Abstract
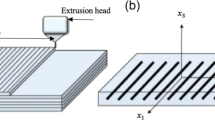
Even when damaged by injury or disease bone tissue has the remarkable ability to regenerate. When this process is limited by large size bone defects, tissue engineering is responsible for restoring, maintaining or improving tissue function. Scaffolds are support structures, designed to be implanted in the damaged site, supporting mechanical loads and protecting the regenerating bone tissue. In this paper, 3D-printed PLA scaffolds with three different porosity values and two different geometries were experimentally and numerically characterized. Micro-CT analysis showed that fused filament fabrication can be used to produce scaffolds with the desired porosity and 100% of interconnected pores. Under monotonical compression, scaffolds apparent compressive modulus increased from 89 to 918 MPa, while yield stress increased from 2.9 to 27.5 MPa as porosity decreased from 70 to 30%. Open porosity decreased up to 8% on aligned scaffolds and 14% on staggered scaffolds, after compression, while scaffold’s surface-to-volume ratio highest reduction (7.48 to 4.55 mm−1) was obtained with aligned low porosity scaffolds. Micro-CT volume reconstruction allowed for scaffold simplified numerical models to be built and analyzed. Excellent agreement was found when predicting scaffold’s apparent compressive modulus. Overall, it can be concluded that 3D printing is a viable scaffold manufacturing technique for trabecular bone replacement.

















Similar content being viewed by others
References
Gregor A, Filová E, Novák M et al (2017) Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng 11:1–21. https://doi.org/10.1186/s13036-017-0074-3
Roseti L, Parisi V, Petretta M et al (2017) Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C 78:1246–1262. https://doi.org/10.1016/j.msec.2017.05.017
Sin D, Miao X, Liu G et al (2010) Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater Sci Eng C 30:78–85. https://doi.org/10.1016/j.msec.2009.09.002
Thadavirul N, Pavasant P, Supaphol P (2014) Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. J Biomed Mater Res Part A 102:3379–3392. https://doi.org/10.1002/jbm.a.35010
Intranuovo F, Gristina R, Brun F et al (2014) Plasma modification of PCL porous scaffolds fabricated by solvent-casting/particulate-leaching for tissue engineering. Plasma Process Polym 11:184–195. https://doi.org/10.1002/ppap.201300149
Tuzlakoglu K, Bolgen N, Salgado AJ et al (2005) Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci Mater Med 16:1099–1104. https://doi.org/10.1007/s10856-005-4713-8
Thomson RC, Wake MC, Yaszemski MJ, Mikos AG (1995) Biodegradable polymer scaffolds to regenerate organs. Adv Polym Sci 122:245–274
Trachtenberg JE, Mountziaris PM, Miller JS et al (2014) Open-source three-dimensional printing of biodegradable polymer scaffolds for tissue engineering. J Biomed Mater Res Part A 102:4326–4335. https://doi.org/10.1002/jbm.a.35108
Nam YS, Park TG (1999) Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res 47:8–17. https://doi.org/10.1002/(SICI)1097-4636(199910)47:1%3c8::AID-JBM2%3e3.0.CO;2-L
Do KH, Bae EH, Kwon IC et al (2004) Effect of PEG–PLLA diblock copolymer on macroporous PLLA scaffolds by thermally induced phase separation. Biomaterials 25:2319–2329. https://doi.org/10.1016/j.biomaterials.2003.09.011
Rowlands AS, Lim SA, Martin D, Cooper-White JJ (2007) Polyurethane/poly(lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials 28:2109–2121. https://doi.org/10.1016/j.biomaterials.2006.12.032
Malmström J, Adolfsson E, Emanuelsson L, Thomsen P (2008) Bone ingrowth in zirconia and hydroxyapatite scaffolds with identical macroporosity. J Mater Sci Mater Med 19:2983–2992. https://doi.org/10.1007/s10856-007-3045-2
Ravi P, Shiakolas PS, Welch TR (2017) Poly-l-lactic acid: pellets to fiber to fused filament fabricated scaffolds, and scaffold weight loss study. Addit Manuf 16:167–176. https://doi.org/10.1016/j.addma.2017.06.002
Germain L, Fuentes CA, van Vuure AW et al (2018) 3D-printed biodegradable gyroid scaffolds for tissue engineering applications. Mater Des 151:113–122. https://doi.org/10.1016/j.matdes.2018.04.037
Korpela J, Kokkari A, Korhonen H et al (2013) Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J Biomed Mater Res Part B Appl Biomater 101B:610–619. https://doi.org/10.1002/jbm.b.32863
Gleadall A, Visscher D, Yang J et al (2018) Review of additive manufactured tissue engineering scaffolds: relationship between geometry and performance. Burn Trauma 6:19. https://doi.org/10.1186/s41038-018-0121-4
Zhang L, Yang G, Johnson BN, Jia X (2019) Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater 84:16–33. https://doi.org/10.1016/j.actbio.2018.11.039
Cavo M, Scaglione S (2016) Scaffold microstructure effects on functional and mechanical performance: integration of theoretical and experimental approaches for bone tissue engineering applications. Mater Sci Eng C 68:872–879. https://doi.org/10.1016/j.msec.2016.07.041
Hollister S, Lin C, Saito E et al (2005) Engineering craniofacial scaffolds. Orthod Craniofacial Res 8:162–173. https://doi.org/10.1111/j.1601-6343.2005.00329.x
Karageorgiou V, Kaplan D (2005) Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26:5474–5491. https://doi.org/10.1016/j.biomaterials.2005.02.002
Moroni L, Poort G, Van Keulen F et al (2006) Dynamic mechanical properties of 3D fiber-deposited PEOT/PBT scaffolds: an experimental and numerical analysis. J Biomed Mater Res Part A 78A:605–614. https://doi.org/10.1002/jbm.a.30716
Wieding J, Wolf A, Bader R (2014) Numerical optimization of open-porous bone scaffold structures to match the elastic properties of human cortical bone. J Mech Behav Biomed Mater 37:56–68. https://doi.org/10.1016/j.jmbbm.2014.05.002
Baptista R, Guedes M, Pereira MFC et al (2020) On the effect of design and fabrication parameters on mechanical performance of 3D printed PLA scaffolds. Bioprinting 20:e00096. https://doi.org/10.1016/j.bprint.2020.e00096
Baptista R, Guedes M (2020) Design and printing parameters effect on PLA fused filament fabrication scaffolds. In: Almeida H, Vasco J (eds) Progress in digital and physical manufacturing. Springer, Cham, pp 131–136
Jia S, Yu D, Zhu Y et al (2017) Morphology, crystallization and thermal behaviors of PLA-based composites: wonderful effects of hybrid GO/PEG via dynamic impregnating. Polymers (Basel) 9:528–547
Müller AJ, Ávila M, Saenz G, Salazar J (2015) Crystallization of PLA-based Materials. In: Jiménez A, Peltzer M, Ruseckaite R (eds) Poly(lactic acid) science and technology: processing, properties, additives and applications. The Royal Society of Chemistry, London, pp 66–98
Gregorova A (2013) Application of differential scanning calorimetry to the characterization of biopolymers. In: Elkordy AA (ed) Applications of calorimetry in a wide context—differential scanning calorimetry, isothermal titration calorimetry and microcalorimetry. InTech, Rijeka, pp 1–20
Fischer EW, Sterzel HJ, Wegner G (1973) Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z und Zeitschrift für Polym 251:980–990. https://doi.org/10.1007/BF01498927
Domingos M, Chiellini F, Gloria A et al (2012) Effect of process parameters on the morphological and mechanical properties of 3D bioextruded poly(ε-caprolactone) scaffolds. Rapid Prototyp J 18:56–67. https://doi.org/10.1108/13552541211193502
Liu H, Ahlinder A, Yassin MA et al (2020) Computational and experimental characterization of 3D-printed PCL structures toward the design of soft biological tissue scaffolds. Mater Des 188:108488. https://doi.org/10.1016/j.matdes.2020.108488
Vikingsson L, Gómez-Tejedor JA, Gallego Ferrer G, Gómez Ribelles JL (2015) An experimental fatigue study of a porous scaffold for the regeneration of articular cartilage. J Biomech 48:1310–1317. https://doi.org/10.1016/j.jbiomech.2015.02.013
Esposito Corcione C, Gervaso F, Scalera F et al (2019) Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram Int 45:2803–2810. https://doi.org/10.1016/j.ceramint.2018.07.297
Serra T, Ortiz-Hernandez M, Engel E et al (2014) Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Mater Sci Eng C 38:55–62. https://doi.org/10.1016/j.msec.2014.01.003
Hoque ME, Hutmacher DW, Feng W et al (2005) Fabrication using a rapid prototyping system and in vitro characterization of PEG-PCL-PLA scaffolds for tissue engineering. J Biomater Sci Polym Ed 16:1595–1610. https://doi.org/10.1163/156856205774576709
Todo M, Kuraoka H, Kim J et al (2008) Deformation behavior and mechanism of porous PLLA under compression. J Mater Sci 43:5644–5646
Baptista R, Guedes M (2021) Morphological and mechanical characterization of 3D printed PLA scaffolds with controlled porosity for trabecular bone tissue replacement. Mater Sci Eng C 118:111528. https://doi.org/10.1016/j.msec.2020.111528
Burg K (2014) Poly(α-ester)s. In: Natural and synthetic biomedical polymers. Elsevier, pp 115–121
Kister G, Cassanas G, Vert M (1998) Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer (Guildf) 39:267–273. https://doi.org/10.1016/S0032-3861(97)00229-2
Ross G, Ross S, Tighe BJ (2017) Bioplastics: new routes, new products. In: Brydson’s plastics materials. Butterworth-Heinemann, pp 631–652
Valainis D, Dondl P, Foehr P et al (2019) Integrated additive design and manufacturing approach for the bioengineering of bone scaffolds for favorable mechanical and biological properties. Biomed Mater 14:065002. https://doi.org/10.1088/1748-605X/ab38c6
Senatov FS, Niaza KV, Stepashkin AA, Kaloshkin SD (2016) Low-cycle fatigue behavior of 3d-printed PLA-based porous scaffolds. Compos Part B Eng 97:193–200. https://doi.org/10.1016/j.compositesb.2016.04.067
Gleadall A, Ashcroft I, Segal J (2018) VOLCO: a predictive model for 3D printed microarchitecture. Addit Manuf 21:605–618. https://doi.org/10.1016/j.addma.2018.04.004
Marei NH, El-Sherbiny IM, Lotfy A et al (2016) Mesenchymal stem cells growth and proliferation enhancement using PLA vs PCL based nanofibrous scaffolds. Int J Biol Macromol 93:9–19. https://doi.org/10.1016/j.ijbiomac.2016.08.053
Derakhshanfar S, Mbeleck R, Xu K et al (2018) 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater 3:144–156. https://doi.org/10.1016/j.bioactmat.2017.11.008
Zein I, Hutmacher DW, Tan KC, Teoh SH (2002) Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23:1169–1185. https://doi.org/10.1016/S0142-9612(01)00232-0
Serra T, Planell JA, Navarro M (2013) High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater 9:5521–5530. https://doi.org/10.1016/j.actbio.2012.10.041
Grémare A, Guduric V, Bareille R et al (2018) Characterization of printed PLA scaffolds for bone tissue engineering. J Biomed Mater Res Part A 106:887–894. https://doi.org/10.1002/jbm.a.36289
Choi WJ, Hwang KS, Kwon HJ et al (2020) Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater Sci Eng C 110:110693. https://doi.org/10.1016/j.msec.2020.110693
Yan Y, Chen H, Zhang H et al (2019) Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 190–191:97–110. https://doi.org/10.1016/j.biomaterials.2018.10.033
Moroni L, De Wijn JR, Van Blitterswijk CA (2006) 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 27:974–985. https://doi.org/10.1016/j.biomaterials.2005.07.023
Park S, Kim G, Jeon YC et al (2009) 3D polycaprolactone scaffolds with controlled pore structure using a rapid prototyping system. J Mater Sci Mater Med 20:229–234. https://doi.org/10.1007/s10856-008-3573-4
Rodrigues N, Benning M, Ferreira AM et al (2016) Manufacture and characterisation of porous PLA scaffolds. In: Procedia CIRP. pp 33–38
Rosenzweig DH, Carelli E, Steffen T et al (2015) 3D-printed ABS and PLA scaffolds for cartilage and nucleus pulposustissue regeneration. Int J Mol Sci 16:15118–15135. https://doi.org/10.3390/ijms160715118
Szojka A, Lalh K, Andrews SHJ et al (2017) Biomimetic 3D printed scaffolds for meniscus tissue engineering. Bioprinting 8:1–7. https://doi.org/10.1016/j.bprint.2017.08.001
Wu D, Isaksson P, Ferguson SJ, Persson C (2018) Young’s modulus of trabecular bone at the tissue level: a review. Acta Biomater 78:1–12. https://doi.org/10.1016/j.actbio.2018.08.001
Li S, Zahedi A, Silberschmidt V (2018) Numerical simulation of bone cutting. In: Numerical methods and advanced simulation in biomechanics and biological processes. Elsevier, pp 187–201
Kopperdahl DL, Keaveny TM (1998) Yield strain behavior of trabecular bone. J Biomech 31:601–608. https://doi.org/10.1016/S0021-9290(98)00057-8
Fatihhi SJ, Rabiatul AAR, Harun MN et al (2016) Effect of torsional loading on compressive fatigue behaviour of trabecular bone. J Mech Behav Biomed Mater 54:21–32. https://doi.org/10.1016/j.jmbbm.2015.09.006
Taylor D (2000) Scaling effects in the fatigue strength of bones from different animals. J Theor Biol 206:299–306. https://doi.org/10.1006/jtbi.2000.2125
Skalka P, Slámečka K, Montufar EB, Čelko L (2019) Estimation of the effective elastic constants of bone scaffolds fabricated by direct ink writing. J Eur Ceram Soc 39:1586–1594. https://doi.org/10.1016/j.jeurceramsoc.2018.12.024
Provaggi E, Capelli C, Rahmani B et al (2019) 3D printing assisted finite element analysis for optimising the manufacturing parameters of a lumbar fusion cage. Mater Des 163:107540. https://doi.org/10.1016/j.matdes.2018.107540
Koc B, Acar AA, Weightman A et al (2019) Biomanufacturing of customized modular scaffolds for critical bone defects. CIRP Ann 68:209–212. https://doi.org/10.1016/j.cirp.2019.04.106
Fostad G, Hafell B, Førde A et al (2009) Loadable TiO2 scaffolds—a correlation study between processing parameters, micro CT analysis and mechanical strength. J Eur Ceram Soc 29:2773–2781. https://doi.org/10.1016/j.jeurceramsoc.2009.03.017
Liu F, Mao Z, Zhang P et al (2018) Functionally graded porous scaffolds in multiple patterns: new design method, physical and mechanical properties. Mater Des 160:849–860. https://doi.org/10.1016/j.matdes.2018.09.053
Egan PF, Gonella VC, Engensperger M et al (2017) Computationally designed lattices with tuned properties for tissue engineering using 3D printing. PLoS ONE 12:1–20. https://doi.org/10.1371/journal.pone.0182902
Lipowiecki M, Ryvolová M, Töttösi Á et al (2014) Permeability of rapid prototyped artificial bone scaffold structures. J Biomed Mater Res Part A 102:4127–4135. https://doi.org/10.1002/jbm.a.35084
Bergström JS, Hayman D (2016) An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann Biomed Eng 44:330–340. https://doi.org/10.1007/s10439-015-1455-8
Eshraghi S, Das S (2012) Micromechanical finite-element modeling and experimental characterization of the compressive mechanical properties of polycaprolactone–hydroxyapatite composite scaffolds prepared by selective laser sintering for bone tissue engineering. Acta Biomater 8:3138–3143. https://doi.org/10.1016/j.actbio.2012.04.022
Andreassen E, Andreasen CS (2014) How to determine composite material properties using numerical homogenization. Comput Mater Sci 83:488–495. https://doi.org/10.1016/j.commatsci.2013.09.006
Duan S, Xi L, Wen W, Fang D (2020) Mechanical performance of topology-optimized 3D lattice materials manufactured via selective laser sintering. Compos Struct 238:111985. https://doi.org/10.1016/j.compstruct.2020.111985
Dutra TA, Ferreira RTL, Resende HB et al (2020) A complete implementation methodology for asymptotic homogenization using a finite element commercial software: preprocessing and postprocessing. Compos Struct 245:112305. https://doi.org/10.1016/j.compstruct.2020.112305
Acknowledgements
This work was supported by FCT, through IDMEC under LAETA (project UIDB/50022/2020), CeFEMA (UID/CTM/04540/2019) and CERENA (UIDB/04028/2020). IPFN activities also received financial support from FCT through Projects UIDB/50010/2020 and UIDP/50010/2020.
Author information
Authors and Affiliations
Contributions
RB, MP, AM, and DR were involved in conceptualization. RB, MP, DR, VI, and MG were involved in methodology. RB, DR, and MG were involved in formal analysis and writing—original draft. RB, MP, AM, DR, VI, and MG were involved in investigation and resources. RB and DR were involved in software. RB and AM were involved in visualization. RB was responsible for supervision. AM was involved in data curation. MP, VI and MG were involved in writing—review and editing. VI and MG were involved in validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
Supplementary Information
Below is the link to the Supplementary Information.
Supplementary Information 2 (MP4 12377 kb)
Supplementary Information 3 (MP4 18021 kb)
Supplementary Information 4 (MP4 18476 kb)
Supplementary Information 5 (MP4 10524 kb)
Supplementary Information 6 (MP4 18818 kb)
Supplementary Information 7 (MP4 17821 kb)
Rights and permissions
About this article
Cite this article
Baptista, R., Pereira, M.F.C., Maurício, A. et al. Experimental and numerical characterization of 3D-printed scaffolds under monotonic compression with the aid of micro-CT volume reconstruction. Bio-des. Manuf. 4, 222–242 (2021). https://doi.org/10.1007/s42242-020-00122-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-020-00122-3