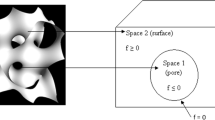
Abstract
Porous structure offers the advantage of minimizing stress shielding phenomena and supports bone ingrowth, thereby improving the long-term durability of scaffolds. Unlike conventional techniques, additive manufacturing is capable of fabricating complex pore architecture in an exceedingly controlled fashion. In this paper, implicit surface modeling technique is used to develop the triply periodic minimal surfaces-based scaffolds of varying architectures. Sheet and solid-based wrapped package graph (IWP) and diamond are investigated by finite element analysis of lattices under compression. Parameters of mathematical trigonometric functions are varied to tune the structural characteristics like pore size, porosity on the elastic moduli, and strength of the scaffold. Results indicate that morphological features could be effectively controlled to achieve the desired bone mimicking architectures. In terms of biomechanical performances, IWP and diamond structures achieved the responses similar to surrounding bone tissue and have the good agreement with the data available in the literature for the range of elastic modulus of bone for various anatomical locations; the numerical results show that the architecture, pore size, and porosity have a major impact on performances of scaffolds. Also, in the biomechanical and clinical context, this work highlights the limitations and capabilities of additively manufactured porous scaffolds, thus proposing a permissible design space for the scaffold fabricated by additive manufacturing.













Similar content being viewed by others
References
A.B. Sadegh and A. Oryan, Basic Concepts Regarding Fracture Healing and the Current Opinions and Future Directions in Managing Bone Fractures, Int. Wound J., 2015, 12, p 238–247.
G.C. Vorys, H. Bai, C. Chandhanayingyong, C.H. Lee, J.T. Compton, J.M. Caldwell, T.R. Gardner, J.J. Mao, and F.Y. Lee, Optimal Internal Fixation of Anatomically Shaped Synthetic Bone Grafts for Massive Segmental Defects of Long Bones, Clin. Biomech., 2015, 30, p 1114–1118.
O.M. Aho, P. Lehenkari, J. Ristiniemi, S. Lehtonen, J. Risteli, and H.V. Leskelä, The Mechanism of Action of Induced Membranes in Bone Repair, J. Bone Joint Surg. Am., 2013, 95, p 597–604.
J.W. May Jr., J.B. Jupiter, A.J. Weiland, and H.S. Byrd, Clinical Classification of Post-Traumatic Tibial Osteomyelitis, J. Bone Joint Surg. Am., 1989, 71, p 1422–1428.
H. Nieto and C. Baroan, Limits of Internal Fixation in Long-Bone Fracture, Orthop. Traumatol. Surg. Res., 2017, 103, p S61–S66.
D. Zhao, T. Zhu, J. Li, L. Cui, Z. Zhang, X. Zhuang, and J. Ding, Poly (Lactic-Co-Glycolic Acid)-based Composite Bone-Substitute Materials, Bioact. Mater., 2021, 6, p 346–360.
T. Zhu, M. Jiang, M. Zhang, L. Cui, X. Yang, X. Wang, G. Liu, J. Ding, and X. Chen, Biofunctionalized Composite Scaffold to Potentiate Osteoconduction, Angiogenesis, and Favorable Metabolic Microenvironment for Osteonecrosis Therapy, Bioact. Mater., 2022, 9, p 446–460.
J. Ding, J. Zhang, J. Li, D. Li, C. Xiao, H. Xiao, H. Yang, X. Zhuang, and X. Chen, Electrospun Polymer Biomaterials, Prog. Polym. Sci., 2019, 90, p 1–34.
L. Cui, J. Zhang, J. Zou, X. Yang, H. Guo, H. Tian, P. Zhang, Y. Wang, N. Zhang, X. Zhuang, Z. Li, J. Ding and X. Chen, Electroactive Composite Scaffold with Locally Expressed Osteoinductive Factor for Synergistic Bone Repair Upon Electrical Stimulation, Biomaterials, 2020, 230, p 119617.
Z. Wang, H. Le, Y. Wang, H. Liu, Z. Li, X. Yang, C. Wang, J. Ding, and X. Chen, Instructive Cartilage Regeneration Modalities with Advanced Therapeutic Implantations under Abnormal Conditions, Bioact. Mater., 2022, 11, p 317–338.
X. Wang, S. Xu, S. Zhou, W. Xu, M. Leary, P. Choong, M. Qian, M. Brandt, and Y.M. Xie, Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review, Biomaterials, 2016, 83, p 127–141.
S. Wang, L. Liu, K. Li, L. Zhu, J. Chen, and Y. Hao, Pore Functionally Graded Ti6Al4V Scaffolds for Bone Tissue Engineering Application, Mater. Des., 2019, 168, p 107643.
I.V. Okulov, A.S. Volegov, H. Attar, M. Bönisch, S. Ehtemam-Haghighi, M. Calin, and J. Eckert, Composition Optimization of Low Modulus and High-Strength TiNb-Based Alloys for Biomedical Applications, J. Mech. Behav. Biomed. Mater., 2017, 65, p 866–871.
S. Ehtemam-Haghighi, K.G. Prashanth, H. Attar, A.K. Chaubey, G.H. Cao, and L.C. Zhang, Evaluation of Mechanical and Wear Properties of TixNb7Fe Alloys Designed for Biomedical Applications, Mater. Des., 2016, 111, p 592–599.
T. Poltue, C. Karuna, S. Khrueaduangkham, S. Seehanam, and P. Promoppatum, Design Exploration of 3D-Printed Triply Periodic Minimal Surface Scaffolds for Bone Implants, Int. J. Mech. Sci., 2021, 211, p 106762.
S. Gross and E.W. Abel, A Finite Element Analysis of Hollow Stemmed Hip Prostheses as a Means of Reducing Stress Shielding of the Femur, J. Biomech., 2001, 34, p 995–1003.
Niinomi M, Nakai M, Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone, Int. J. Biomater., 2011, p 1–10.
L. Wang, J. Kang, C. Sun, D. Li, Y. Cao, and Z. Jin, Mapping Porous Microstructures to Yield Desired Mechanical Properties for Application in 3D Printed Bone Scaffolds and orthopaedic Implants, Mater. Des., 2017, 133, p 62–68.
L.J. Gibson, Biomechanics of Cellular Solids, J. Biomech., 2005, 38, p 377–399.
M.R. Dias, P.R. Fernandes, J.M. Guedes, and S.J. Hollister, Permeability Analysis of Scaffolds for Bone Tissue Engineering, J. Biomech., 2012, 45, p 938–944.
S.J. Hollister, Porous Scaffold Design for Tissue Engineering, Nat. Mater., 2005, 4, p 518–524.
T.S. Karande, J.L. Ong, and C.M. Agrawal, Diffusion in Musculoskeletal Tissue Engineering Scaffolds: Design Issues Related to Porosity, Permeability, Architecture, and Nutrient Mixing, Ann. Biomed. Eng., 2004, 32, p 1728–1743.
F.S.L. Bobbert, K. Lietaert, A.A. Eftekhari, B. Pouran, S.M. Ahmadi, H. Weinans, and A.A. Zadpoor, Additively Manufactured Metallic Porous Biomaterials Based on Minimal Surfaces: A Unique Combination of Topological Mechanical, and Mass Transport Properties, Acta Biomater., 2017, 53, p 572–584.
K.B. Hazlehurst, C.J. Wang, and M. Stanford, An Investigation into the Flexural Characteristics of Functionally Graded Cobalt Chrome Femoral Stems Manufactured Using Selective Laser Melting, Mater. Des., 2014, 60, p 177–183.
K.B. Hazlehurst, C.J. Wang and M. Stanford, A Numerical Investigation into the Influence of the Properties of Cobalt Chrome Cellular Structures on the Load Transfer to the Periprosthetic Femur Following Total Hip Arthroplasty, Med. Eng. Phys., 2014, 36, p 458–466.
T. Zhu, Y. Cui, M. Zhang, D. Zhao, G. Liu, and J. Ding, Engineered Three-Dimensional Scaffolds for Enhanced Bone Regeneration in Osteonecrosis, Bioact. Mater., 2020, 5, p 584–601.
M. Fantini and M. Curto, Interactive Design and Manufacturing of a Voronoi-Based Biomimetic Bone Scaffold for Morphological Characterization, Int. J. Interact. Des. Manuf., 2018, 12, p 585–596.
S. Gómez, M.D. Vlad, J. López, and E. Fernández, Design and Properties of 3D Scaffolds for Bone Tissue Engineering, Acta Biomater., 2016, 42, p 341–350.
P.H. Warnke, T. Douglas, P. Wollny, E. Sherry, M. Steiner, S. Galonska, S.T. Becker, I.N. Springer, J. Wiltfang, and S. Sivananthan, Rapid Prototyping: Porous Titanium Alloy Scaffolds Produced by Selective Laser Melting for Bone Tissue Engineering, Tissue Eng. Part C, 2009, 15, p 115–124.
A.A. Zadpoor, Bone Tissue Regeneration: The Role of Scaffold Geometry, Biomater. Sci., 2015, 3, p 231–245.
H. Chen, Q. Han, C. Wang, Y. Liu, B. Chen, and J. Wang, Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review, Front. Bioeng. Biotechnol., 2020, 8, p 609.
W. Peng, L. Xu, J. You, L. Fang, and Q. Zhang, Selective Laser Melting of Titanium Alloy Enables Osseointegration of Porous Multi-Rooted Implants in a Rabbit Model, Biomed. Eng. Online, 2016, 15, p 1–13.
L. Mullen, R.C. Stamp, W.K. Brooks, E. Jones, and C.J. Sutcliffe, Selective Laser Melting: A Regular Unit Cell Approach for the Manufacture of Porous, Titanium, Bone in-Growth Constructs, Suitable for Orthopedic Applications, J. Biomed. Mater. Res. B. Appl. Biomater., 2009, 89, p 325–334.
H.A. Zaharin, A.M.A. Rani, F.I. Azam, T.L. Ginta, N. Sallih, A. Ahmad, N.A. Yunus, and T.Z.A. Zulkifli, Effect of Unit Cell Type and Pore Size on Porosity and Mechanical Behavior of Additively Manufactured Ti6Al4V Scaffolds, Materials, 2018, 11, p 1–15.
B. Otsuki, M. Takemoto, S. Fujibayashi, M. Neo, T. Kokubo, and T. Nakamura, Pore Throat Size and Connectivity Determine Bone and Tissue Ingrowth into Porous Implants: Three-Dimensional Micro-CT Based Structural Analyses of Porous Bioactive Titanium Implants, Biomaterials, 2006, 27, p 5892–5900.
D.J. Yoo, Advanced Porous Scaffold Design Using Multi-Void Triply Periodic Minimal Surface Models with High Surface Area to Volume Ratios, Int. J. Precis. Eng. Manuf., 2014, 15, p 1657–1666.
H. Karcher, The Triply Periodic Minimal Surfaces of Alan Schoen and Their Constant Mean Curvature Companions, Manuscr. Math., 1989, 64, p 291–357.
S.B.G. Blanquer, M. Werner, M. Hannula, S. Sharifi, G.P.R. Lajoinie, D. Eglin, J. Hyttinen, A.A. Poot and D.W. Grijpma, Surface Curvature in Triply-Periodic Minimal Surface Architectures as a Distinct Design Parameter in Preparing Advanced Tissue Engineering Scaffolds, Biofabrication, 2017, 9, p 1–13.
J. Shi, L. Zhu, L. Li, Z. Li, J. Yang, and X. Wang, A TPMS-Based Method for Modeling Porous Scaffolds for Bionic Bone Tissue Engineering, Sci. Reports, 2018, 8, p 1–10.
X. Zheng, Z. Fu, K. Du, C. Wang, and Y. Yi, Minimal Surface Designs for Porous Materials: From Microstructures to Mechanical Properties, J. Mater. Sci., 2018, 53, p 10194–10208.
O. Al-Ketan, M. Adel Assad, and R.K. Abu Al-Rub, Mechanical Properties of Periodic Interpenetrating Phase Composites with Novel Architected Microstructures, Compos. Struct., 2017, 176, p 9–19.
I. Maskery, A.O. Aremu, L. Parry, R.D. Wildman, C.J. Tuck, and I.A. Ashcroft, Effective Design and Simulation of Surface-Based Lattice Structures Featuring Volume Fraction and Cell Type Grading, Mater. Des., 2018, 155, p 220–232.
O. Al-Ketan, R.K. Abu Al-Rub, U. Abu Dhabi, and A. Dhabi, MSLattice: A Free Software for Generating Uniform and Graded Lattices Based on Triply Periodic Minimal Surfaces, Mater. Des. Process. Commun., 2021, 3, p e205.
S.R. Burnett Johnston, J.A. Pura, B. Singh, M. Tanzer, and D. Pasini, High-Strength Porous Biomaterials for Bone Replacement: A Strategy to Assess the Interplay between Cell Morphology, Mech. Prop. Bone Ingrowth and Manuf Constraints, Acta Biomater., 2016, 30, p 345–356.
C. Kasper, F. Witte, and R. Pörtner, Tissue Engineering III: Cell - Surface Interactions for Tissue Culture, Advances in Biochemical Engineering/Biotechnology, Springer, T. Scheper, 2012, p 126
S. Vijayavenkataraman, L. Zhang, S. Zhang, J.Y.H. Fuh and W.F. Lu, Triply Periodic Minimal Surfaces Sheet Scaffolds for Tissue Engineering Applications: An Optimization Approach toward Biomimetic Scaffold Design, ACS Appl. Bio Mater., 2018, 1, p 259–269.
U. Simsek, A. Akbulut, C.E. Gayir, C. Basaran, and P. Sendur, Modal Characterization of Additively Manufactured TPMS Structures: Comparison between Different Modeling Methods, Int. J. Adv. Manuf. Technol., 2021, 115, p 657–674.
C.I. Albert, J. Jameson, and G. Harris, Design and Validation of Bending Test Method for Characterization of Miniature Pediatric Cortical Bone Specimens, Proc. Inst. Mech. Eng. H., 2013, 227, p 105–113.
J. Parthasarathy, B. Starly, S. Raman, and A. Christensen, Mechanical Evaluation of Porous Titanium (Ti6Al4V) Structures with Electron Beam Melting (EBM), J. Mech. Behav. Biomed. Mater., 2010, 3, p 249–259.
K. Hazlehurst, C.J. Wang, and M. Stanford, Evaluation of the Stiffness Characteristics of Square Pore CoCrMo Cellular Structures Manufactured Using Laser Melting Technology for Potential Orthopaedic Applications, Mater. Des., 2013, 51, p 949–955.
H. Mehboob, F. Tarlochan, A. Mehboob, and S.H. Chang, Finite Element Modelling and Characterization of 3D Cellular Microstructures for the Design of a Cementless Biomimetic Porous Hip Stem, Mater. Des., 2018, 149, p 101–112.
Y. Jin, H. Kong, X. Zhou, G. Li, and J. Du, Design and Characterization of Sheet-Based Gyroid Porous Structures with Bioinspired Functional Gradients, Mater., 2020, 13, p 3844.
M. Keshavarzan, M. Kadkhodaei, M. Badrossamay, and M.R. KaramoozRavari, Investigation on the Failure Mechanism of Triply Periodic Minimal Surface Cellular Structures Fabricated by Vat Photopolymerization Additive Manufacturing under Compressive Loadings, Mech. Mater., 2020, 140, p 103150.
F.P.W. Melchels, A.M.C. Barradas, C.A. Van Blitterswijk, J. De Boer, J. Feijen, and D.W. Grijpma, Effects of the Architecture of Tissue Engineering Scaffolds on Cell Seeding and Culturing, Acta Biomater., 2010, 6, p 4208–4217.
D. Mahmoud and M.A. Elbestawi, Selective Laser Melting of Porosity Graded Lattice Structures for Bone Implants, Int. J. Adv. Manuf. Technol., 2018, 100, p 2915–2927.
Y. Li, C. Yang, H. Zhao, S. Qu, X. Li, and Y. Li, New Developments of Ti-Based Alloys for Biomedical Applications, Materials, 2014, 7, p 1709–1800.
A.H. Burstein, D.T. Reilly, and M. Martens, Aging of Bone Tissue: Mechanical Properties, J. Bone Jt. Surg. Am., 1976, 58, p 82–86.
K.M. Abate, A. Nazir, and J.Y. Jeng, Design, Optimization, and Selective Laser Melting of Vin Tiles Cellular Structure-Based Hip Implant, Int. J. Adv. Manuf. Technol., 2021, 112, p 2037–2050.
Acknowledgements
This work was financially supported by the National Institute of Technology Raipur, India through “Research Seed Grant Project” (Sanction Order No. NITRR/Dean(R&C)/2019/116 on Dated 03/04/2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, J., Verma, R., Singh, N.K. et al. Mechanical Property Analysis of Triply Periodic Minimal Surface Inspired Porous Scaffold for Bone Applications: A Compromise between Desired Mechanical Strength and Additive Manufacturability. J. of Materi Eng and Perform 32, 3335–3347 (2023). https://doi.org/10.1007/s11665-022-07322-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-022-07322-1