Abstract
Utilising finite element analyses and experimental testing, this study investigates the influence of scaffold porosity on mechanical behaviour and evaluates the potential of polylactic acid (PLA) and polyvinylidine fluoride (PVDF) as bone substitute materials. Scaffold geometries were devised using design parameters adapted from extant literature and then generated using computer-aided engineering tools. Methodical variations in strand thickness were applied, maintaining other design criteria constant for robust analysis. Results, derived under varied loading conditions, suggest that scaffold mechanical properties are influenced significantly by geometry, strand diameter and porosity. Cubic scaffolds exhibited marked strength. Structures with reduced porosity demonstrated heightened mechanical characteristics, while facilitating bone cell proliferation. For a comparative context, PVDF scaffolds were benchmarked against human femur bone properties, revealing a mechanical behaviour alignment, particularly in their Young’s modulus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bones possess the inherent ability to heal minor damages. However, severe clinical conditions can impede this natural healing process [1,2,3]. While autogenous bone grafts remain the preferred treatment in such situations, they present challenges including donor site complications and limited graft availability [4]. As a result, the focus has shifted to bone tissue engineering (BTE) techniques. These techniques integrate osteogenic cells, biological factors, and 3D scaffolds to promote enhanced bone growth [5, 6]. The scaffolds, typically made of materials such as polymers, ceramics and composites, are integral to the process [5, 7, 8]. Despite their robust mechanical properties, these materials might disrupt essential signaling pathways vital for bone remodeling. This has led to a rising interest in bio-inspired materials, especially piezoelectric ones akin to bones. Such materials generate charges when subjected to pressure, strengthening the impacted region [9, 10].
Polylactic acid (PLA) and polyvinylidine fluoride (PVDF) stand out as frontrunners in the realm of bio-inspired materials. PLA, known for its biocompatibility, biodegradability, absorbability and robust mechanical properties, has gained prominence in medical devices and bone replacements [11, 12]. Moreover, its cost-effectiveness and adaptability with commercial 3D printers have bolstered its widespread use in large-scale applications [13]. On the other hand, PVDF, among piezoelectric polymers, boasts the most fitting mechanical attributes for BTE. Its processability and biocompatibility further enhance its appeal [14, 15]. Notably, PVDF exhibits five distinct crystalline polymorphic phases, with \(\beta\)-phase having the highest piezoelectric coefficient, reaching 20 pC/N [14]. The electroactive environment created by PVDF scaffolds enhances collagen mineralisation, leading to stimulated bone regeneration [16].
Choosing the appropriate material and fabrication method for scaffolding are not the only challenge. The design of the scaffold, from its geometric shape to the dimensions of its pores, plays a pivotal role in determining its effectiveness [17]. While there exist general guidelines for porosity and pore size [18, 19], maintaining an equilibrium between structural integrity and biological performance is complex. Notably, scaffolds with concave surfaces have been observed to promote higher cell proliferation than those with flat or convex surfaces [18]. Additionally, a high degree of interconnectedness among the pores is vital to encourage cell movement and ensure the seamless flow of nutrients [7]. While the most commonly recommended range of pore size for bone tissue scaffolds is 100–300 \(\upmu\)m [18, 19], studies have demonstrated successful osteoconduction in scaffolds with pores as large as 650 \(\upmu\)m [20]. It is noted that while high porosity and interconnectivity can enhance biological performance, they might compromise the scaffold’s mechanical stability, particularly in situations demanding load-bearing. Hence, it is imperative to maintain a balance between the scaffold’s porosity and its mechanical strength.
For the fabrication of bone scaffolds, a high porosity in the range of 40–90% is recommended [21,22,23]. However, a study conducted by Gregor et al. [24] found that scaffolds with 30–50% porosity demonstrated cell attachment and proliferation comparable to scaffolds with 90% porosity. Additionally, these scaffolds retained optimal mechanical properties, indicating that compliance with 90% porosity might not be essential for achieving osteoconduction. During the fabrication phase, achieving consistent control over aspects such as porosity, pore dimensions, pore morphology, and interconnectivity proves challenging. Modifying one attribute often inadvertently affects others, complicating the task of studying the independent impact of a single parameter using traditional techniques [18].
The intricacies of biological processes in BTE, coupled with the high costs of experimental tests and challenges in evaluating each design parameter in isolation, have led to the adoption of computer-aided design and computational modelling. These tools offer a deeper understanding and more precise predictions about the effects of design parameter modifications on scaffold mechanical performance [25]. Utilising these tools, Noordin et al. [26] designed a series of geometries with cubic base shape and porosities above 80%, while maintaining optimal mechanical properties. Numerical analysis showed a direct correlation between Young’s modulus and surface area, where the scaffold with highest surface area and smallest pore sizes possessed the highest Young’s modulus, a finding corroborated by subsequent studies [27] and [28].
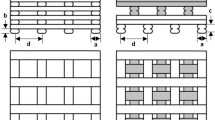
Comparison of natural bone ECM and multi-layered scaffold designs from literature: a anatomy of human long bone in transverse plane (image adapted from [29]), b Layer-by-layer printed cubic scaffold featuring square lattice layers, showcasing different lay-down angles and associated unit cells in the XY plane (image adapted from [28]), and (c) Cylindrical scaffold with an approximate porosity of 30% and 50% (image adapted from [24])
Whulanza et al. [30] explored the influence of geometry and porosity on bone scaffolds, designing a range with cubic bases and diverse pore sizes. Their finite element analysis (FEA) results showed that scaffolds with smaller pores possessed superior elastic modulus and yield strength, affirming that more compact scaffolds will result in stronger scaffolds. Figure 1 displays natural extracellular matrix (ECM) of long bones compared to cubic and cylindrical scaffolds proposed by Zhang et al. [28] and Gregor et al. [24]. In their designs, the strands are placed next to and on top one another to form the geometry, but every strand is not connected to the ones parallel to it. This paper introduces geometries where each scaffold layer consists of a continuous strand, enhancing scaffold stiffness.
Despite the advancements in this field, a clear understanding of how varying porosities influence the mechanical performance of polylactic acid (PLA) and polyvinylidine fluoride (PVDF) bone scaffolds remains elusive. While PLA and PVDF have been individually studied for their potential as bone scaffold materials, comprehensive comparative analyses investigating their relative suitability across varying scaffold geometries, porosity levels and under different loading conditions, seem to be largely absent from the current body of literature. Such a comparison is crucial to fully understand their potential and limitations as bone substitutes, and to ascertain how design parameters can be optimised to leverage their individual strengths. The specific effects of parameters such as scaffold geometry, strand diameter and pore size on the mechanical properties of these scaffolds appear to be inadequately explored. Therefore, a precise characterisation of these parameters is needed to predict how these scaffolds might behave post-implantation and to optimise their design for enhanced compatibility and function.
In this paper, FEA is utilised to explore the impact of porosity on the mechanical behaviour of polylactic acid (PLA) and polyvinylidine fluoride (PVDF) bone scaffolds. A series of experimental tests have been conducted to validate the findings from the FEA. Scaffolds with various porosities are obtained by maintaining all design parameters constant and altering the thickness of strands. The suitability of PLA and PVDF as bone substitutes is evaluated through stress analysis, with the results compared to the mechanical properties of human femoral bones. Findings indicate that the geometry, strand diameter, and pore size significantly enhance the mechanical properties of PLA and PVDF bone scaffolds.
2 Methodology
The applicability of cubic scaffolds has been proven in previous studies focusing on BTE purposes [26,27,28, 31, 32]. In other investigations addressing large defects of femur bone, cylindrical scaffolds have demonstrated their potential as bone substitutes [13, 24, 33,34,35]. Based on experimental data and FEA studies, the recommended dimensions for the scaffold’s radius, length, and height are 10 mm, 10 mm, and 10–12 mm, respectively [13, 31, 33,34,35,36]. Hence, in this paper, cubic and cylindrical base shapes are adapted.
Using design parameters sourced from the existing literature, scaffold geometries have been formulated. These parameters play a key role in ensuring that the scaffolds align with contemporary research and established norms. Utilising the SolidWorks computer-aided design software, these geometries are meticulously crafted. A visual representation of this process is presented in Fig. 2.
Geometric construction of multi-layered strand-based cubic and cylindrical scaffolds. The construction begins with the creation of a unique strand using the sweep function in SolidWorks. This process ensures uniformity and precision in the strand’s formation. Subsequent layers are then stacked with orientation angles of 90\(^\circ\), 180\(^\circ\) and 270\(^\circ\), showcasing the intricate interplay of the strands in the third dimension
Eight distinct geometries are modeled as depicted in Fig. 3. The cubic model has a square dimension of 10 mm \(\times\) 10 mm, while the cylinder has a diameter of 10 mm. Both geometries have a height of 10.5 mm. Four strand thicknesses are investigated: 350, 525, 700, and 875 \(\upmu\)m. These variations lead to different pore sizes and porosities. The following equation has been used to determine the porosity of the scaffolds [37]:
where V\(_\textrm{solid}\) is the volume of the solid part, and V\(_\textrm{total}\) is the total volume of the scaffold.
Finite element analysis (FEA) is performed using the Ansys finite element software package to assess the mechanical behaviour of the scaffolds under various loading conditions.
3 Results and discussion
To identify materials that mimic the mechanical properties of natural bones, results are compared with those from structures having properties similar to human femoral bones. This study evaluated scaffolds made from PLA, PVDF, and actual human femoral bones. The material properties used are detailed in Table 1.
Boundary conditions are chosen to reflect the natural stresses on human femoral bones. The scaffold’s bottom surface is anchored, while a 700 N force is applied at its top surface, simulating the stress from an average 70 kg adult’s body weight. This force mirrors the stress exerted on the femur due to the body weight of an average 70 kg adult. The number of elements used in the FEA ranged between 30,623 and 196,385.
To validate the FEA findings, a series of experimental tests was executed, assessing the scaffold’s mechanical strength under compression. All eight geometries were fabricated using an FDM printer with PLA RS Pro 1.75 mm filament. The TA.XTPlus Texture Analyzer by Stable Micro Systems, fitted with a 35 mm cylindrical probe, was used for compression testing.
3.1 Porosity and pore size
While the recommended porosity for bone scaffolds is in the range of 40–90% [23], the porosity of scaffolds in this study were found ranging from 16.90 to 68.35%, varying by structure and strand thickness. The average porosity of the cubic and cylindrical models are calculated to be 41.92% and 44.61%, respectively. As detailed in Table 2, strand thickness of 350 \(\upmu\)m resulted in the highest porosity while as expected, 875 \(\upmu\)m strand thickness produced lowest porosity. The respective pore sizes for strand thicknesses of 350, 525, 700, and 875 \(\upmu\)m were 650, 475, 300, and 125 \(\upmu\)m, respectively.
As noted by Muthu et al. [37], thinner strands lead to larger pore size, providing suitable environment and space for new cell formation. Therefore, scaffolds with higher porosity are preferred for TE applications. However, pore size should remain within practical bounds to maintain the scaffold’s mechanical stability. Comparing porosity with stress analysis can pinpoint the ideal balance between pore size and mechanical robustness for BTE use. The correlation between strand thickness and scaffold porosity is illustrated in Fig. 4.
3.2 Experimental validation
The designed scaffolds were effectively 3D printed and subsequently tested using a texture analyser, as depicted in Fig. 5. The outcomes from these experimental testings closely mirrored the results derived from FEA, underscoring the accuracy of our simulations. Notably, the variance between the simulation predictions and actual experimental data averaged 2.6%, confirming their close alignment. The force-deformation relationship for models 4 and 8 is presented in Fig. 6.
3.3 Stress analysis
FEA findings indicate the most deformation in cylindrical scaffolds with the thinnest strands and the least in cubic ones with the thickest strands. Cubic scaffolds consistently show less deformation than their cylindrical equivalents across all strand thicknesses, implying superior mechanical strength in the cubic geometry (Table 3). This could be attributed to the lower porosity of cubic scaffolds, irrespective of strand size, when contrasted with cylindrical ones. Comparing PLA and PVDF, the deformation in PVDF scaffolds is more akin to that in femur bone, while PLA shows the least deformation among the three. This lower deformation observed in PLA can be ascribed to its higher Young’s modulus, leading to stiffer scaffolds. Figure 7 visually contrasts deformation in cubic and cylindrical scaffolds under 700 N across different strand sizes.
Table 4 lists the value of equivalent stress for the eight geometries under a 700 N force. The stress values range from 26.61–851.49 MPa for bone, 26.08–850.88 MPa for PLA, and 25.70–850.12 MPa for PVDF. Notably, the stress in PLA scaffolds is more akin to that of femur bones. When assessing the percentage difference in stress values, cubic geometries more closely mirror bone stress, averaging 1.27% and 2.18% differences for PLA and PVDF, respectively. In contrast, cylindrical geometries show larger deviations, averaging 2.87% for PLA and 4.96% for PVDF. Both PLA and PVDF have stress values lower than that of the femur bone.
Figures 8, 9, 10, 11 depict the contour plot of equivalent stress results of both cubic and cylindrical models under 700 N mechanical loading. Comparison of equivalent stress results for eight models with three assigned materials indicates reveals that geometries with greater porosity experience higher stress. This is consistent with findings from previous studies in the literature [13, 28], which indicates that scaffolds with higher porosity exhibit lower mechanical performance. Notably, the stress in PLA scaffolds is more comparable to femur bones, with PVDF scaffolds showing the least stress and femur bones the most. This difference can be attributed to the PVDF’s high Poisson’s ratio and the lower ratios of femur bone and PLA. Additionally, cylindrical geometries undergo more stress than cubic ones under identical mechanical loads, which can be linked to the cubic scaffolds’ enhanced strength and reduced porosity.
4 Discussion
While earlier studies advocate for higher porosity in bone scaffold fabrication [21,22,23], the current study indicates that even scaffolds with porosities as low as 16.9–20.91% can demonstrate favorable mechanical properties. Considering the parameters and measurements of design of these geometries, the obtained results suggest that models with thickest strands and lower porosities can produce pore sizes within the acceptable range for osteoconduction, and can be expected to provide suitable environment for osteocyte attachment and proliferation. The study by Gregor et al. [24] supports this finding, demonstrating that scaffolds with a porosity range of 30–50% exhibit cell attachment and growth rates comparable to those observed in scaffolds with 90% porosity. Prior research has identified the minimum pore size for osteoinduction as 100 \(\upmu\)m [42], with peak bone formation in implants having pore sizes between 150 and 200 \(\upmu\)m [43]. Therefore, the proposed designs meet the necessary porosity criteria while offering optimal mechanical strength.
5 Conclusion
This study examined the behaviour of bone, PLA and PVDF scaffolds in cubic and cylindrical shapes with varying porosities under load. The primary objectives were to compare the mechanical responses of structures based on their porosity and to assess the suitability of PLA and PVDF as bone scaffold materials. Porosity effects were explored by adjusting strand thickness while keeping other design parameters constant. The performance of PLA and PVDF was gauged by comparing their deformation and stress to that of femur bone. These findings can help predict scaffold mechanical responses post-implantation due to patient body weight. It was found that structures with as low as 16.9% porosity showcase enhanced mechanical properties while preserving optimal pore sizes for bone cell growth. The cubic scaffolds with the thickest strands showed the least total deformation, while cylindrical scaffolds with the largest strand diameters exhibited the lowest equivalent stress values. PVDF’s mechanical behaviour closely mirrored that of femur bones, attributed to its Young’s modulus being more akin to bones. Future studies will encompass additional geometrical designs and optimization of the proposed designs.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
Atala A (2012) Regenerative medicine strategies. J Pediatr Surg 47(1):17–28
Bhatia SN, Chen CS (1991) Tissue engineering at the micro-scale. Biomed Microdevice 2(2):131–144
Archunan M, Petronis S (2021) Bone grafts in trauma and orthopaedics. Cureus 13(9)
Baldwin P, Li D, Auston D, Mir H, Yoon R, Koval K (2019) Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthopaed Trauma 1
Black C, Goriainov V, Gibbs D, Kanczler J, Tare R, Oreffo R (2015) Bone tissue engineering. Curr Mol Biol Rep 1(3)
Motamedian S, Iranparvar P, Nahvi G, Khojasteh A (2016) Bone tissue engineering: a literature review. Regen Reconstr Restor 1(3)
Salah M, Tayebi L, Moharamzadeh K, Naini F (2020) Three-dimensional bio-printing and bone tissue engineering: technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac Plast Reconstr Surg 42(18)
Stevens M (2008) Biomaterials for bone tissue engineering. Mater Today 11(5)
Jacob J, More N, Kalia K, Kapusetti G (2018) Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regener 38(2)
Tandon B, Blaker JJ, Cartmell SH (2018) Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater 73
Pawar RP, Tekale SU, Shisodia SU, Totre JD, Domb AJ (2014) Biomedical applications of poly(lactic acid). Recent Pat Regen Med 4(1):40–51
Baptista R, Guedes M (2022) Morphological and mechanical characterization of 3d printed pla scaffolds with controlled porosity for trabecular bone tissue replacement. Mater Sci Eng C 118
Dussault A, Pitaru A, Weber M, Haglund L, Rosenzweig DH, Villemure I (2022) Optimizing design parameters of pla 3d-printed scaffolds for bone defect repair. Surgeries 3
Zaszczyńska A, Sajkiewicz P, Gradys A (2020) Piezoelectric scaffolds as smart materials for neural tissue engineering. Polymers 12
Samadi A, Salati M, Safari A, Jouyandeh M, Barani M, Chauhan N, Golab E, Zarrintaj P, Kar S, Seidi F, Hejna A, Paran S (2022) Comparative review of piezoelectric biomaterials approach for bone tissue engineering. J Biomater Sci Polym Ed 33
Szewczyk P, Metwally S, Karbowniczek J, Marzec M, Stodolak-Zych E, GruszczyĔski A, Bernasik A, Stachewicz U (2018) Surface-potential-controlled cell proliferation and collagen mineralization on electrospun polyvinylidene fluoride (pvdf) fiber scaffolds for bone regeneration. ACS Biomater Sci Eng 5
Marew T, Birhanu G (2021) Three dimensional printed nanostructure biomaterials for bone tissue engineering. Regen Ther 18
Zadpoor A (2014) Bone tissue regeneration: the role of scaffold geometry. Biomater Sci
Bose S, Roy M, Bandyopadhyay A (2012) Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30(10)
Deng F, Liu L, Li Z, Liu J (2021) 3d printed ti6al4v bone scaffolds with different pore structure effects on bone ingrowth. J Biol Eng 15
Abbasi N, Hamlet S, Love R, Nguyen N (2020) Porous scaffolds for bone regeneration. J Sci Adv Mater Devices 5(1)
Kim T, Kim M, Goh T, Lee J, Kim Y, Yoon S, Lee C (2019) Evaluation of structural and mechanical properties of porous artificial bone scaffolds fabricated via advanced tba-based freeze-gel casting technique. Appl Sci 9(9)
Karageorgiou V, Kaplan D (2005) Porosity of 3d biomaterial scaffolds and osteogenesis. Biomaterials 26
Gregor A, Filova E, Novak M, Kronek J, Chlup H, Buzgo M, Blahnova V, Lukasova V, Bartos M, Necas A, Hosek J (2017) Designing of pla scaffolds for bone tissue replacement fabricated by ordinary commercial 3d printer. J Biol Eng 11(31)
Oliveras A, Damien L (2013) Computational methods in modeling of scaffolds for tissue engineering. Comput Model Tissue Eng 107–126
Noordin M, Md Saad A, Ngadiman N, Mustafa N, Mohd Yusof N, Ma’aram A (2021) Finite element analysis of porosity effects on mechanical properties for tissue engineering scaffold. Biointerface Res Appl Chem 11
Tang M, Kadir A, Ngadiman N (2020) Simulation analysis of different bone scaffold porous structures for fused deposition modelling fabrication process. IOP Conf Ser Mater Sci Eng 788
Zhang B, Guo L, Chen H, Ventikos Y, Narayan R, Huang J (2020) Finite element evaluations of the mechanical properties of polycaprolactone/hydroxyapatite scaffolds by direct ink writing: effects of pore geometry. J Mech Behav Biomed Mater 104
Paulsen F, Waschke J (2010) Sobotta Atlas der Anatomie des Menschen. Urban & Fischer Verlag/Elsevier GmbH, München, Munich
Whulanza Y, Istiyanto J, Ramadhan T (2013) Fabrication and characterization of pla scaffolds for bone tissue engineering. In: The 12th annual national seminar of mechanical engineering
Rosenzweig D, Carelli E, Steffen T, Jarzem P, Haglund L (2015) 3d-printed abs and pla scaffolds for cartilage and nucleus pulposus tissue regeneration. Int J Mol Sci 16
Rodrigues N, Benning M, Ferreira A, Dixon L, Dalgarno K (2016) Manufacture and characterisation of porous pla scaffolds. Proc CIRP 49
Fan J, Liu X (2013) A finite element model revealing stress distribution on tissue engineering scaffold in perfused bioreactor. IFMBE Proc 39
Jahir-Hussain M, Maaruf N, Esa N, Jusoh N (2021) The effect of geometry on the mechanical properties of 3d printed bone scaffold due to compressive loading. Mater Sci Eng 1051
Serra T, Planell J, Navarro M (2013) High-resolution pla-based composite scaffolds via 3-d printing technology. Acta Biomater 9
Germain L, Fuentes C, Vuure A, Rieux A, Dupont-Gillain C (2018) 3d-printed biodegradable gyroid scaffolds for tissue engineering applications. Mater Design 151
Muthu P, Mishra S, Sri Sai Shilpa R, Veerendranath B, Latha S (2018) Prediction and estimation of scaffold strength with different pore size. J Phys Conf Ser 1000
Masood MS, Ahmad A, Mufti RA (2013) Unconventional modeling and stress analysis of femur bone under different boundary conditions. Int J Sci Eng Res 4(12)
Nithin Kumar K, Griya N, Shaikh A, Chaudhry V, Chavadaki S (2020) Structural analysis of femur bone to predict the suitable alternative material. Mater Today Proc 26
Wang X, Zhao L, Fuh J, Lee H (2019) Effect of porosity on mechanical properties of 3d printed polymers: experiments and micromechanical modeling based on x-ray computed tomography analysis. Polymers 11(7)
Wire T. Cable: PVDF (Kynar) Detailed Properties, TexWire, TexLoc, Ltd., USA. http://www.texwire.us/cablewire/pvdfproperties.html
Iviglia G, Kargozar S, Baino F (2019) Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J Funct Biomater 10
Murphy C, O’Brien F (2010) Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes Migr 4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akbari, S., Khazaeinejad, P. Geometrical and mechanical analysis of polylactic acid and polyvinylidine fluoride scaffolds for bone tissue engineering. Engineering with Computers 39, 4153–4165 (2023). https://doi.org/10.1007/s00366-023-01902-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00366-023-01902-y