Abstract
Purpose and methods
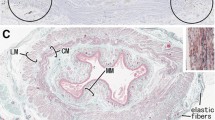
To identify site-dependent and individual differences in neuronal nitric oxide synthase (nNOS)-positive nerves of the myenteric plexus, we examined full-thickness walls of the stomach, pylorus, duodenum, ileum, colon, and rectum in 7 male and 8 female cadavers (mean ages, 80 and 87 years, respectively).
Results
The areas occupied by nNOS-positive nerve fibers in the myenteric plexus were fragmentary and overlapped with areas occupied by vasoactive intestinal polypeptide-positive fibers. The nNOS-positive fiber-containing areas per 1-mm length of intermuscular space tended to be larger at more anal sites, with positive areas four times greater in the rectum than in the stomach. Interindividual differences in rectal areas were extremely large, ranging from 0.017 mm2 in one 80-year-old man to 0.067 mm2 in another 80-year-old man. Similarly, the numbers of nNOS-positive ganglion cell bodies per 1-mm length in the rectum ranged from 4 to 28. These areas and numbers were weakly correlated (r = 0.62; p = 0.02). Interindividual differences in the rectum appeared not to depend on either age or gender.
Conclusions
Anatomic studies using donated cadavers carried the advantage of obtaining any parts of intestine within an individual, in contrast to surgically removed specimens. We speculated excess control of evacuation with laxatives as one of causes of atrophy of the rectal myenteric plexus.







Similar content being viewed by others
References
Porter AJ, Wattchow DA, Brookes SJH, Costa M (2002) Cholibergic and nitergic interneurons in the myenteric plexus of the human colon. Gut 51(1):70–75. doi:10.1136/gut.51.1.70
Anlauf M, Schåfer MKH, Eiden L, Weihe E (2003) Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol 459(1):90–111. doi:10.1002/cne.10599
Brehmer A, Schrödl F, Neuhuber W (2006) Morphology of VIP/nNOS-immunorective myenteric neurons in the human gut. Histochem Cell Biol 125(5):557–565. doi:10.1007/s00418-005-0107-8
Wattchow DA, Furness JB, Costa M (1988) Distribution and coexistence of peptides in nerve fibers of the external muscle of the human gastrointestinal tract. Gastroenterol 95(1):32–41
Wattchow D, Brookes S, Murphy E, Carbone S, De Fontgalland D, Costa M (2008) Regional variation in the neurochemical coding of the myenteric plexus of the human colon and changes in patients with slow transit constipation. Neurogastroenterol Motil 20(12):1298–1305. doi:10.1111/j.1365-2982.2008.01165.x
Ippolito C, Segnani C, De Giorgio R, Blandizzi C, Mattii L, Castagna M, Moscato S, Dolfi A, Bernardini N (2009) Quantitative evaluation of myenteric ganglion cells in normal human left colon: implications for histopathological analysis. Cell Tissue Res 336(2):191–201. doi:10.1007/s00441-009-0770-5
Beck M, Schlabrakowski A, Schrödl F, Neuhuber W, Brehmer A (2009) ChAT and NOS in human myenteric neurons: co-existence and co-absence. Cell Tissue Res 338(1):37–51. doi:10.1007/s00441-009-0852-4
Hieda K, Cho KH, Arakawa T, Fujimiya M, Murakami G, Matsubara M (2013) Nerves in the intersphincteric space of the human anal canal with special reference to their continuation to the enteric nerve plexus of the rectum. Clin Anat 26:843–854. doi:10.1002/ca.22227
Aldridge RT, Campbell PE (1968) Ganglion cell distribution in the normal rectum and anal canal. A basis for the diagnosis of Hirschsprung’s disease by anorectal biopsy. J Pedatr Surg 3(4):475–489
Ferri GL, Adrian TE, Allen JM, Soimero L, Cancellieri A, Yeats JC, Blank M, Polak JM, Bloom SR (1988) Intramural distribution of regulatory peptides in the sigmoid-recto-anal region of the human gut. Gut 29(6):762–768
Hörsch D, Fink T, Bűchler M, Weihe E (1993) Regional specificities in the distribution, chemical phenotypes, and coexistence patterns of neuropeptide containing nerve fibers in the human anal canal. J Comp Neurol 335(3):381–401
Hörsch D, Day R, Seidah NG, Weihe E, Schāfer MK (1997) Immunohistochemical localization of the pro-peptide processing enzymes PC1/PC3 and PC2 in the human anal canal. Peptides 18(5):755–760
Stebbing JF (1998) Nitric oxide synthase neurons and neuromuscular behavior of the anorectum. Ann R Coll Surg Engl 80(2):137–345
Jones OM, Brading AF, Mortensen NJ (2003) Role of nitric oxide in anorectal function of normal and neuronal nitric oxide synthase knowckout mice: a novel approach to anorectal disease. Dis Colon Rectum 46(7):963–970
Terauchi A, Kobayashi D, Mashimo H (2005) Distinct roles of nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol 289(2):G291–299. doi:10.1152/ajpgi.00005.2005
Stavreva G, Radomirov R (2012) Region-related modular nerve-dependent motor activity in anorectum-cholinergic and nitergic contriobution to rat model. Acta Neurobiol Exp 72(2):185–194
Bernard CE, Gibbon SJ, Gomez-Pinilla PJ, Luren MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ, Larson DW, Camilleri M, Zinsmeister AR, Pozo MJ, Hicks GA, Farrugia G (2009) Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil 21(7):746–754. doi:10.1111/j.1365-2982.2008.01245.x
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 324(1):1–8
Tomita R, Tanjoh K, Fujisaki S, Fukuzawa M (1999) The role of nitric oxide (NO) in the human pyloric sphincter. Hepatogastroenterology 46(29):2999–3003
Tomita R (2009) Regulation of vasoactive intestinal peptide and substance P in the human pyloric sphincter. Hepatogastroenterology 56(94–95):1403–1406
Gershon MD, Kirchgessner AL, Wade PR (1994) Functional anatomy of the enteric nervous system. In: Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH (eds) Physiology of the gastrointestinal tract, 4th edn. Raven press, New York, pp 381–422
Furness JB, Bornstein JC, Kunze WAA, Clerc N (1999) The enteric nervous system and its extrinsic connections. In: Yamada T, Alpers DH, Laine L, Owyang C, Powell DW (eds) Textbook of gastroenterology, vol 1, 3rd edn. Lippincott, Williams and Wilkins, Philadelphia, pp 11–35
Ekblad E, Bauer AJ (2004) Role of vasoactive intestinal polypeptide and inflammatory mediators in enteric neuronal plasticity. Neurogastroenterol Motil 16(suppl 1):123–128
Acknowledgments
We are grateful to the people who donated their cadavers to Tokyo Dental College for research and education on human anatomy without any economic benefit. We also thank their families for agreeing to these donations as well as for their patience in awaiting the return of their relatives’ bones after study. This work was supported by a grant (0620220-1) from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, S.E., Hieda, K., Kim, J.H. et al. Region-specific differences in the human myenteric plexus: an immunohistochemical study using donated elderly cadavers. Int J Colorectal Dis 29, 783–791 (2014). https://doi.org/10.1007/s00384-014-1869-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-014-1869-z