Abstract
Purpose
The present study was performed to get a better insight in the incidence of anastomotic leakage leading to reintervention when using the C-seal: a biodegradable sheath that protects the stapled colorectal anastomosis from leakage.
Methods
The C-seal is a thin walled tube-like sheath that forms a protective sheath within the bowel lumen. Thirty-seven patients undergoing surgery with creation of a stapled colorectal anastomosis with C-seal were analyzed. Follow-up was completed until 3 months after surgery.
Results
One patient (3 %) developed anastomotic leakage leading to reintervention. None of the 37 anastomoses was dismantled. One patient was diagnosed with a rectovaginal fistula. In three patients (8 %), a perianastomotic abscess spontaneously drained.
Conclusion
The incidence of anastomotic leakage leading to reintervention when using the C-seal (3 %) is lower than expected based on the literature (11 %). We have currently set-up a multicenter randomized trial to confirm the efficiency of the C-seal (www.csealtrial.nl).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purpose
Anastomotic leakage is a major complication of a low anterior resection. Anastomotic leakage may lead to prolonged hospital stay, reintervention, sepsis, and even death [1–6]. Besides a very complicated postoperative recovery, anastomotic leakage can have lifelong impact, with a high risk of a permanent stoma and increased risk of fecal incontinence [7–10]. The current incidence of anastomotic leakage of colorectal anastomoses is reported to be approximately 11 % [1–5].
To prevent clinical consequences of anastomotic leakage, a defunctioning stoma during the initial operation may be created [6]. A stoma, however, also has its drawbacks: it is cumbersome for the patient, complications associated with the stoma can occur, and a second intervention is needed for stoma reversal [9]. Moreover, in 8–20 % of patients, the stoma is never reversed [10, 11].
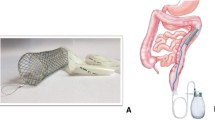
In the past years, we have developed a device which we called the “C-seal”. The C-seal is a biodegradable soft sheath that protects the stapled colorectal anastomosis from leakage [12–14]. In case of ischaemia or technical error, it might not prevent the formation of dehiscence of the anastomotic walls, but it will prevent leakage of fecal content from the lumen of the intestine into the abdominal cavity or towards the retroperitoneal space. Also, if the anastomotic dehiscence is small or distal from the level of the anastomosis and covered by the C-seal, the defect may heal without interventions by secondary intent.
Previously, we performed a feasibility study applying the C-seal successfully [12].
In this report, we have further evaluated the incidence of anastomotic leakage when using the C-seal in a phase II study.
Methods
The study was approved by the medical ethics committees of the participating hospitals and by the Dutch Health Care Inspectorate and performed in accordance with the ethical standards of the Helsinki Declaration.
Study population
To be eligible, patients were electively planned for colorectal resection with the creation of a stapled anastomosis at maximally 15 cm from the anal verge. Inclusion criteria further included age ≥18 years and ASA score ≤3. Patients with an active inflammation at the time of surgery and patients who were treated in an acute setting were excluded. All consecutive patients were asked to participate, and informed consent was obtained after at least 1 week of reflection. All patient data were anonymised.
Endpoints
The primary endpoint of this study was anastomotic leakage leading to reintervention. Reintervention was defined as relaparotomy with either dismantling of the anastomosis and creation of an end-colostomy, placement of drains in the pelvis, creation of a diverting stoma, or drainage (radiological guided) of a pelvic abscess.
Secondary endpoints included the assessment of technical feasibility of applying the C-seal and successful clearance (biofragmentation) of the C-seal. Procedure or device related serious and nonserious adverse events during the application and during the postoperative period were registered.
The C-seal
The C-seal is made from biodegradable polyurethane, composed of “hard” and “soft” molecular components. The “hard” component provides the desired elastic and mechanical properties and consists of polyurethane bindings (HNCO2) composed of a di-isocyanate (OCN-NCO) and a diol (HO-OH). The “soft” segment has a hydrophilic character and determines the degradation behavior of the polyurethane.
The C-seal is compatible with all circular staplers currently used for colorectal anastomoses. The staplers used in this study were the Ethicon Endo-Surgery Circular Staplers (29 or 33 mm diameter) (Ethicon Endo-Surgery, Johnson & Johnson Medical BV, Amersfoort, the Netherlands) and Covidien Premium Plus CEEA (28 or 31 mm head diameter) (Covidien Nederland BV, Zaltbommel, the Netherlands).
The application of the C-seal has been described elsewhere [15, 16]. Briefly, the C-seal is attached to the anvil of the circular stapler as illustrated in Figs. 1 and 2. Application of the C-seal has been slightly altered since its introduction [14, 17]. The C-seal is attached to the stapler with sided tape (3 M Hi Tack conformable double-coated tape #1510) instead of glue, and a marble is inserted in the seal to facilitate placement of the C-seal in the proximal bowel loop and to check its position in the bowel.
The anvil together with the attached C-seal is introduced in the proximal bowel loop. The circular stapler is introduced in the rectal stump, connected to the anvil, and fired. In this way, the C-seal is attached to the staplers just proximal of the anastomosis. By pulling the stapler with the C-seal through the anus, the C-seal forms a protective sheath covering the anastomosis starting just proximal to the stapled intestinal walls. The C-seal is cut from the stapler/anvil and shortened at an adequate distance (4 cm) from the anal verge (Fig. 3).
The C-seal remains in situ for at least 1 week; the exact duration to completely dissolve depends on the humidity.
Follow-up
Patients received standard postoperative care according to local protocol. One week after the operation, a rectal contrast enema was performed to check for anastomotic leakage. Water-soluble contrast was first syringed within the lumen of the C-seal and next in the lumen between the C-seal and the rectum mucosa. Anastomoses within 4 cm of the anal verge were only syringed within the C-seal to prevent manipulation of the anastomosis. A case report form was kept and completed by the attending physician. Serious adverse events had to be reported within 24 h to the study coordinator. After hospital discharge, patients were evaluated at the outpatient clinic. Patients were followed until 3 months postoperatively.
Results
Between January 2010 and October 2010, 37 patients were included in one of the following hospitals: University Medical Center Groningen (n = 16), Medical Center Leeuwarden (n = 6), Laurentius Hospital Roermond (n = 4), Ommelander Hospital Group (in Delfzijl and Winschoten, n = 5), Wilhelmina Hospital Assen (n = 3), and Antonius Hospital Sneek (n = 3).
Patients were operated for malignancy (n = 30), diverticulosis (n = 5), endometriosis (n = 1), and a rectovaginal fistula (n = 1). The median age was 65 years (range 27–79). Baseline characteristics are listed in Table 1.
Endpoints
Anastomotic leakage leading to reintervention
One anastomotic leakage (3 %) leading to reintervention occurred in a patient who underwent rectal resection en bloc with part of the posterior vaginal wall because of endometriosis located in the rectovaginal septum. The colorectal anastomosis was created at 4 cm distance from the anal verge. On postoperative day 16, this patient was readmitted to hospital with a vaginal bleeding originating from the pudendal artery. This artery was coiled, a loop ileostomy was created, and debridement of the inflammatory mass between rectum and vagina was performed. The anastomotic leakage from the rectum to the posterior vaginal wall had probably caused inflammation of the surrounding tissue resulting in a rectovaginal fistula and bleeding. The anastomosis was kept intact and maintained. The fistula healed by secondary intention, and after 3 months, the stoma was successfully taken down.
Anastomotic leakage not leading to reintervention
A female patient, treated for rectal cancer with a low anterior resection with deviating ileostomy (anastomosis at 3 cm from the anal verge), presented with abdominal pain and pus per vaginum 15 days after surgery. Vaginal and rectal examination revealed a rectovaginal fistula: the posterior vaginal wall was apparently stapled together with the anastomosis. This patient was initially treated conservatively. Since this fistula persisted, closure was attempted 8 months after the primary low anterior resection.
In three patients (8 %), rectal pus discharge occurred, which was interpreted as a perianastomotic abscess (CT proven in one of these three patients). One of these patients was initially treated for a fistula after previous low anterior resection for rectal cancer, and the other two underwent low anterior resection for rectal cancer. All three had a diverting ileostomy, and the anastomosis was situated at, respectively, 2, 6, and 6 cm from the anal verge. Another two patients (5 %) treated for rectal cancer with a low anterior resection (anastomosis at 4 and 7 cm; one with a diverting stoma) had a CT-proven presacral infiltration and were antibiotically treated. All recovered well, and none of these patients had a reintervention.
Clearly, patients with rectal cancer undergoing low anterior resection have a higher chance of developing anastomotic leakage compared to patients undergoing anterior resection for sigmoid pathologies with the anastomosis situated at greater distance from the anal verge. In our group, 28 patients underwent a low anterior resection for rectal cancer, and 7 patients underwent surgery for sigmoid pathologies (5 for diverticulosis and 2 for cancer). In this “sigmoid” subgroup, the anastomosis was situated between 10 and 15 cm above the anal verge, and none had an anastomotic related complication. Only one of the patients in this “sigmoid” group received a diverting stoma.
All anastomosis-related complications occurred in patients with the anastomosis situated within 8 cm distance from the anal verge.
Secondary endpoints
Application of the C-seal
The C-seal was successfully applied without any complications in 35 patients. The extra time needed by the surgeon to apply the C-seal was on average 5 min.
In two cases (5 %), it was impossible to pull the C-seal through the anus after firing the stapler. It appeared that the C-seal was double-stapled at the anastomotic site after interposition of the “tail” of the C-seal due to inadequate positioning of this “tail” in the afferent loop. To solve this problem, the C-seal was cut transanally, which prevented any obstruction. Both patients are included in the analysis according to the intention to treat principle and had an uneventful recovery.
Upon survey of applicability, 65 % of the surgeons rated the use of the C-seal as “good” on a five-point scale ranging from excellent–good–average–fair to poor. Another 34 % rated the procedure as “average”. Upon feedback, it appeared that initially the insertion of the anvil with the attached C-seal into the proximal bowel loop was found to be cumbersome.
Rectal contrast enemas were performed on postoperative day 7 (range day 6–10). Contrast was inserted within the lumen of the C-seal as well as in the space between the C-seal and the rectum mucosa, so that leaks above and below the anastomosis will be demonstrated. In four patients, the enema was not performed because of logistic reasons. In five patients, the rectal contrast enemas demonstrated leakage of contrast outside the bowel contour. Among these five patients are the patient with anastomotic leakage leading to reintervention, the patient with the rectovaginal fistula, and a patient with a spontaneously drained abscess at the anastomosis as mentioned above. The two remaining patients with a radiological leakage had no clinical signs or complications of anastomotic leakage.
Clearance of the C-seal
The C-seal cleared from the bowel after a median of 14 days (range 5–63 days). In patients without a diverting stoma, median time to losing the C-seal was 10 days. In patients with a diverting stoma, the exact time of C-seal disappearance is not always known, since the C-seal fragments and resolves in the bowel lumen and becomes unrecognizable as such. In one patient with a diverting loop ileostomy, the anastomosis was checked by proctoscopy at 63 days after surgery (according to local hospital protocol), and a part of the C-seal was still visible at the anastomotic site.
Reinterventions not related to the C-seal
One patient (3 %) underwent surgical intervention in the postoperative phase for stoma retraction. This was not related to the C-seal, nor did this patient suffer from anastomotic leakage.
Perioperative mortality
One patient (3 %) died because of a pulmonary embolism and myocardial infarction 10 days after surgery. No signs of anastomotic leakage or adverse events due to the C-seal were suspected at clinical and laboratory evaluation.
Discussion
The C-seal is a biodegradable soft sheath that covers the colorectal anastomosis and aims to prevent anastomotic leakage and clinical consequences. This study evaluates the incidence of anastomotic leakage leading to complications and reintervention in patients treated with the C-seal.
We hypothesized that the C-seal prevents extravasation of feces in case of an anastomotic dehiscence and allows healing by secondary intent. The anastomotic leakage rate leading to reintervention of 3 % when using the C-seal is lower than the 11 % leakage as reported in current literature[1–5], suggesting a protective effect of the C-seal on anastomotic leakage.
Five patients were diagnosed with a perianastomotic abscess or infiltration. Clearly, a perianastomotic abscess should be considered as anastomotic leakage [18]. From the literature, the incidence of contained leaks (perianastomotic abscesses or infiltration) is up to 50 % of diagnosed leakages [15–17, 19]. The discrepancy between our findings and the literature might be explained by a migration of the degree of severity of anastomotic leakage: with C-seal, a lower percentage of full blown anastomotic leakages and a higher percentage of “contained leakagese” may occur.
We found a radiologic anastomotic leakage in five patients (14 %), with one patient developing anastomotic leakage leading to reintervention. As described in the literature, our radiologic anastomotic leakage incidence is higher than the incidence of clinical anastomotic leakage [20].
In 49 % of our patients, the anastomosis was protected by a deviating stoma. Although a deviating stoma does not prevent anastomotic leakage to occur, many surgeons believe it reduces the consequences of anastomotic leakage. In this study, deviating stomas were created at the discretion of the operating surgeon. We encouraged participating surgeons to take the decision of a stoma or not without considering a possible benefit of the C-seal. In the literature, Matthiessen et al. randomized patients who underwent a low stapled colorectal anastomosis to a deviating stoma or not [6]. In the group of patients with a stoma, anastomotic leakage occurred in 12 of 116 patients (10 %); and in the group without a stoma, anastomotic leakage occurred in 33 of 118 (28 %). Data from the Dutch Surgical Colorectal Audit reveal that in the period 2009–2011, 3,313 patients with a colorectal anastomosis were registered. A deviating stoma was created in 2,190 patients (66 %) [18]. anastomotic leakage occurred in 10 % of patients with a deviating stoma and in 13 % of the patients without a deviating stoma [5]. With a 10 % anastomotic leakage percentage in the presence of a deviating stoma, we consider the use of the C-seal to remain beneficial.
Time to degradation of the C-seal depends on the humidity of the environment. In our series, we observed a patient with a deviating stoma, where parts of the C-seal were still present in the bowel lumen at 63 days after the operation. We believe that such a late degradation is not harmful, as eventually the C-seal will be cleared from the bowel lumen.
The C-seal can give some inconvenience of hygienic nature, since cleaning the sheath can be difficult. Furthermore, as the C-seal crosses the anal sphincter, soiling may occur. This discomfort is temporary, since the C-seal is cleared from the bowel lumen or is cut at the anal verge at time of discharge from the hospital.
The C-seal can be applied in both open and laparoscopic techniques, as long as the colorectal anastomosis is created by a circular stapler. In laparoscopic surgery, a skin incision is usually made to introduce the anvil in the proximal bowel loop. The proximal bowel loop should slightly protrude above skin level to facilitate insertion of the anvil with the attached C-seal. The procedure remains essentially unchanged. In this study, in 14 (38 %) patients, a laparoscopic procedure was performed, of which a conversion was necessary in three patients. No indication for conversion was related to use of the C-seal.
All outcome parameters monitored in the phase II study had also been prospectively documented in our C-seal feasibility study that was performed immediately prior to this consecutive series. Therefore, we were able to merge the study results to determine the overall incidence of anastomotic leakage leading to reintervention. Thus, in total, fifty-two patients have now been treated with the C-seal. The results are promising, with an anastomotic leakage leading to reintervention rate of 2 %. We have currently set-up a multicenter, randomized, open phase III trial in patients undergoing surgery with creation of a stapled anastomosis to confirm the efficiency of the C-seal in preventing anastomotic leakage leading to reintervention (csealtrial.nl).
References
Matthiessen P, Hallbook O, Andersson M et al (2004) Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis 6(6):462–469
Bertelsen CA, Andreasen AH, Jorgensen T et al (2010) Danish colorectal cancer group. Anastomotic leakage after anterior resection for rectal cancer: risk factors. Colorectal Dis 12(1):37–43
Paun BC, Cassie S, MacLean AR et al (2010) Postoperative complications following surgery for rectal cancer. Ann Surg 251(5):807–818
Peeters KC, Tollenaar RA, Marijnen CA et al (2005) Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg 92(2):211–216
Wouters MW, van Leersum NJ, Snijders HS, et al (2012) Uitkomst van zorg registratie, transparantie en kwaliteit. Jaarrapportage Dutch Surgical Colorectal Audit 2011. Utrecht, Dutch Surgical Colorectal Audit
Matthiessen P, Hallbook O, Rutegard J et al (2007) Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 246(2):207–214
Ravo B, Ger R (1985) Temporary colostomy—an outmoded procedure? A report on the intracolonic bypass. Dis Colon Rectum 28(12):904–907
Ravo B, Ger R (1984) A preliminary report on the intracolonic bypass as an alternative to a temporary colostomy. Surg Gynecol Obstet 159(6):541–545
Bakx R, Busch OR, Bemelman WA et al (2004) Morbidity of temporary loop ileostomies. Dig Surg 21(4):277–281
Bailey CM, Wheeler JM, Birks M et al (2003) The incidence and causes of permanent stoma after anterior resection. Colorectal Dis 5(4):331–334
Lindgren R, Hallbook O, Rutegard J et al (2011) What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum 54(1):41–47
Kolkert JL, Havenga K, ten Cate Hoedemaker HO et al (2011) Protection of stapled colorectal anastomoses with a biodegradable device: the C-seal feasibility study. Am J Surg 201(6):754–758
Morks AN, Havenga K, ten Cate Hoedemaker HO et al (2011) C-seal for prevention of anastomotic leakage following colorectal anastomosis. Ned Tijdschr Geneeskd 155:A2812
Morks AN, Havenga K, Ten Cate Hoedemaker HO, et al (2010) The C-seal: a biofragmentable drain protecting the stapled colorectal anastomosis from leakage. J.Vis.Exp. Nov 4;(45). pii: 2223. doi(45):10.3791/2223
Buchs NC, Gervaz P, Secic M et al (2008) Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 23(3):265–270
Khan AA, Wheeler JM, Cunningham C et al (2008) The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis 10(6):587–592
Damrauer SM, Bordeianou L, Berger D (2009) Contained anastomotic leaks after colorectal surgery: are we too slow to act? Arch Surg 144(4):333–338, discussion 338
Rahbari NN, Weitz J, Hohenberger W et al (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of rectal cancer. Surgery 147(3):339–351
Eckmann C, Kujath P, Schiedeck TH et al (2004) Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Colorectal Dis 19(2):128–133
Akyol AM, McGregor JR, Galloway DJ et al (1992) Early postoperative contrast radiology in the assessment of colorectal anastomotic integrity. Int J Colorectal Dis 7(3):141–143
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Collaborators
Christiaan Hoff, MD PhD (Medical Center Leeuwarden, Department of Surgery, Leeuwarden, the Netherlands)
Frank W.H. Kloppenberg, MD (Ommelander Hospital Group, Department of Surgery, Delfzijl, the Netherlands)
Wim A. Bleeker, MD PhD (Wilhelmina Hospital, Department of Surgery, Assen, the Netherlands)
Erik Jan Mulder, MD (Antonius Hospital, Department of Surgery, Sneek, the Netherlands)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Morks, A.N., Havenga, K., ten Cate Hoedemaker, H.O. et al. Thirty-seven patients treated with the C-seal: protection of stapled colorectal anastomoses with a biodegradable sheath. Int J Colorectal Dis 28, 1433–1438 (2013). https://doi.org/10.1007/s00384-013-1724-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-013-1724-7