linked to the pathogenesis of T2DM.
There is no publication bias in the analysis. In addition, a meta-regression analysis was performed to assess the association between pooled effect sizes, doses, and supplementation periods. Finally, based on the GRADE guidelines, we graded the overall certainty of evidence across the studies. Regarding limitations, since all the trials except one were equal to or less than 3 months, our analysis cannot assess the long-term effects of probiotic or synbiotic supplementation on inflammation and oxidative stress profile and circulating adipokines level. Moreover, our analysis showed high statistical heterogeneity. This may be due to a variety of methodologies (different study designs) and/or differences in treatment regimens (doses/durations) or the intervention type (probiotic or synbiotic). In addition, the number of studies conducted on patients with prediabetes was limited. Finally, many clinical studies included in the current study were from Iran, limiting the study to reflect diverse populations worldwide and generalizing the results.
Conclusions
In conclusion, our findings show that probiotics or synbiotics intake may reduce cardiovascular disease risk in patients with prediabetes and T2DM, by decreasing CRP, TNF-α, and MDA and increasing TAC, GSH, and NO levels, but have no significant effects on IL-6, adiponectin, and leptin when compared with a control group. Patients with T2DM seem to benefit more from this intervention than individuals with prediabetes. In addition, probiotic or synbiotic products also showed favorable anti-oxidative effects in individuals with T2DM and prediabetes in overweight and obese populations. Large-scale RCTs with longer follow-ups are necessary to establish the long-term effects of these supplements in both prediabetes and T2DM. Furthermore, investigating the mechanisms involved in probiotic or synbiotic effects on the studied outcomes is crucial to determining how these interventions target specific signaling pathways.
Availability of data and material
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JC, Mbanya JC (2022) IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
Toejing P, Khampithum N, Sirilun S, Chaiyasut C, Lailerd N (2021) Influence of Lactobacillus paracasei HII01 supplementation on glycemia and inflammatory biomarkers in type 2 diabetes: a randomized clinical trial. Foods 10(7):1455
Tay A, Pringle H, Penning E, Plank LD, Murphy R (2020) PROFAST: a randomized trial assessing the effects of intermittent fasting and Lacticaseibacillusrhamnosus probiotic among people with prediabetes. Nutrients 12(11):3530
Naderpoor N, Mousa A, Gomez-Arango LF, Barrett HL, Dekker Nitert M, de Courten B (2019) Faecal microbiota are related to insulin sensitivity and secretion in overweight or obese adults. J Clin Med 8(4):452
Snelson M, de Pasquale C, Ekinci EI, Coughlan MT (2021) Gut microbiome, prebiotics, intestinal permeability and diabetes complications. Best Pract Res Clin Endocrinol Metab 35(3):101507
Abhari K, Saadati S, Yari Z, Hosseini H, Hedayati M, Abhari S, Alavian SM, Hekmatdoost A (2020) The effects of Bacillus coagulans supplementation in patients with non-alcoholic fatty liver disease: a randomized, placebo-controlled, clinical trial. Clin Nutr ESPEN 39:53–60
Cox A, Zhang P, Bowden D, Devereaux B, Davoren P, Cripps A, West N (2017) Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab 43(2):163–166
Naseri K, Dabiri H, Rostami-Nejad M, Yadegar A, Houri H, Olfatifar M, Sadeghi A, Saadati S, Ciacci C, Iovino P, Zali MR (2021) Influence of low FODMAP-gluten free diet on gut microbiota alterations and symptom severity in Iranian patients with irritable bowel syndrome. BMC Gastroenterol 21(1):292
Okubo H, Nakatsu Y, Kushiyama A, Yamamotoya T, Matsunaga Y, Inoue MK, Fujishiro M, Sakoda H, Ohno H, Yoneda M, Ono H, Asano T (2018) Gut microbiota as a therapeutic target for metabolic disorders. Curr Med Chem 25(9):984–1001
Ostadmohammadi S, Azimirad M, Houri H, Naseri K, Javanmard E, Mirjalali H, Yadegar A, Sadeghi A, AsadzadehAghdaei H, Zali MR (2021) Characterization of the gut microbiota in patients with primary sclerosing cholangitis compared to inflammatory bowel disease and healthy controls. Mol Biol Rep 48(7):5519–5529
Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, Tarniceriu CC, Maranduca MA, Lacatusu CM, Floria M, Serban IL (2020) Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients 12(12):3719
Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I (2018) Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr 12(5):617–624
Roobab U, Batool Z, Manzoor MF, Shabbir MA, Khan MR, Aadil RM (2020) Sources, formulations, advanced delivery and health benefits of probiotics. Curr Opin Food Sci 32:17–28
BordaloTonucci L, Dos Santos KMO, De Luces Fortes Ferreira CL, Ribeiro SMR, De Oliveira LL, Martino HSD (2017) Gut microbiota and probiotics: focus on diabetes mellitus. Crit Rev Food Sci Nutr 57(11):2296–2309
Rezaei M, Sanagoo A, Jouybari L, Behnampoo N, Kavosi A (2017) The effect of probiotic yogurt on blood glucose and cardiovascular biomarkers in patients with type II diabetes: a randomized controlled trial. Evidence Based Care 6(4):26–35
AbdelQadir YH, Hamdallah A, Sibaey EA, Hussein AS, Abdelaziz M, AbdelAzim A, Ragab KM, Helmy SK, Nourelden AZ (2020) Efficacy of probiotic supplementation in patients with diabetic nephropathy: a systematic review and meta-analysis. Clin Nutr ESPEN 40:57–67
Ardeshirlarijani E, Tabatabaei-Malazy O, Mohseni S, Qorbani M, Larijani B, Baradar Jalili R (2019) Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. DARU J Pharmac Sci 27(2):827–837
Bohlouli J, Namjoo I, Borzoo-Isfahani M, Kermani MAH, Zehi ZB, Moravejolahkami AR (2021) Effect of probiotics on oxidative stress and inflammatory status in diabetic nephropathy: a systematic review and meta-analysis of clinical trials. Heliyon 7(1):e05925
Hove KD, Brøns C, Færch K, Lund SS, Rossing P, Vaag A (2015) Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind placebo-controlled study. Eur J Endocrinol 172(1):11–20
Sato J, Kanazawa A, Azuma K, Ikeda F, Goto H, Komiya K, Kanno R, Tamura Y, Asahara T, Takahashi T, Nomoto K, Yamashiro Y, Watada H (2017) Probiotic reduces bacterial translocation in type 2 diabetes mellitus: a randomised controlled study. Sci Rep 7(1):12115
Sabico S, Al-Mashharawi A, Al-Daghri NM, Wani K, Amer OE, Hussain DS, Ansari MGA, Masoud MS, Alokail MS, McTernan PG (2019) Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: a randomized, double-blind, placebo-controlled trial. Clin Nutr 38(4):1561–1569
Krumbeck JA, Maldonado-Gomez MX, Ramer-Tait AE, Hutkins RW (2016) Prebiotics and synbiotics: dietary strategies for improving gut health. Curr Opin Gastroenterol 32(2):110–119
Kooshki AA, Tofighiyan T, Rakhshani MH (2015) Effects of synbiotics on inflammatory markers in patients with type 2 diabetes mellitus. Global J Health Sci 7(7):1
Asemi Z, Khorrami-Rad A, Alizadeh SA, Shakeri H, Esmaillzadeh A (2014) Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 33(2):198–203
Soleimani A, Motamedzadeh A, ZarratiMojarrad M, Bahmani F, Amirani E, Ostadmohammadi V, Tajabadi-Ebrahimi M, Asemi Z (2019) The effects of synbiotic supplementation on metabolic status in diabetic patients undergoing hemodialysis: a randomized, double-blinded, placebo-controlled trial. Probiotics Antimicrob Proteins 11(4):1248–1256
Kanazawa A, Aida M, Yoshida Y, Kaga H, Katahira T, Suzuki L, Tamaki S, Sato J, Goto H, Azuma K, Shimizu T, Takahashi T, Yamashiro Y, Watada H (2021) Effects of synbiotic supplementation on chronic inflammation and the gut microbiota in obese patients with type 2 diabetes mellitus: a randomized controlled study. Nutrients 13(2):558
Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, Hadaegh H, Hijijafari M, Abedi F, Asemi Z (2014) Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab 65(1):34–41
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/bmj.d5928
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5(1):1–10
Brondani LA, Assmann TS, de Souza BM, Boucas AP, Canani LH, Crispim D (2014) Meta-analysis reveals the association of common variants in the uncoupling protein (UCP) 1–3 genes with body mass index variability. PLoS ONE 9(5):e96411
Zahedi H, Djalalinia S, Sadeghi O, Asayesh H, Noroozi M, Gorabi AM, Mohammadi R, Qorbani M (2018) Dietary inflammatory potential score and risk of breast cancer: systematic review and meta-analysis. Clin Breast Cancer 18(4):e561–e570
Tobias A (1999) Assessing the influence of a single study in the meta-anyalysis estimate. STATA Tech Bull 47:15–17
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Toshimitsu T, Gotou A, Sashihara T, Hachimura S, Shioya N, Suzuki S, Asami Y (2020) Effects of 12-week ingestion of yogurt containing Lactobacillus plantarum OLL2712 on glucose metabolism and chronic inflammation in prediabetic adults: a randomized placebo-controlled trial. Nutrients 12(2):374
Soleimani A, Mojarrad MZ, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, Jafari P, Esmaillzadeh A, Asemi Z (2017) Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int 91(2):435–442
Raygan F, Rezavandi Z, Bahmani F, Ostadmohammadi V, Mansournia MA, Tajabadi-Ebrahimi M, Borzabadi S, Asemi Z (2018) The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr 10(1):1–7
Mohseni S, Bayani M, Bahmani F, Tajabadi-Ebrahimi M, Bayani MA, Jafari P, Asemi Z (2018) The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev 34(3):e2970
Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani G-A, Mohammadi F (2014) Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4(2):83
Miraghajani M, Zaghian N, Mirlohi M, Feizi A, Ghiasvand R (2017) The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: a randomized controlled clinical trial. J Ren Nutr 27(5):317–324
MazrueiArani N, Emam-Djomeh Z, Tavakolipour H, Sharafati-Chaleshtori R, Soleimani A, Asemi Z (2019) The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double-blind, controlled trial. Probiotics Antimicrob Proteins 11(4):1195–1201
Mazloom Z, Yousefinejad A, Dabbaghmanesh MH (2013) Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci 38(1):38
Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, Asemi Z (2018) Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct 9(9):4763–4770
Hsieh M-C, Tsai W-H, Jheng Y-P, Su S-L, Wang S-Y, Lin C-C, Chen Y-H, Chang W-W (2018) The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep 8(1):1–11
Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak M-Y (2017) Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr 56(4):1535–1550
Feizollahzadeh S, Ghiasvand R, Rezaei A, Khanahmad H, Hariri M (2017) Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Probiotics Antimicrob Proteins 9(1):41–47
Farrokhian A, Raygan F, Soltani A, Tajabadi-Ebrahimi M, Sharifi Esfahani M, Karami AA, Asemi Z (2019) The effects of synbiotic supplementation on carotid intima-media thickness, biomarkers of inflammation, and oxidative stress in people with overweight, diabetes, and coronary heart disease: a randomized, double-blind, placebo-controlled trial. Probiotics Antimicrob Proteins 11(1):133–142
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V (2012) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28(5):539–543
Bayat A, Azizi-Soleiman F, Heidari-Beni M, Feizi A, Iraj B, Ghiasvand R, Askari G (2016) Effect of Cucurbita ficifolia and probiotic yogurt consumption on blood glucose, lipid profile, and inflammatory marker in type 2 diabetes. Int J Prev Med 7:30
Bahmani F, Tajadadi-Ebrahimi M, Kolahdooz F, Mazouchi M, Hadaegh H, Jamal A-S, Mazroii N, Asemi S, Asemi Z (2016) The consumption of synbiotic bread containing Lactobacillus sporogenes and inulin affects nitric oxide and malondialdehyde in patients with type 2 diabetes mellitus: randomized, double-blind, placebo-controlled trial. J Am Coll Nutr 35(6):506–513
Asemi Z, Zare Z, Shakeri H, Sabihi S-S, Esmaillzadeh A (2013) Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab 63(1–2):1–9
Mobini R, Tremaroli V, Ståhlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, BertéusForslund H, Perkins R, Bäckhed F (2017) Metabolic effects of L actobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 19(4):579–589
Ismail A, Darwish O, Tayel D, Elneily D, Elshaarawy G (2021) Impact of probiotic intake on the glycemic control, lipid profile and inflammatory markers among patients with type 2 diabetes mellitus. Clin Diabetol 10(6):468–475
Tonucci LB, Dos Santos KMO, de Oliveira LL, Ribeiro SMR, Martino HSD (2017) Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin Nutr 36(1):85–92
Zheng HJ, Guo J, Jia Q, Huang YS, Huang W-J, Zhang W, Zhang F, Liu WJ, Wang Y (2019) The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 142:303–313
Ding L-N, Ding W-Y, Ning J, Wang Y, Yan Y, Wang Z-B (2021) Effects of probiotic supplementation on inflammatory markers and glucose homeostasis in adults with type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol 12:770861–770861
Kocsis T, Molnár B, Németh D, Hegyi P, Szakács Z, Bálint A, Garami A, Soós A, Márta K, Solymár M (2020) Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci Rep 10(1):1–14
Tabrizi R, Ostadmohammadi V, Lankarani KB, Akbari M, Akbari H, Vakili S, Shokrpour M, Kolahdooz F, Rouhi V, Asemi Z (2019) The effects of probiotic and synbiotic supplementation on inflammatory markers among patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur J Pharmacol 852:254–264
Wang X, Bao W, Liu J, OuYang Y-Y, Wang D, Rong S, Xiao X, Shan Z-L, Zhang Y, Yao P (2013) Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36(1):166–175
Akbari V, Hendijani FJ (2016) Effects of probiotic supplementation in patients with type 2 diabetes: systematic review and meta-analysis. Nutr Rev 74(12):774–784
Wang X, Juan Q-F, He Y-W, Zhuang L, Fang Y-Y, Wang Y-H (2017) Metabolism, multiple effects of probiotics on different types of diabetes: a systematic review and meta-analysis of randomized placebo-controlled trials. J Pediatr Endocrinol Metab 30(6):611–622
Barengolts E, Smith ED, Reutrakul S, Tonucci L, Anothaisintawee T (2019) The effect of probiotic yogurt on glycemic control in type 2 diabetes or obesity: a meta-analysis of nine randomized controlled trials. Nutrients 11(3):671
Fernandez MA, Marette A (2017) Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties. Adv Nutr 8(1):155S-164S
Pourrajab B, Fatahi S, Sohouli MH, Găman M-A, Shidfar F (2021) The effects of probiotic/synbiotic supplementation compared to placebo on biomarkers of oxidative stress in adults: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 62(2):490–507
Heshmati J, Farsi F, Shokri F, Rezaeinejad M, Almasi-Hashiani A, Vesali S, Sepidarkish M (2018) A systematic review and meta-analysis of the probiotics and synbiotics effects on oxidative stress. J Funct Foods 46:66–84
Zhao J, Yu L, Zhai Q, Tian F, Zhang H, Chen W (2020) Effects of probiotic administration on hepatic antioxidative parameters depending on oxidative stress models: a meta-analysis of animal experiments. J Funct Foods 71:103936
Amirani E, Milajerdi A, Mirzaei H, Jamilian H, Mansournia MA, Hallajzadeh J, Ghaderi A (2020) The effects of probiotic supplementation on mental health, biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med 49:102361
Markowiak-Kopeć P, Śliżewska K (2020) The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 12(4):1107
Hegazy SK, El-Bedewy MM (2010) Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J Gastroenterol 16(33):4145
Ashtary-Larky D, Rezaei Kelishadi M, Bagheri R, Moosavian SP, Wong A, Davoodi SH, Khalili P, Dutheil F, Suzuki K, Asbaghi O (2021) The effects of nano-curcumin supplementation on risk factors for cardiovascular disease: a GRADE-assessed systematic review and meta-analysis of clinical trials. Antioxidants 10(7):1015
Asbaghi O, Ashtary-Larky D, Bagheri R, Moosavian SP, Nazarian B, Afrisham R, Kelishadi MR, Wong A, Dutheil F, Suzuki K (2021) Effects of folic acid supplementation on inflammatory markers: a grade-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Nutrients 13(7):2327
Asbaghi O, Ashtary-Larky D, Mousa A, Rezaei Kelishadi M, Moosavian SP (2021) The effects of soy products on cardiovascular risk factors in patients with type 2 diabetes: a systematic review and meta-analysis of clinical trials. Adv Nutr. https://doi.org/10.1093/advances/nmab121
Cicero AFG, Fogacci F, Bove M, Giovannini M, Borghi C (2021) Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: a randomized placebo-controlled clinical trial. Eur J Nutr 60(2):655–663
Tangvarasittichai S (2015) Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 6(3):456
Ahmadi S, Jamilian M, Tajabadi-Ebrahimi M, Jafari P, Asemi Z (2016) The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. Br J Nutr 116(8):1394–1401
Mahboobi S, Rahimi F, Jafarnejad S (2018) Effects of prebiotic and synbiotic supplementation on glycaemia and lipid profile in type 2 diabetes: a meta-analysis of randomized controlled trials. Adv Pharmac Bull 8(4):565
Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, Raygan F, Karamali F, Razzaghi R, Taheri S, Asemi Z (2017) A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes 125(01):21–27
Madhu AN, Amrutha N, Prapulla SG (2012) Characterization and antioxidant property of probiotic and synbiotic yogurts. Probiotics Antimicrob Proteins 4(2):90–97
Namkhah Z, Ashtary-Larky D, Naeini F, Clark CC, Asbaghi O (2021) Does vitamin C supplementation exert profitable effects on serum lipid profile in patients with type 2 diabetes? A systematic review and dose-response meta-analysis. Pharmacol Res 169:105665
Acknowledgements
The authors of this paper thank the authors of the articles included in the meta-analysis for sending the requested aggregate data for the meta-analysis.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors reported there is no funding associated with the work featured in this article.
Author information
Authors and Affiliations
Contributions
KN and SS developed the search strategy, conducted the search and screening, extracted the data, appraised the quality of evidence, performed the data analysis, and drafted and revised the manuscript. FG and DA-L drafted and revised the manuscript. OA and AS appraised the quality of evidence and revised the manuscript. RA co-developed the protocol and search strategy, revised the manuscript. BC designed the protocol and determined the scope of the review, revised the manuscript, and is the guarantor of the review.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest related to this article.
Consent for publication
All authors reviewed and approved the final submitted version of the paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naseri, K., Saadati, S., Ghaemi, F. et al. The effects of probiotic and synbiotic supplementation on inflammation, oxidative stress, and circulating adiponectin and leptin concentration in subjects with prediabetes and type 2 diabetes mellitus: a GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Eur J Nutr 62, 543–561 (2023). https://doi.org/10.1007/s00394-022-03012-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03012-9