Abstract
Background
The present systematic review and meta-analysis sought to evaluate the effects of conjugated linoleic acid (CLA) supplementation on glycemic control, adipokines, cytokines, malondialdehyde (MDA) and liver function enzymes in patients at risk of cardiovascular disease.
Methods
Relevant studies were obtained by searching the PubMed, SCOPUS and Web of Science databases (from inception to January 2023). Weighted mean differences (WMD) and 95% confidence intervals (CIs) were pooled using a random-effects model. Heterogeneity, sensitivity analysis, and publication bias were reported using standard methods.
Results
A pooled analysis of 13 randomized controlled trials (RCTs) revealed that CLA supplementation led to a significant increment in fasting blood glucose (FBG) (WMD: 4.49 mg/dL; 95%CI: 2.39 to 6.59; P < 0.001), and aspartate aminotransferase (AST) (WMD: 2.54 IU/L; 95%CI: 0.06 to 5.01; P = 0.044). Moreover, CLA supplementation decreased leptin (WMD: -1.69 ng/ml; 95% CI: -1.80 to -1.58; P < 0.001), and interleukin 6 (IL-6) (WMD: -0.44 pg/ml; 95%CI: -0.86 to -0.02; P = 0.037). However, there was no effect on hemoglobin A1c (HbA1c), homeostatic model assessment for insulin resistance (HOMA-IR), C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and alanine aminotransferase (ALT) adiponectin compared to the control group.
Conclusion
Our findings showed the overall favorable effect of CLA supplementation on the adipokines and cytokines including serum IL-6, and leptin, while increasing FBG and AST. It should be noted that the mentioned metabolic effects of CLA consumption were small and may not reach clinical importance.
Prospero registeration cod
CRD42023426374.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVDs) create huge morbidity and mortality risks worldwide. They place a significant economic burden on the healthcare system. Unhealthy lifestyles like obesity, alcohol consumption, unhealthy diet, and physical inactivity are traditional risk factors of CVDs. Other risk factors linked to CVDs are genetic predisposition and presence of chronic diseases [1]. Diabetes is one well-established example. Based on studies, controlling the glycemic profile may be beneficial in preventing CVD events, by decreasing oxidative stress and vascular complications [2]. Deregulation of adipokines which is linked to obesity can cause a low-grade, chronic inflammatory state that may develop CVDs [3]. Pro-inflammatory cytokines and a parameter of oxidative stress, MDA, are also biomarkers for predicting the risk of CVDs [4, 5]. Moreover, CVDs are associated with the accumulation of liver fat and increased levels of the liver enzymes [6]. Therefore, effective strategies are highly needed for treating high-risk people for CVDs to help reduce the complications.
Among different possible alternative strategies to prevent CVDs (medical therapy, surgical treatments, and dietary supplements), nutraceuticals have gained public interest [7]. One nutraceutical which may have a role in modulating CVD risks is conjugated linoleic acid (CLA). CLA is an omega-6 polyunsaturated fatty acid found mostly in meat and dairy products. It is a family of positional and geometric isomers of linoleic acid. Cis-9, trans-11 and trans-10, cis-12 are major isomers of CLA in food [8, 9]. Some effects of CLA can be isomer-specific and difference in the intake of CLA isomers may influence the results of studies conducting on CLA. Thus, dietary supplementation of CLA, with different isomer ratios, has drawn the attention of researchers in healthcare systems.
The relationship between CLA consumption and glycemic profile still needs to be clarified. Eight weeks of supplementation with CLA showed efficacy of this supplement in decreasing body weight in individuals with insulin resistance [10]. A study working on obese children without diabetes revealed that CLA improved fasting insulin and homeostatic model assessment for insulin resistance (HOMA-IR)[11]. A meta-analysis on 32 randomized controlled trials (RCTs) indicated no effects of CLA consumption on fasting blood glucose (FBG) [12]. Furthermore, supplementing with trans-10,cis-12 isomer of CLA for 12 weeks increased insulin resistance and fasting glucose in abdominally obese men [13]. Consumption of another active isomer of CLA (cis-9,trans-11) for three months also increased insulin resistance in abdominally obese men [14].
CLA seems to elevate C-reactive protein (CRP) levels [15,16,17,18]. However, CLA effects on inflammatory cytokines (tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6)) and adipokines (adiponectin and leptin) remain unanswered. In some meta-analyses CLA consumption increased TNF-α [17], decreased TNF-α and IL-6 [15, 17], and caused no changes in IL-6 [18]. Decreasing effect of CLA consumption on circulating leptin was observed in one study [19], while in another study no effect was shown [15]. In a meta-analysis conducted by Rastgoo et al. (2023), CLA supplementation did not change adiponectin, but Mazidi et al. (2017) showed a significant reduction effect of CLA on adiponectin levels [18].
Results of the efficacy of CLA on liver enzymes and MDA are inconclusive. No changes of aspartate aminotransferase (AST) / alanine aminotransferase (ALT) activation were observed after 12 weeks of CLA intake in obese and overweight women [20]. However, a meta-analysis analyzing 13 RCTs, CLA increased AST significantly and ALT non-significantly [21]. A meta-analysis of 11 trials indicated that intervention with CLA could not change malondialdehyde (MDA) [22]. Conversely, another meta-analysis, also including 11 RCTs, showed that CLA supplementation decreased MDA levels, significantly [23]. Interestingly, another meta-analysis conducted by Haghighat et al. (2022) proposed that CLA may increase AST/ ALT and reduce MDA levels or cause no change [24].
Consequently, to detect the inconsistency, the present systematic review and meta-analysis aimed to update previous meta-analyses and include all subsequent trials that investigated the effects of CLA supplementation on glycemic control, adipokine, cytokine, MDA, and liver function enzymes in patients at risk of cardiovascular diseases.
Materials and methods
Search strategy and study selection
To conduct this study, the protocol of Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) was selected between the various methods for reporting systematic reviews and meta-analyses [25]. The literature was searched comprehensively in the various online databases, including PubMed, Scopus, and ISI Web of Science, to find relevant studies without any date or language limitation up to January 2023. Therefore, the following search terms in titles and abstracts were searched (supplementary file 1). Moreover, the Google scholar database was searched manually. The Endnote software was applied as a screening tool for included studies. Search strategy and study selection were conducted by two separate investigators.
Eligibility criteria
All studies with the following features were included in this meta-analysis: 1) randomized controlled trials (RCTs) that evaluated the effects of CLA supplementation on these factors as an outcome ( FBG, Insulin, HbA1c, HOMA-IR, CRP, TNF-α, IL-6, leptin, adiponectin, AST, ALT), with a control group, 2) studies conducted on adults (≥ 18 years), 3) studies used CLA supplementation as an intervention, 4) studies with parallel or crossover designs, 5) studies with outcome reporting at the beginning and the end of the intervention, 6) studies conducted on subjects at risk of CVDs (being over-weight and obese, having metabolic syndrome, type 2 diabetes mellitus, hypertension, and hyperlipidemia, atherosclerotic patients and non-alcoholic fatty liver disease).
Exclusion criteria
By analyzing the full text of the articles, the following studies were excluded: 1) animal, review, ecological, and observational studies, 2) studies conducted on individuals younger than 18 years, 3) studies without randomization or placebo or control groups, 4) studies conducted on healthy individuals.
Data extraction
Records were screened primarily by two separate investigators following the title and abstract assessment to detect eligibility. Next, to determine if the potential studies could be included in the study, their full texts were reviewed closely. Ultimately, the following data were extracted: the name of the first author, the year of the publication, the location of the study, the study design, the sample size in each group, the characteristics of the subjects such as mean age, sex, body mass index (BMI), health status, the doses of CLA used for the intervention, the duration of the interventions, the mean changes, and the standard deviation (SD) of the markers throughout the study, for both intervention and control groups. By observing multiple data at various time points for a specific study, the most recent was considered.
Quality assessment
The quality assessment of the qualified studies was performed by two separate investigators using the Cochran scoring method [26]. It possessed seven criteria to evaluate the risk of bias, which are as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Consequently, terms such as “Low”, “High”, or “Unclear” were used to assess each field. In addition, any dissimilarity was clarified by the corresponding authors.
Data synthesis and statistical analysis
In this meta-analysis, to detect the overall effect sizes, weighted mean differences (WMD) and the SDs of measures from both intervention and control groups were extracted using the random-effects model, according to DerSimonian And Laird method [27]. Furthermore, without meaningful changes reporting, it was calculated by using this formula: mean change = final values − baseline values, and SD changes were calculated by the following formula [28]:
We considered the correlation coefficient (R) to be 0.8. We also converted standard errors (SEs), 95% confidence intervals (CIs), and interquartile ranges (IQRs) to SDs by applying the Hozo et al. method [29]. To consider between-study variations the random-effects model was used to determine the overall effect size. The Between-study heterogeneity was also tested by Cochran's Q test and was measured by the I-squared statistic (I2) [30]. I2 > 40% or p-value < 0.05 was considered high between-study heterogeneity. To detect potential sources of heterogeneity [31], subgroup analyses were carried out following the pre-planned criteria, including study duration (≤ 16 and > 8 weeks), baseline levels of FBG, Insulin, HbA1c, HOMA-IR, CRP, TNF-α, IL-6, leptin, adiponectin, AST, ALT, baseline BMI, sex (male, female, both), health status (Metabolic syndrome, Type2 Diabetes, Hyperlipidemia, Hypertension, Non-Alcoholic Fatty Liver Disease (NAFLD)) and intervention doses (mg/d). We conducted a sensitivity analysis to determine the effect of each specific study on the overall estimation [32]. The possibility of publication bias was tested using Egger's regression test and the visually inspected funnel plot test [33]. STATA, version 11.2 (Stata Corp, College Station, TX) was used to carry out statistical analyses. The p-values < 0.05 were considered statistically significant in all analyses.
Results
Study selection
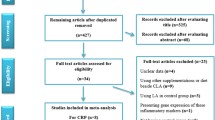
As mentioned in Fig. 1, 8516 studies were found in online databases at the first step of the search protocol. As a result, 2182 studies were duplicates and were subsequently removed. Afterward, the titles and abstracts of the studies were assessed exhaustively, and 6260 unrelated studies were deleted. Furthermore, irrelevant studies based on inclusion criteria, review, and animal studies were excluded. Moreover, we removed 61 studies without necessary data reporting by executing a comprehensive full-text assessment. After all, this study included 13 appropriate studies with the closest characteristics to the mentioned inclusion criteria.
Study characteristic
Finally, we qualified and included 13 studies, with 723 overall participants (348 cases and 375 controls). 2002 until 2018 was the publication date of all included studies. The intervention duration in qualified articles differed from 8 [34,35,36,37,38,39,40,41] to 16 [42] weeks. The sample size varied from 14 [43] to 80 [36] individuals. Parallel [35,36,37,38,39,40,41,42,43,44,45,46] and crossover RCTs [34] were the designs of qualified studies. Various subjects participated in included studies, like obese men with metabolic syndrome [44], type 2 diabetes mellitus patients [35, 37, 38, 46], overweight subjects with low-density lipoprotein phenotype B [45], Obesity-related hypertensive patients [36], postmenopausal women with type 2 diabetes mellitus [42], overweight hyperlipidemic individuals [34], atherosclerotic patients [41], patients with metabolic syndrome [43], and non-alcoholic fatty liver disease patients [39, 40]. All studies were executed in the UK [35], Iran [37,38,39,40,41], Netherlands [45], Canada [34], Sweden [44], Germany[42], Brazil [43], France [46], and China [36]. In included investigations, two studies were performed on just females [42, 43], four studies on males [34, 44], and the others were carried out on both [35,36,37,38,39,40,41, 45, 46]. The features of included studies are mentioned in Table 1.
Quality assessment
By assessing the general risk of bias, five studies acquired a moderate risk of bias [35, 38, 41, 45, 46], two studies showed a low risk of bias [40, 42], and five studies mentioned a high risk of bias [34, 36, 37, 39, 44] (Table 2).
Meta-analysis
Effect of CLA on FBG, fasting insulin, HbA1c, and HOMA-IR
Assessing 12 overall effect sizes from 10 studies for FBG and fasting insulin, and six effect sizes from five studies for HbA1c, revealed that CLA supplementation failed to affect HbA1c and fasting insulin levels significantly (for HbA1c WMD: -0.03%; 95%CI: -0.17 to 0.09; P = 0.567) (Fig. 2C), (for fasting Insulin WMD: 0.16 mU/L; 95%CI: -0.69 to 1.02; P = 0.702) (Fig. 2B), whereas it made a significant increasing effect on FBG levels (for FBG WMD: 4.49 mg/dL; 95%CI: 2.39 to 6.59; P < 0.001) (Fig. 2A). We also observed high heterogeneity for FBG (I2 = 97.1%), moderate for HbA1c (HbA1c I2 = 57.6%), and no heterogeneity for insulin among studies (I2 = 0.0%). Additionally, subgroup analysis indicated that CLA supplementation increased FBG levels in the long-term intervention (≥ 12 weeks), in lower doses (< 3g), among overweight (25 < BMI < 29.9) or hyperlipidemic individuals, and in studies conducted on participants with higher baseline levels of FBG (≥ 100). Moreover, in NAFLD patients, CLA supplementation significantly lowered HbA1c levels. Evaluating 11 overall effect sizes from nine studies demonstrated that CLA supplementation failed to alter HOMA-IR (for HOMA-IR WMD: 0.34; 95%CI: -0.11 to 0.81; P = 0.140) (Fig. 2D). In addition, a significant degree of between-studies heterogeneity was observed (I2 = 78.7%). Moreover, subgroup analysis indicated that CLA supplementation increased HOMA-IR in female participants (Table 3).
Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effect of CLA supplementation on A FBG (mg/dl); B Insulin (pmol/l); C HbA1c (%); D HOMA-IR; E CRP (mg/l); F IL-6 (pg/ml); G TNF-α (pg/ml); H Adiponectin (ng/ml); I Leptin (ng/ml); J MDA (umol/l); K ALT (U/L); and L) AST (U/L)
Effect of CLA on CRP, TNF-α and IL-6
By analyzing seven overall effect sizes from five studies for CRP, four effect sizes from three studies for TNF-α, and five effect sizes from four studies for IL-6, it was revealed that CLA supplementation did not change CRP and TNF-α levels, significantly (for CRP WMD: 0.00 mg/L; 95%C: -0.45 to 0.46; P = 0.976) (Fig. 2E) (for TNF-α, WMD:0.26 ng/l; 95%CI: -0.16 to 0.69; P = 0.232) (Fig. 2G), but made a significant reduction in IL-6 levels (for IL-6, WMD: -0.44 pg/ml; 95%CI: -0.86 to -0.02; P = 0.037) (Fig. 2F). Additionally, a moderate degree of heterogeneity for both TNF-α (I2 = 45.0%), and IL-6 (I2 = 52.3%), was found among studies, whereas no between-studies heterogeneity was observed for CRP (I2 = 0.00%). Evaluating the results of subgroup analysis showed that CLA supplementation failed to decrease IL-6 levels significantly in overweight individuals (25 < BMI < 29.9), whereas lowered IL-6 levels in obese (BMI > 30) or normal BMI (18.5–24.9) participants (Table 3).
Effect of CLA supplementation on adiponectin and leptin
Four studies with five effect sizes evaluated the effect of CLA supplementation on adiponectin and leptin. Pooled results from the random effects model demonstrated no significant alteration in adiponectin levels, whereas CLA supplementation diminished leptin levels, significantly (for adiponectin WMD: -0.12 µg/ml; 95%CI: -2.41 to 2.17; P = 0.918) (Fig. 2H), (for leptin WMD: -1.69 ng/ml; 95% CI:-1.80 to -1.58; P < 0.001) (Fig. 2I). Furthermore, a significant heterogeneity for adiponectin (I2 = 98.7%), and no heterogeneity for leptin (I2 = 0.00%) was observed among studies. Following the assessment of results in subgroup analysis, CLA supplementation failed to lower leptin levels in type 2 diabetic or metabolic syndrome patients, or male participants. Moreover, long-term CLA supplementation (≥ 12 weeks), or supplementation among female participants, hypertensive or type 2 diabetic patients, altered adiponectin levels (Table 3).
Effect of CLA supplementation on AST and ALT
Four overall effect sizes from three studies for AST and ALT were assessed to reveal the effect of CLA on AST and ALT. It was shown that CLA supplementation did not affect ALT levels significantly (WMD: 0.48 IU/L; 95%CI: -5.11 to 6.07; P = 0.866) (Fig. 2K), but increased AST levels significantly (WMD: 2.54 IU/L; 95%CI: 0.06 to 5.01; P = 0.044) (Fig. 2L). In addition, a high heterogeneity for ALT (I2 = 75.5%) and a moderate for AST (I2 = 62.4%) were found among studies (Table 3).
Effect of CLA supplementation on MDA
Three pooled overall effect sizes were analyzed and indicated that CLA supplementation failed to alter MDA levels significantly (WMD: -0.08 mmol/l; 95%CI: -0.80 to 0.62; P = 0.809) (Fig. 2J). Moreover, a significant degree of between-studies heterogeneity was seen (I2 = 85.7%) (Table 3).
Sensitivity analysis
To assess the effect of each study on the overall effect size in this meta-analysis, we omitted each article. As a result, we did not observe any significant change in the overall results of FBG, Insulin, HbA1c, HOMA-IR, CRP, IL-6, TNF-α, Adiponectin, Leptin, MDA, ALT, and AST, following the CLA supplementation.
Publication bias
Evaluating the results of Egger’s regression test indicated a significant publication bias in studies aimed to assess the effect of CLA supplementation on TNF-α, as an outcome (P = 0.040) (Fig. 3G).
Non-linear dose–response analysis
The results of the non-linear dose–response analysis (Figs. 4 and 5) demonstrated a significant association between CLA supplementation and changes in FBG (P = 0.012) (Fig. 6A).
Meta-regression analysis
The outcomes of the meta-regression test revealed no significant association between the dose and duration of CLA supplementation and changes in levels of FBG, Insulin, HbA1c, HOMA-IR, CRP, IL-6, TNF-α, adiponectin, leptin, MDA, ALT, and AST (Figs. 5 and 7).
GRADE analysis
The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) protocol was applied to assess the quality of the evidence for outcomes in this meta-analysis (Table 4). The studies examining the impact of CLA supplementation on FBG, HOMA-IR, TNF-α, Adiponectin, MDA, and ALT were considered to have a very low evidence quality. Furthermore, the articles evaluating the effect of CLA supplementation on HbA1C had a low quality evidence. On the other hand, the overall quality of the evidence showing the influence of CLA supplementation on Insulin, AST, IL-6, and CRP was upgraded to moderate. Lastly, high-quality evidence was observed for studies evaluating the effect of CLA supplementation on leptin (Table 4).
Discussion
To our knowledge, this is the first GRADE-assessed systematic review and dose–response meta-analysis to evaluate the effects of CLA supplementation on glycemic control, adipokine, cytokine, MDA, and liver function enzymes in patients at risk of CVDs. Our study suggested that CLA supplementation was negatively associated with serum IL-6 and leptin and positively associated with FBG and AST, but generally, no associations with serum fasting insulin, HbA1c, HOMA-IR, CRP, TNF-α, adiponectin, MDA and ALT were observed. According to subgroup analyses, CLA decreased HbA1c in patients with NAFLD. Furthermore, in females, HOMA-IR levels increased. Moreover, among females with T2DM and in long-term intervention, adiponectin decreased. CLA also decreased adiponectin in hypertensive individuals.
CVDs and their risk factors are associated with 30% of all mortality worldwide [47]. Risk factors that are the leading causes of CVDs are dyslipidemia, high blood glucose, high blood pressure, obesity, and inflammation [48]. CLA, as a nutraceutical compound, has a beneficial effect on empowering the immune system, regulating glucose and lipid metabolism, and the CVD risk factors [49].
The present study failed to show improvement in glycemic profile after CLA supplementation. According to the data from animal studies, CLA may not have positive effects on the glycemic profile [50, 51]. In human studies, supplementation with CLA for 8 weeks did not cause a significant change in serum insulin and insulin resistance [52, 53]. In some studies conducted on obese individuals or individuals with metabolic syndrome, CLA increased blood glucose and insulin resistance [54,55,56]. The increase in blood glucose and insulin resistance due to the consumption of different isomers of 10-trans, 12-cis or 9-cis, 11-trans CLA has been reported [57]. While blood glucose increased in our study, there was no significant change in insulin sensitivity. The reason for the contradiction in the findings of these studies may be due to the difference in the responses of people [37, 58]. This difference may be related to the different types of diseases, participants' weight, the severity of the insulin resistance, the medicines taken by the patients, and the different amounts of CLA intake from the diet.
CLA has been shown to exert anti-inflammatory properties in animal models of disease [59]. However, CLA's anti-inflammatory effects must be clarified in human studies. Similar to our results, Aslani (2020) et al. suggested that 3.2 g daily consumption of CLA reduces inflammatory markers such as IL-6 serum levels, significantly [60]. Our recently published systematic review and meta-analysis of 42 studies showed that CLA increased CRP levels and decreased TNF-α and IL-6 levels [15]. Therefore, it seems that CLA can have both proinflammatory and anti-inflammatory roles. Since there is limited data about CLA's anti-inflammatory effects in patients at risk for CVDs, more RCTs are needed.
In agreement with our finding regarding the impact of CLA supplementation on leptin, Esmaeili Shahmirzadi et al. indicated that 6.4 gr/day CLA supplementation reduced serum leptin [61]. This decrease in serum leptin levels may be related to the significant reduction of adipose tissue and fat mass [62]. Our results were confirmed by one meta-analysis study [63] showed that short-term intervention of CLA supplementation (less than eight weeks) might decrease leptin in overweight subjects.
Over the past decades, it has been well-documented that ALT and AST, provoked immense interest as promising diagnostic biomarkers for various conditions, including CVDs and diabetes [64]. The present study found a non-significant increase in serum ALT and a significant increase in serum AST after CLA supplementation. Similar to our study, several previous studies did not see any effect on liver enzymes [21, 44, 45, 65]. Kadegowda et al. indicated that received CLA supplementation compared to the control group had an increase in liver weight due to hepatic steatosis [66]. Moreover, Wang et al. reported that a high dose of CLA supplementation can lead to fatty liver disease. This can be due to the compensatory pathway for reducing the fat accumulation in fat mass, instead of increasing lipogenesis and fat deposition in liver tissue [67]. Increasing AST as a measure of liver function due to CLA consumption (10-trans, 12-cis isomer) may suggest unwanted side effects. In a recent systematic review and meta-analysis by Haghighat et al., in the general population, ALT and AST levels did not change after CLA supplementation compared to the control group [24]. Based on these findings, the harmful properties of CLA supplementation on liver markers are more in participants at risk for CVDs.
The cardiovascular protective effects of CLAs are apparently mediated not only by CLAs themselves but also by their metabolites [68]. CLA intake improves blood pressure, a risk factor for CVD, by increasing adiponectin and endothelial nitric oxide synthase activity [69]. CLA activates 5’-adenosine monophosphate-activated protein kinase (AMPK) with concomitant increases in prostaglandin levels, sufficient to decrease lipids in adipocytes [70]. Moreover, the anti-steatotic effects of CLA may increase lipid utilization by peripheral tissues [71]. In animal models, CLA improves hepatic steatosis and restores liver triacylglycerol secretion and the fatty acid profile during protein repletion [72]. However, it should be noted that most protentional mechanisms of CLA supplementation on CVD risks are not based on patients at risk for CVDs. Therefore, more studies are needed to confirm our findings.
Our study had some limitations to be acknowledged. Subgroup analyses were not performed on some metabolic disease risk factors. In addition, no studies controlled for the diet, that might have effect on their results. Moreover, most studies did not evaluate extra CLA intake from diet. There were some strengths in this meta-analysis, including the publication bias not observe in this meta-analysis, and most of the included studies were double-blind, randomized and placebo-controlled trials, which increased the internal validity and decreased the biases.
Conclusion
The findings of this meta-analysis supported the overall favorable effect of CLA supplementation on some of the adipokines and cytokines. CLA consumption was negatively associated with serum IL-6 and leptin. However, after CLA consumption, we found a significant increase in serum FBG and AST. It should be noted that the mentioned metabolic effects of CLA consumption were minor and may not reach clinical importance.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CVDs:
-
Cardiovascular diseases
- CIs:
-
Confidence intervals
- CLA:
-
Conjugated linoleic acid
- CRP:
-
C-reactive protein
- FBG:
-
Fasting blood glucose
- GRADE:
-
Grading of Recommendations, Assessment, Development and Evaluations
- HbA1c:
-
Hemoglobin A1c
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- IL-6:
-
Interleukin 6
- IQRs:
-
Interquartile ranges
- I2 :
-
I-squared statistic
- MDA:
-
Malondialdehyde
- NAFLD:
-
Non-Alcoholic Fatty Liver Disease
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyzes
- RCTs:
-
Randomized controlled trials
- SEs:
-
Standard errors
- TNF-α:
-
Tumor necrosis factor alpha
- WMD:
-
Weighted mean differences
References
Mokhtari M, Khalil D, Farzadfar F, Daroudi R, Asadi-Lari M. The Burden of Cardiovascular Disease Attributable to Modifiable Risk Factors and Cost-effectiveness Analysis of IraPEN Program in the General Population of Iran. Medical Journal of the Islamic Republic of Iran. 2022;36.
Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes care. 2013;36(Supplement_2):S259–63.
Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63(4):250–9.
Williams JW, Huang LH, Randolph GJ. Cytokine Circuits in Cardiovascular Disease. Immunity. 2019;50(4):941–54.
Khan MA, Baseer A. Increased malondialdehyde levels in coronary heart disease. J Pak Med Assoc. 2000;50(8):261–4.
Choi KM, Han K, Park S, Chung HS, Kim NH, Yoo HJ, et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: A nationwide population-based cohort study. Sci Rep. 2018;8(1):1–9.
Russell C, Keshavamurthy S, Saha S. Nutraceuticals in the Management of Cardiovascular Risk Factors: Where is the Evidence? Cardiovasc Hematol Disord: Drug Targets. 2021;21(3):150–61.
Lehnen TE, da Silva MR, Camacho A, Marcadenti A, Lehnen AM. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J Int Soc Sports Nutr. 2015;12(1):36.
Rubin D, Herrmann J, Much D, Pfeuffer M, Laue C, Winkler P, et al. Influence of different CLA isomers on insulin resistance and adipocytokines in pre-diabetic, middle-aged men with PPARγ2 Pro12Ala polymorphism. Genes Nutr. 2012;7(4):499–509.
Belury MA, Mahon A, Banni S. The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J Nutr. 2003;133(1):257S-S260.
Garibay-Nieto N, Queipo-García G, Alvarez F, Bustos M, Villanueva E, Ramírez F, et al. Effects of conjugated linoleic acid and metformin on insulin sensitivity in obese children: Randomized clinical trial. J Clin Endocrinol Metab. 2017;102(1):132–40.
Rahbar AR, Ostovar A, Derakhshandeh-Rishehri SM, Janani L, Rahbar A. Effect of Conjugated Linoleic Acid as a Supplement or Enrichment in Foods on Blood Glucose and Waist Circumference in Humans: A Metaanalysis. Endocr Metab Immune Disord Drug Targets. 2017;17(1):5–18.
Risérus U, Arner P, Brismar K, Vessby B. Treatment With Dietary trans10cis12 Conjugated Linoleic Acid Causes Isomer-Specific Insulin Resistance in Obese Men With the Metabolic Syndrome. Diabetes Care. 2002;25(9):1516–21.
Risérus U, Vessby B, Ärnlöv J, Basu S. Effects of cis-9, trans-11 conjugated linoleic acid supplementation on insulin sensitivity, lipid peroxidation, and proinflammatory markers in obese men. Am J Clin Nutr. 2004;80(2):279–83.
Rastgoo S, Shimi G, Shiraseb F, Karbasi A, Ashtary-Larky D, Yousefi M, et al. The effects of conjugated linoleic acid supplementation on inflammatory cytokines and adipokines in adults: A GRADE-assessed systematic review and dose-response meta-analysis. Front Immunol. 2023;14:1092077.
Derakhshandeh-Rishehri S-M, Rahbar AR, Ostovar A. Effects of conjugated linoleic acid intake in the form of dietary supplement or enriched food on c-reactive protein and lipoprotein (a) levels in humans: A literature review and meta-analysis. Iran J Med Sci. 2019;44(5):359.
Haghighatdoost F, Gh NM, BF. Effect of conjugated linoleic acid on blood inflammatory markers: a systematic review and meta-analysis on randomized controlled trials. Eur J Clin Nutr. 2018;72(8):1071–82.
Mazidi M, Karimi E, Rezaie P, Ferns GA. Effects of conjugated linoleic acid supplementation on serum C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Cardiovasc Ther. 2017;35(6):e12275.
Medina EA, Horn WF, Keim NL, Havel PJ, Benito P, Kelley DS, et al. Conjugated linoleic acid supplementation in humans: effects on circulating leptin concentrations and appetite. Lipids. 2000;35(7):783–8.
Mądry E, Malesza IJ, Subramaniapillai M, Czochralska-Duszyńska A, Walkowiak M, Miśkiewicz-Chotnicka A, et al. Body fat changes and liver safety in obese and overweight women supplemented with conjugated linoleic acid: A 12-week randomised, double-blind, placebo-controlled trial. Nutrients. 2020;12(6):1811.
Mirzaii S, Mansourian M, Derakhshandeh-Rishehri S-M, Kelishadi R, Heidari-Beni M. Association of conjugated linoleic acid consumption and liver enzymes in human studies: A systematic review and meta-analysis of randomized controlled clinical trials. Nutrition. 2016;32(2):166–73.
Morvaridzadeh M, Estêvão MD, Morvaridi M, Belančić A, Mohammadi S, Hassani M, et al. The effect of Conjugated Linoleic Acid intake on oxidative stress parameters and antioxidant enzymes: a systematic review and meta-analysis of randomized clinical trials. Prostaglandins & Other Lipid Mediators. 2022:106666.
Suksatan W, Putera HD, Abdulkadhim AH, Hammid AT, Ismailov JA, Jannat B, et al. The effect of conjugated linoleic acid supplementation on oxidative stress markers: A systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition ESPEN. 2022.
Haghighat N, Shimi G, Shiraseb F, Karbasi A, Nadery M, Ashtary-Larky D, et al. The effects of conjugated linoleic acid supplementation on liver function enzymes and malondialdehyde in adults: A GRADE-assessed systematic review and dose-response meta-analysis. Pharmacol Res. 2022;186:106518.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9 w64.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, version 5.0. 2 [updated Sept 2009], The Cochrane Collaboration, 2009. 2010.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis: John Wiley & Sons; 2021.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–7.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Joseph SV, Jacques H, Plourde M, Mitchell PL, McLeod RS, Jones PJ. Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. J Nutr. 2011;141(7):1286–91.
Moloney F, Yeow T-P, Mullen A, Nolan JJ, Roche HM. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2004;80(4):887–95.
Zhao W-S, Zhai J-J, Wang Y-H, Xie P-S, Yin X-J, Li L-X, et al. Conjugated Linoleic Acid Supplementation Enhances Antihypertensive Effect of Ramipril in Chinese Patients With Obesity-Related Hypertension. Am J Hypertens. 2009;22(6):680–6.
Shadman Z, Rastmanesh R, Hedayati M, Taleban F, Saadat N, Tahbaz F, et al. Effects of Conjugated Linoleic Acid on Insulin Sensitivity and Diabetes Markers in Type 2 Diabetic Patients. Iran J Endocrinol Metab. 2010;11(2):135–42.
Shadman Z, Taleban FA, Saadat N, Hedayati M. Effect of conjugated linoleic acid and vitamin E on glycemic control, body composition, and inflammatory markers in overweight type2 diabetics. J Diabetes Metab Disord. 2013;12(1):1–9.
Abedi R, Aref-Hosseini S-R, Khoshbaten M, Ebrahimi-Mameghani M, Laleh HJ, Jalalypour F, et al. The effect of conjugated linoleic acid (CLA) on inflammatory factors in Non-Alcoholic Fatty Liver Disease (NAFLD): A randomized controlled clinical trial. Prog Nutr. 2018;20:173–81.
Ebrahimi-Mameghani M, Jamali H, Mahdavi R, Kakaei F, Abedi R, Kabir-Mamdooh B. Conjugated linoleic acid improves glycemic response, lipid profile, and oxidative stress in obese patients with non-alcoholic fatty liver disease: a randomized controlled clinical trial. Croat Med J. 2016;57(4):331–42.
Eftekhari MH, Aliasghari F, Babaei-Beigi MA, Hasanzadeh J. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA atherosclerosis. 2013;9(6):311.
Norris LE, Collene AL, Asp ML, Hsu JC, Liu LF, Richardson JR, et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am J Clin Nutr. 2009;90(3):468–76.
Carvalho RF, Uehara SK, Rosa G. Microencapsulated conjugated linoleic acid associated with hypocaloric diet reduces body fat in sedentary women with metabolic syndrome. Vasc Health Risk Manag. 2012;8:661.
RISerus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans 10 cis 12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes care. 2002;25(9):1516–21.
Naumann E, Carpentier YA, Saebo A, Lassel TS, Chardigny J-M, Sébédio J-L, et al. Cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) do not affect the plasma lipoprotein profile in moderately overweight subjects with LDL phenotype B. Atherosclerosis. 2006;188(1):167–74.
Schmitt B, Ferry C, Daniel N, Weill P, Kerhoas N, Legrand P. Effet d’un régime riche en acides gras ω3 et en CLA 9-cis, 11-trans sur l’insulinorésistance et les paramètres du diabète de type 2. Oléagineux, Corps gras, Lipides. 2006;13(1):70–5.
Mensah GARG, Fuster V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J Am Coll Cardiol. 2019;74:2529–32.
Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57–70.
Funck LG, Barrera-Arellano D, Block JM. Conjugated linoleic acid (CLA) and its relationship with cardiovascular disease and associated risk factors. Arch Latinoam Nutr. 2006;56(2):123–34.
DeLany JP, Blohm F, Truett AA, Scimeca JA, West DB. Am J Physiol. Conjugated linoleic acid rapidly reduces body fat content in mice without affecting energy intake. 1999;276(4):R1172-9.
Larsen TM, AstrupAJJolr. Efficacy and safety of dietary supplements containing CLA for the treatment of obesity: evidence from animal and human studies. 2003;44(12):2234-41.
Tricon S, Burdge GC, Kew S, Banerjee T, Russell JJ, Grimble RF, et al. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid on immune cell function in healthy humans. Am J Clin Nutr. 2004;80(6):1626–33.
Tricon S, Burdge GC, Kew S, Banerjee T, Russell JJ, Jones EL, et al. Opposing effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am J Clin Nutr. 2004;80(3):614–20.
Noto A, Zahradka P, Yurkova N, Xie X, Truong H, Nitschmann E, et al. Dietary conjugated linoleic acid decreases adipocyte size and favorably modifies adipokine status and insulin sensitivity in obese, insulin-resistant rats. Metabolism. 2007;56(12):1601–11.
Shim WS, Kim SK, Kim HJ, Kang ES, Ahn CW, Lim SK, et al. Decrement of postprandial insulin secretion determines the progressive nature of type-2 diabetes. Eur J Endocrinol. 2006;155(4):615–22.
De Roos B, Rucklidge G, Reid M, Ross K, Duncan G, Navarro MA, et al. Divergent mechanisms of cis9, trans11-and trans10, cis12-conjugated linoleic acid affecting insulin resistance and inflammation in apolipoprotein E knockout mice: a proteomics approach. FASEB J. 2005;19(12):1746–8.
de Almeida MM, de Souza YO, Luquetti SCPD, Sabarense CM, do Amaral Corrêa JO, da Conceição EPS, et al. Cis-9, trans-11 and trans-10, cis-12 CLA mixture does not change body composition, induces insulin resistance and increases serum HDL cholesterol level in rats. 2015;64(5):539–51.
Eyjolfson V, Spriet LL, Dyck DJ. Conjugated linoleic acid improves insulin sensitivity in young, sedentary humans. Med Sci Sports Exerc. 2004;36(5):814–20.
Viladomiu M, Hontecillas R, Bassaganya-Riera JJEJoP. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur J Pharmacol. 2016;785:87–95.
Aslani MR, Matin S, Nemati A, Mesgari-Abbasi M, Ghorbani S, Ghobadi H. Effects of conjugated linoleic acid supplementation on serum levels of interleukin-6 and sirtuin 1 in COPD patients. Avicenna J Phytomed. 2020;10(3):305.
Shahmirzadi FE, Ghavamzadeh S, Zamani T. The effect of conjugated linoleic acid supplementation on body composition, serum insulin and leptin in obese adults. Arch Iran Med. 2019;22(5):255.
Wang JY, Lu KC, Lin YF, Hu W-M. Correlation of serum leptin concentrations with body composition and gender in Taiwanese hemodialysis patients without diabetes. Ren Fail. 2003;25(6):953–66.
Haghighatdoost F, Hariri M. Effect of conjugated linoleic acid supplementation on serum leptin concentration: a systematic review and meta-analysis. Endocr Metab Immune Disord Drug Targets. 2018;18(3):185–93.
Asbaghi O, Shimi G, Shiraseb F, Karbasi A, Nadery M, Ashtary-Larky D, et al. The effects of conjugated linoleic acid supplementation on liver function enzymes and malondialdehyde in adults: A GRADE-assessed systematic review and dose-response meta-analysis. 2022:106518.
Asbaghi O, Shimi G, Shiraseb F, Karbasi A, Nadery M, Ashtary-Larky D, et al. The effects of conjugated linoleic acid supplementation on liver function enzymes and malondialdehyde in adults: A GRADE-assessed systematic review and dose-response meta-analysis. Pharmacological Research. 2022:106518.
Kadegowda AK, Connor EE, Teter BB, Sampugna J, Delmonte P, Piperova LS, et al. Dietary trans fatty acid isomers differ in their effects on mammary lipid metabolism as well as lipogenic gene expression in lactating mice. J Nutr. 2010;140(5):919–24.
Wang Y, Jones PJ. Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obes. 2004;28(8):941–55.
Eder K, Ringseis R. Mol Nutr Food Res. Metabolism and actions of conjugated linoleic acids on atherosclerosis-related events in vascular endothelial cells and smooth muscle cells. 2010;54(1):17–36.
DeClercq V, Taylor CG, Wigle J, Wright B, Tworek L, Zahradka PJTJoNB. Conjugated linoleic acid improves blood pressure by increasing adiponectin and endothelial nitric oxide synthase activity. J Nutr Biochem. 2012;23(5):487–93.
Jiang S, Chen H, Wang Z, Riethoven J-J, Xia Y, Miner J, et al. Activated AMPK and prostaglandins are involved in the response to conjugated linoleic acid and are sufficient to cause lipid reductions in adipocytes. J Nutr Biochem. 2011;22(7):656–64.
Stringer DM, Zahradka P, DeClercq VC, Ryz NR, Diakiw R, Burr LL, et al. Modulation of lipid droplet size and lipid droplet proteins by trans-10, cis-12 conjugated linoleic acid parallels improvements in hepatic steatosis in obese, insulin-resistant rats. Biochim Biophys Acta. 2010;1801(12):1375–85.
Andreoli MF, Illesca PG, González MA, Bernal CAJL. Conjugated linoleic acid reduces hepatic steatosis and restores liver triacylglycerol secretion and the fatty acid profile during protein repletion in rats. Lipids. 2010;45(11):1035–45.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
O.A. and G.S. contributed in conception, data collection and manuscript drafting. N.G., N.R., K.G., M.H., S.D., H.S.O., and N.A. contributed in data collection and manuscript drafting. D.A.L. revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghodoosi, N., Rasaei, N., Goudarzi, K. et al. The effects of conjugated linoleic acid supplementation on glycemic control, adipokines, cytokines, malondialdehyde and liver function enzymes in patients at risk of cardiovascular disease: a GRADE-assessed systematic review and dose–response meta-analysis. Nutr J 22, 47 (2023). https://doi.org/10.1186/s12937-023-00876-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-023-00876-3