Abstract
Animal life-history traits fall within limited ecological space with animals that have high reproductive rates having short lives, a continuum referred to as a “slow-fast” life-history axis. Animals of the same body mass at the slow end of the life-history continuum are characterized by low annual reproductive output and low mortality rate, such as is found in many tropical birds, whereas at the fast end, rates of reproduction and mortality are high, as in temperate birds. These differences in life-history traits are thought to result from trade-offs between investment in reproduction or self-maintenance as mediated by the biotic and abiotic environment. Thus, tropical and temperate birds provide a unique system to examine physiological consequences of life-history trade-offs at opposing ends of the “pace of life” spectrum. We have explored the implications of these trade-offs at several levels of physiological organization including whole-animal, organ systems, and cells. Tropical birds tend to have higher survival, slower growth, lower rates of whole-animal basal metabolic rate and peak metabolic rate, and smaller metabolically active organs compared with temperate birds. At the cellular level, primary dermal fibroblasts from tropical birds tend to have lower cellular metabolic rates and appear to be more resistant to oxidative cell stress than those of temperate birds. However, at the subcellular level, lipid peroxidation rates, a measure of the ability of lipid molecules within the cell membranes to thwart the propagation of oxidative damage, appear not to be different between tropical and temperate species. Nevertheless, lipids in mitochondrial membranes of tropical birds tend to have increased concentrations of plasmalogens (phospholipids with antioxidant properties), and decreased concentrations of cardiolipin (a complex phospholipid in the electron transport chain) compared with temperate birds.








Similar content being viewed by others
References
Anderson KJ, Jetz W (2005) The broad-scale ecology of energy expenditure of endotherms. Ecol Lett 8:310–318
Arendt JD (1997) Adaptive intrinsic growth rates: an integration across taxa. Quart Rev Biol 72:149–177
Austin SH, Robinson TR, Robinson WD, Ricklefs RE (2011) Potential biases in estimating the rate parameter of sigmoid growth functions. Methods Ecol Evol 2:43–51
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495
Barja G, Cadenas S, Rojas C, Perez-Campo R, Lopez-Torres M (1994) Low mitochondrial free radical production per unit O2 consumption can explain the simultaneous presence of high longevity and high aerobic metabolic rate in birds. Free Radic Res 21:317–327
Bartholomew GA (1972) Body temperature and energy metabolism. In: Gordon M (ed) Animal physiology: principles and adaptations. MacMillan, New York, pp 63–72
Bennett AF (1991) The evolution of activity capacity. J Exp Biol 160:1–23
Bennett PM, Harvey PH (1987) Active and resting metabolism in birds: allometry, phylogeny and ecology. J Zool Lond 213:327–363
Bennett AF, Ruben JA (1979) Endothermy and activity in vertebrates. Science 206:649–654
Blake JG, Loiselle BA (2008) Estimates of apparent survival rates for forest birds in eastern Ecuador. Biotropica 40:485–493
Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL et al (2004) Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Rad Biol Med 37:755–767
Braverman NE, Moser AB (2012) Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 1822:1442–1452
Brawn JD, Karr JR, Nichols JD, Robinson WD (1998) Demography of tropical forest birds in Panama: how do transients affect estimates of survival rates? In: Adams NJ, Slotow RH (eds) Proceedings 22nd International Ornithological Congress. BirdLife South Africa, Johannesburg, pp 297–305
Brawn JD, Karr JR, Nichols JD, Robinson WD (1999) Demography of forest birds in Panama: how do transients affect estimates of survival rates. In: Proceedings of the International Ornithological Congress vol. 22, pp 297–305
Brookes PS, Hulbert AJ, Brand MD (1997) The proton permeability of Liposomes made from mitochondrial inner membrane phospholipids: no effect of fatty acid composition. Bioch Biophys Acta/Biomemb 1330:157–164
Brosche T, Platt D (1998) The biological significance of plasmalogens in defense against oxidative damage. Exp Gerontol 33:363–369
Brown MF, Gratton TP, Stuart J (2007) Metabolic rate does not scale with body mass in cultured mammalian cells. Am J Physiol Regul Integr Comp Physiol 292:R2115–R2121
Bryant DM (1997) Energy expenditure in wild birds. Proc Nutr Soc 56:1025–1039
Bryant DM, Hails CJ (1983) Energetics and growth patterns of three tropical bird species. Auk 400:425–439
Brzek P, Bielawska K, Ksiazek A, Konarzewski M (2007) Anatomic and molecular correlates of divergent selection for basal metabolic rate in laboratory mice. Physiol Biochem Zool 80:491–499
Buttemer WA, Battam H, Hulbert AJ (2008) Fowl play and the price of petrel: long-living Procellariiformes have peroxidation-resistant membrane composition compared with short-living Galliformes. Biol Lett 4:351–354
Calder WA (1984) Size, function, and life history. Harvard University Press, Cambridge
Calhoon EA, Jimenez AG, Harper JM, Jurkowitz MS, and Williams JB (2014) Linkages between mitochondrial lipids and life-history in temperate and tropical birds. Physiol Biochem Zool 87. doi:10.1086/674696
Camfield AF, Pearson SF, Martin K (2010) Life history variation between high and low elevation subspecies of horned larks Eremophila spp. J Avian Biol 41:273–281
Campisi J (2001) From cells to organisms: can we learn about aging from cells in culture? Exp geront 36:607–618
Chapman LB (1955) Studies of a tree swallow colony (third paper). Bird-Banding 45–70
Chappell MA, Bech C, Buttemer WA (1999) The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J Exp Biol 202:2269–2279
Chappell MA, Garland T, Robertson GF, Saltzman W (2007) Relationships among running performance, aerobic physiology and organ mass in male Mongolian gerbils. J Exp Biol 210:4179–4197
Charnov EL (1993) Life history invariants. Oxford University Press, Oxford
Cheng YR, Martin TE (2012) Nest predation risk and growth strategies of passerine species: grow fast or develop traits to escape risk? Am Nat 180:285–295
Chicco AJ, Sparagna GC (2007) Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292:C33–C44
Cilimburg AB, Lindberg MS, Tewksbury JJ, Hejl SJ (2002) Effects of dispersal on survival probability of adult Yellow Warblers (Dendroica petechia). Auk 119:778–789
Cohen AA, Mcgraw KJ, Wiersma P, Williams JB, Robinson WD, Robinson TD, Brown JD, Ricklefs RE (2008) Interspecific association between circulating antioxidant levels and life-history variation in birds. Am Nat 172:178–193
Constantini D, Verhulst S (2009) Does high antioxidant capacity indicate low oxidative stress? Funct Ecol 23:506–509
Costantini D, Rowe M, Butler MW, McGraw KJ (2010) From molecules to living systems: historical and contemporary issues in oxidative stress and antioxidant ecology. Funct Ecol 24:950–959
Couture P, Hulbert AJ (1995) Relationship between body mass, tissue metabolic rate, and sodium pump activity in mammalian liver and kidney. Am J Physiol 268:R641–R650
Cox WA, Martin TE (2009) Breeding biology of the three-striped warbler in Venezuela: a contrast between tropical and temperate parulids. Wilson J Ornithol 121:667–678
Daan S, Masman D, Groenewold A (1990) Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am J Physiol 259:R333–R340
Daan S, Masman D, Strijkstra AM, Kenagy GJ (1991) Daily energy turnover during reproduction in birds and mammals: its relationship to basal metabolic rate. Acta XX Congr Int Ornithol 4:1976–1987
Demetrius L (2006) The origin of allometric scaling laws in biology. J Theor Biol 243:455–467
Dietz MW, Ricklefs RE (1997) Growth rate and maturation of skeletal muscles over a size range of galliform birds. Physiol Biochem Zool 70:502–510
Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86:97–116
Dohm M, Richardson C, Garland T (1994) Exercise physiology of wild and random-bred laboratory house mice and their reciprocal hybrids. Am J Physiol 36:R1098–R1108
Dowling DK, Simmons LW (2009) Reactive oxygen species as universal constraints in life-history evolution. Proc R Soc B Biol Sci 276:1737–1745
Drilling NE, Thompson CF (1988) Natal and breeding dispersal in House Wrens (Troglodytes aedon). Auk 105:480–491
Drummen GP, van Liebergen L, Op den Kamp JA, Post JA (2002) C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe:(micro) spectroscopic characterization and validation of methodology. Free Radical Biol Med 33:473–490
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Dufour E, Larsson NG (2004) Understanding aging, revealing order out of chaos. Biochim Biophys Acta 1658:122–132
Dumas J, Peyta L, Couet C, Servais S (2013) Implication of liver cardiolipins in mitochondrial energy metabolism disorder in cancer cachexia. Biochimie 95:27–32
Eli M (1992) Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN (eds) Energy metabolism: tissue determinants and cellular corollaries. Raven Press, New York, pp 61–79
Else PL, Hulbert AJ (1985) An allometric comparison of the mitochondria of mammalian and reptilian tissues: the implications for the evolution of endothermy. J Comp Phyiol B 156:3–11
Else PL, Wu BJ (1999) What role for membranes in determining the higher sodium pump molecular activity of mammals compared to ectotherms? J Comp Physiol B 169:296–302
Engelmann B, Brautigam C, Thiery J (1994) Plasmalogen phospholipids as potential protectors against lipid-peroxidation of low-density lipoproteins. Biochem Biophys Res Commun 204:1235–1242
Faaborg J, Arendt WJ (1995) Survival rates of Puerto Rican birds: are islands really that different? Auk 112:503–507
Fankhauser DP (1971) Annual adult survival rates of blackbirds and starlings. Bird-Banding 42:36–42
Felde R, Spiteller G (1995) Plasmalogen oxidation in human serum lipoproteins. Chem Phys Lipids 76:259–267
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Fontaine JJ, Martel M, Markland HM, Niklison AM, Decker KL, Martin TE (2007) Testing ecological and behavioral correlates of nest predation. Oikos 116:1887–1894
Francis CM, Terborgh JS and Fitzpatrick JW (1999) Survival rates of understory forest birds in Peru. In: Adams NJ, Slotow RH (eds) Proceedings 22nd International Ornithological Congress. BirdLife South Africa, Johannesburg, pp 326-335
Freed LA, Conant S, Fleischer RC (1987) Evolutionary ecology and radiation of Hawaiian passerine birds. Trends Ecol Evol 2:196–203
Fukagawa NK (1999) Aging: is oxidative stress a marker or is it causal? Proc Soc Exp Biol Med 222:293–298
Garrido G, Guzman M, Odriozola J (1996) Effects of physical training on fatty acid metabolism in liver and skeletal muscle of rats fed four different high- carbohydrate diets. J Nutr Biochem 7:348–355
Ghalambor CK, Martine TE (2001) Fecundity-survival trade-offs and parental risk-taking in birds. Science 292:494–497
Gill SA, Haggerty TM (2012) A comparison of life history and parental care in temperate and tropical wrens. J Avian Biol 43:461–471
Golden TR, Hinerfeld DA, Melov S (2002) Oxidative stress and aging: beyond correlation. Aging Cell 1:117–123
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Hails CJ (1983) The metabolic rate of tropical birds. Condor 85:61–65
Halliwell B, Gutteridge JM (1999) Free radicals in biology and medicine vol 135. Oxford university press, Oxford, p 729
Hammond KA, Chappell MA, Cardullo RA, Lin R-S, Johnsen TS (2000) The mechanistic basis of aerobic performance variation in red junglefowl. J Exp Biol 203:2053–2064
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Geront 11:298–300
Harman D (2001) Aging: overview. Ann N Y Acad Sci 928:1–21
Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA (2007) Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell 6:1–13
Herrero A, Barja G (1997) Sites and mechanisms responsible for the low rate of free radical production of heart mitochondria in the long-lived pigeon. Mech Ageing Dev 98:95–111
Hilton B Jr, Miller MW (2003) Annual survival and recruitment in a ruby-throated hummingbird population, excluding the effect of transient individuals. Condor 105:54–62
Huang H, Manton KG (2004) The role of oxidative damage in mitochondria during aging: a review. Front Biosci 9:1100–1111
Huang Z, Jiang J, Tyurin VA, Zhao Q, Mnuskin A, Ren J, Belikova NA, Feng W, Kurnikov IV, Kagan VE (2008) Cardiolipin deficiency leads to decreased cardiolipin peroxidation and increased resistance of cells to apoptosis. Free Radical Biol Med 44:1935–1944
Hulbert AJ, Else PL (2005) Membranes and the setting of energy demand. J Exp Biol 208:1593–1599
Hulbert AJ (2010) Metabolism and longevity: is there a role for membrane fatty acids? Integr Comp Biol 50:808–817
Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213
Jang YC, Pérez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W (2009) Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol Ser A 64:1114–1125
Jeon TI, Lim BO, Yu BP, Lim Y, Jeon EJ, Park DK (2001) Effect of dietary restriction on age-related increase of liver susceptibility to peroxidation in rats. Lipids 36:589–593
Jimenez AG, Dillaman R, Kinsey ST (2013a) Large fiber size in skeletal muscle is metabolically advantageous. Nat Commun 4:2150. doi:10.1038/ncomms3150
Jimenez AG, Harper JM, Queenborough SA, Williams JB (2013b) Linkages between the life-history evolution of tropical and temperate birds and the resistance of their cells to oxidative and non-oxidative chemical injury. J Exp Biol 216:1373–1380
Jimenez AG, van Brocklyn J, Wortman M, Williams JB (2014a) Cellular metabolic rate is influenced by life-history traits in tropical and temperate birds. PLoS ONE 9:e87349. doi:10.1371/journal.pone.0087349
Jimenez AG, Cooper-Mullin C, Anthony N, Williams JB (2014b) A comparison of cellular metabolic rate between isolated primary dermal fibroblasts and myoblast cells from fast-growing and slow-growing Coturnix quail. Comp Biochem Physiol A (in press)
Johnston JP, White SA, Peach WJ, Gregory RD (1997) Survival rates of tropical and temperate passerines: a Trinidadian perspective. Am Nat 150:771–789
Joshi AS, Zhou J, Gohil VM, Chen S, Greenberg ML (2009) Cellular functions of cardiolipin in yeast. Biochim Biophys Acta Mol Cell Res 1793:212–218
Karr JR, Nichols JD, Klimkiewicz MK, Brawn JD (1990) Survival rates of birds of tropical and temperate forests: will the dogma survive? Am Nat 136:277–291
Kersten M, Piersma T (1987) High levels of energy expenditure in shorebirds: metabolic adaptations to an energetically expensive way of life. Ardea 75:175–187
Khairallah RJ, Kim J, O’Shea KM, O’Connell KA, Brown BH, Galvao T, Daneault C, des Rosiers C, Polster BM, Hoppel CL (2012) Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLOS ONE 7:e34402
Kirkwood TB (1977) Evolution of ageing. Nature 270:301–304
Klaassen M, Drent R (1991) An analysis of hatchling resting metabolism: in search of ecological correlates that explain deviations from allometric relations. Condor 93:612–629
Kleiber M (1975) The fire of life: an introduction to animal energetics. R.E. Krieger Pub. Co, Melbourne
Koch LG, Britton SL (2008) Aerobic metabolism underlies complexity and capacity. J Physiol 586:83–95
Kowald A (2001) The mitochondrial theory of aging. Biol Signals Recept 10:162–175
Krebs HA (1950) Body size and tissue respiration. Biochim Biophys Acta 4:249–269
Lack D (1968) Bird migration and natural selection. Oikos 19:1–9
Larsen S, Nielsen J, Hansen CN et al (2012) Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590:3349–3360
Law R (1979) Optimal life histories under age-specific predation. Am Nat 114:399–417
Leray C, Cazenave J, Gachet C (2002) Platelet phospholipids are differentially protected against oxidative degradation by plasmalogens. Lipids 37:285–290
Lessig J, Fuchs B (2009) Plasmalogens in biological systems: their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr Med Chem 16:2021–2041
Longo VD, Mitteldorf J, Skulachev VP (2005) Programmed and altruistic ageing. Nat Rev Genet 6:866–872
Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R (2006) Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 103:1768–1773
Lopez-Torres M, Perez-Campo R, Cadenas S, Rojas C, Barja G (1993) A comparative study of free radicals in vertebrates-II. Non-enzymatic antioxidants and oxidative stress. Comp Biochem Physiol B Comp Biochem 105:757–763
Maeba R, Ueta N (2003) Ethanolamine plasmalogens prevent the oxidation of cholesterol by reducing the oxidizability of cholesterol in phospholipid bilayers. J Lipid Res 44:164–171
Marsh R, Dawson W (1982) Substrate metabolism in seasonally acclimatized American goldfinches. Am J Physiol 242:R563–R569
Martin TE, Li P (1992) Life history traits of open-vs. cavity-nesting birds. Ecology 73:579–592
Martin TE, Auer SK, Bassar RD, Niklison AM, Lloyd P (2007) Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution 61:2558–2569
Martin TE, Lloyd P, Bosque C, Barton DC, Biancucci AL, Cheng YR, Ton R (2011) Growth rate variation among passerine species in tropical and temperate sites: an antagonistic interaction between parental food provisioning and nest predation risk. Evolution 65:1607–1622
McNab BK (1997) On the utility of uniformity in the definition of basal rate of metabolism. Physiol Zool 70:718–720
McNab BK (2008) An analysis of the factors that influence the level and scaling of mammalian BMR. Comp Biochem Physiol 151A:5–28
McNab BK (2009) Ecological factors affect the level and scaling of avian BMR. Comp Biochem Physiol 152A:22–45
Milby MM, Wright ME (1976) Survival of house sparrows and house finches in Kern County, California. Bird-Banding 47:119–122
Mitchell T, Buffenstein R, Hulbert AJ (2007) Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): a comparative study using shotgun lipidomics. Exp Geront 42:1053–1062
Mitteldorf J, Pepper J (2009) Senescence as an adaptation to limit the spread of disease. J Theor Biol 260:186–195
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92
Morandat S, Bortolato M, Anker G, Doutheau A, Lagarde M, Chauvet JP, Roux B (2003) Plasmalogens protect unsaturated lipids against UV-induced oxidation in monolayer. Biochim Biophys Acta Biomembr 1616:137–146
Morrison P, Rosenmann M, Estes JA (1974) Metabolism and thermoregulation in the sea otter. Physiol Zool 47:218–229
Moyes CD (2003) Controlling muscle mitochondrial content. J Exp Biol 206:4385–4391
Mueller P, Diamond J (2001) Metabolic rate and environmental productivity: well- provisioned animals evolved to run and idle fast. Proc Natl Acad Sci 98:12550–12554
Munro D, Blier PU (2012) The extreme longevity of Artica islandica is associated with increased peroxidation resistance in mitochondrial membranes. Aging Cell 11:845–855
Murphy RC (2001) Free-radical-induced oxidation of arachidonoyl plasmalogen phospholipids: antioxidant mechanism and precursor pathway for bioactive eicosanoids. Chem Res Toxicol 14:463–472
Nealen P, Ricklefs R (2001) Early diversification of the avian brain: body relationship. J Zool 253:391–404
Nespolo RF, Bacigalupe LD, Sabat P, Bozinovic F (2002) Interplay among energy metabolism, organ mass and digestive enzyme activity in the mouse-opossum Thylamys elegans: the role of thermal acclimation. J Exp Biol 205:2697–2703
Oniki Y, Ricklefs RE (1981) More growth rates of birds in the humid new world tropics. Ibis 123:349–354
Oring LW, Lank DB, Maxson SJ (1983) Population studies of the polyandrous spotted sandpiper. Auk 100:272–285
Pacheco MA, Beissinger SR, Bosque C (2010) Why grow slowly in a dangerous place? Postnatal growth, thermoregulation, and energetics of nestling green-rumped parrotlets (Forpus Passerinus). Auk 127:558–570
Pape Møller A, Szép T (2002) Survival rate of adult barn swallows Hirundo rustica in relation to sexual selection and reproduction. Ecology 83:2220–2228
Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM (2010) Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res 48:297–310
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Pearl R (1928) The rate of living. University Press, London
Perez-Campo R, Lopez-Torres M, Rojas C, Cadenas S, Barja G (1994) Longevity and antioxidant enzymes, non-enzymatic antioxidants and oxidative stress in the vertebrate lung: a comparative study. J Comp Physiol B 163:682–689
Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G (1998) The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol B 168:149–158
Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G (2008) Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int 53:126–131
Piersma T (2002) Energetic bottlenecks and other design constraints in avian annual cycles. Integr Comp Biol 42:51–67
Piersma T, Bruinzeel L, Drent R, Kersten M, van der Meer J, Wiersma P (1996) Variability in basal metabolic rate of a long-distance migrant shorebird (red knot, Calidris canutus) reflects shifts in organ sizes. Physiol Zool 69:191–217
Porter RK (2001) Allometry of mammalian cellular oxygen consumption. Cell Mol Life Sci 58:815–822
Pöyry S, Cramariuc O, Postila PA, Kaszuba K, Sarewicz M, Osyczka A, Vattulainen I, Róg T (2013) Atomistic simulations indicate cardiolipin to have an integral role in the structure of the cytochrome bc 1 complex. Biochim Biophys Acta 1827:769–778
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Remeŝ V, Martin TE (2002) Environmental influences on the evolution of growth and developmental rates in passerines. Evolution 56:2505–2518
Ricklefs RE (1968) Patterns of growth in birds. Ibis 110:419–451
Ricklefs RE (1969a) Natural selection and the development of mortality rates in young birds. Nature 233:922–925
Ricklefs RE (1969b) The nesting cycle of songbirds in tropical and temperate regions. Living Bird 8:165–175
Ricklefs RE (1973) Patterns of growth in birds. II. Growth rate and mode of development. Ibis 115:177–201
Ricklefs RE (1974) Energetics of reproduction in birds. pp 152–292
Ricklefs RE (1976) Growth rates of birds in the humid New World tropics. Ibis 118:179–207
Ricklefs RE (1979) Patterns of growth in birds. V. A comparative study of development in the starling, common tern, and Japanese quail. Auk 96:10–30
Ricklefs RE (1983) Avian postnatal development. Avian Biol 7:1–83
Ricklefs RE (1997) Comparative demography of New World populations of thrushes (Turdus spp.). Ecol Monogr 67:23–43
Ricklefs RE (2000) Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor 103:9–22
Ricklefs RE, Shea RE (2007) Estimating annual survival in sexually dimorphic species from proportions of first-year birds. Ecology 88:1408–1419
Ricklefs RE, Starck JM (1996) Applications of phylogenetically independent contrasts: a mixed progress report. Oikos 77:167–172
Ricklefs RE, Webb T (1985) Water content, thermogenesis, and growth rate of skeletal muscles in the European Starling. Auk 102:369–376
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Ricklefs RE, Shea RE, Choi IH (1994) Inverse relationship between functional maturity and exponential growth rate of avian skeletal muscle: a constraint on evolutionary response. Evolution 48:1080–1088
Ricklefs RE, Tsunekage T, Shea RE (2011) Annual adult survival in several New World passerine birds based on age ratios in museum collections. J Ornithol 152:481. doi:10.1007/s10336-010-0614-9
Ringsby TH, Sæther BE, Altwegg R, Solberg EJ (1999) Temporal and spatial variation in survival rates of a house sparrow, Passer domesticus, metapopulation. Oikos 85:419–425
Robinson WD, Hau M, Klasing KC, Wikelski M, Brawn JD, Austin S, Tarwater CE, Ricklefs RE (2010) Diversification of life histories in New World birds. Auk 127:253–262
Roff DA (1992) The evolution of life histories: theory and analysis. Springer, New York
Rolfe D, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–758
Rose M (1984) Genetic covariation in Drosophila life-history: untangling the data. Am Nat 123:565–569
Rubner M (1908) Das Problem der Lebensdauer und seine Beziehungen zu Wachstum und Ernährung. R Oldenburg, Munich
Russell EM, Yom-Tov Y, Geffen E (2004) Extended parental care and delayed dispersal: northern, tropical, and southern passerines compared. Behav Ecol 15:831–838
Sæther BE (1989) Survival rates in relation to body weight in European birds. Ornis Scand 20:13–21
Sæther BE, Bakke Ø (2000) Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81:642–653
Salmon AB, Sadighi AA, Buffenstein R, Miller RA (2008) Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, ultraviolet light and endoplasmic reticulum stress. J Gereontol 63A:232–241
Samuels DC (2005) Life span is related to the free energy of mitochondrial DNA. Mech Ageing Dev 126:1123–1129
Sauer JR, Williams BK (1989) Generalized procedures for testing hypotheses about survival or recovery rates. J Wildl Manage 53:137–142
Savidge IR, Davis DE (1974) Survival of some common passerines in a Pennsylvania woodlot. Bird-banding 45:152–155
Schlame M, Ren M, Xu Y, Greenberg M, Haller I (2005) Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids 138:38–49
Schleucher E, Withers PC (2002) Metabolic and thermal physiology of pigeons and doves. Physiol Biochem Zool 75:439–450
Scholander PF et al (1950) Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic rate. Biol Bull 99:259–271
Seebacher F, Brand MD, Else PL, Guderley H, Hulbert AJ, Moyes CD (2010) Plasticity of oxidative metabolism in variable climates: molecular mechanisms. Physiol Biochem Zool 83:721–732
Selman C, Blount JD, Nussey DH, Speakman JR (2012) Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol Evol 27:570–577
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Siriwardena GM, Baillie SR, Wilson JD (1999) Temporal variation in the annual survival rates of six granivorous birds with contrasting population trends. Ibis 141:621–636
Skutch AF (1985) Clutch size, nesting success, and predation on nests of neotropical birds, reviewed. Ornithol Monogr 36:575–594
Snow DW, Lill A (1974) Longevity records for some neotropical land birds. Condor 76:262–267
Sohal RS, Allen RG (1990) Oxidative stress as a causal factor in differentiation and aging: a unifying hypothesis. Exp Gerontol 25:499–522
Sohal RS, Orr WC (2012) The redox stress hypothesis of aging. Free Radical Biol Med 52:539–555
Sorrell JM, Caplan AI (2004) Fibroblast heterogeneity: more than skin deep. J Cell Sci 117(5): 667–675
Speakman JR (2005) Body size, energy metabolism and lifespan. J Exp Biol 208:1717–1730
Speakman JR (2008) The physiological costs of reproduction in small mammals. Philos Trans R Soc 27:375–398
Speakman JR, Selman C (2011) The free radical damage theory: accumulating evidence against a simple link of oxidative stress to ageing and lifespan. BioEssays 33:255–259
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Styrsky JN (2003) Life-history evolution and population dynamics of a neotropical forest bird (Hylophylax naevioides). Thesis/Dissertation: Thesis (Ph. D.) University of Illinois at Urbana-Champaign
Suarez RK (1996) Upper limits to mass specific metabolic rates. Annu Rev Physiol 58:583–605
Surai PF (2002) Selenium in poultry nutrition I. Antioxidant properties, deficiency and toxicity. Worlds Poult Sci J Wyton 58:333–348
Swanson DL, Liknes ET (2006) A comparative analysis of thermogenic capacity and cold tolerance in small birds. J Exp Biol 209:466–474
Tieleman BI, Williams JB, Buschur ME, Brown CR (2003) Phenotypic variation of larks along an aridity gradient: are desert birds more flexible? Ecology 84:1800–1815
Tieleman BI, Dijkstra TH, Lasky JR, Mauck RA, Visser GH, Williams JB (2006) Physiological and behavioural correlates of life-history variation: a comparison between tropical and temperate zone House Wrens. Funct Ecol 20:491–499
Tjorve KMC (2007) Does chick development relate to breeding latitude in waders and gulls? Bull Wader Study Group 112:12
Valencak T, Ruf T (2007) Membrane fatty acids and maximum lifespan in mammals: a reassessment. Comp Biochem Physiol A 146:S55
Van Voorhies WA (1992) Production of sperm reduces nematode lifespan. Nature 360:456–458
Vézina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154
Vleck CM, Vleck D (1979) Metabolic rate in five tropical bird species. Condor 81:89–91
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407
Wang Z, O’Connor TP, Heshka S, Heymsfield SB (2001) The reconstruction of Kleiber’s law at the organ-tissue level. J Nutr 131:2967–2970
Watkins S, Carter L, German J (1998) Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J Lipid Res 39:1583–1588
Weathers WW (1979) Climatic adaptation in avian standard metabolic rate. Oecologia 42:81–89
Weber TP, Piersma T (1996) Basal metabolic rate and the mass of tissues differing in metabolic scope: migration-related covariation between individual knots Calidris canutus. J Avian Biol 27:215–224
Weibel ER, Hoppeler H (2005) Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol 208:1635–1644
West GB, Savage VM, Gillooly J, Enquist B, Woodruff WH, Brown JH (2003) Why does metabolic rate scale with body size? Nature 421:713
Wharton WP (1935) Survival as indicated by returns to Summerville, South Carolina. Bird-Banding 6:125–130
Wheatley DN (2007) Convergence of metabolic rate of cultured cells from animals of different sizes. Am J Physiol Regul Integr Comp Physiol 292:R2113–R2114
White CR, Seymour RS (2004) Does basal metabolic rate contain a useful signal? Mammalian BMR allometry and correlations with a selection of physiological, ecological, and life-history variables. Physiol Biochem Zool 77:929–941
Wiersma P, Muñoz-Garcia A, Walker A, Williams JB (2007a) Tropical birds have a slow pace of life. Proc Natl Acad Sci 104:9340–9345
Wiersma P, Selman C, Speakman JR, Verhulst S (2004) Birds sacrifice oxidative protection for reproduction. Proc Biol Sci 271(Suppl 5):S360–S363
Wiersma P, Chappell MA, Williams JB (2007b) Cold-and exercise-induced peak metabolic rates in tropical birds. Proc Natl Acad Sci 104:20866–20871
Wiersma P, Nowak B, Williams JB (2012) Small organ size contributes to the slow pace of life in tropical birds. J Exp Biol 215:1662–1669
Williams JB, Tieleman BI, Visser GH, Ricklefs RE (2007) Does growth rate determine the rate of metabolism in shorebird chicks living in the Arctic? Physiol Biochem Zool 80:500–513
Williams JB, Miller RA, Harper JM, Wiersma P (2010) Functional linkages for the pace of life, life-history, and environment in birds. Integr Comp Biol 50:855–868
Wu BJ, Hulbert AJ, Storlien LH, Else PL (2004) Membrane lipids and sodium pumps of cattle and crocodiles: an experimental test of the membrane pacemaker theory of metabolism. Am J Physiol 287:R633–R641
Zoeller RA, Morand OH, Raetz CRH (1988) A possible role for plasmalogens in protecting animal-cells against photosensitized killing. J Biol Chem 263:11590–11596
Acknowledgments
We are grateful to Dr. Raineldo Urriola, and the Autoridad Nacional del Ambiente for permission to collect birds in Panama, and the Smithsonian Tropical Research Institute for hosting us. Dr. Jim Van Brocklyn’s help was indispensable in raising cells from Panamanian birds, and Dr. Jim Harper has been a tremendous source of knowledge for our lab while learning to do tissue culture. We greatly appreciate Dr. Matt Wortman’s constant hospitality in his lab and his generosity in letting us use his XF Analyzer. We would like to thank the Velleman lab at the Ohio State University Wooster campus and Cynthia Coy for her help in teaching us myoblast tissue culture methods. We also appreciate Paul Blischak’s help in creating our consensus trees. This study was funded by NSF IBN 0212587 (JBW), and a Smithsonian Tropical Research Institute post-doctoral fellowship (AGJ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Appendix
Appendix
Growth
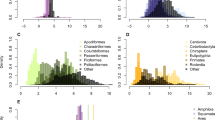
We tested for phylogenetic signal in our comparisons of growth rate in tropical and temperate birds. We first sampled 1,000 trees from birdtree.org, and used BEAST TreeAnnotator v1.8.0 (Drummond et al. 2012) to identify three consensus trees, one for growth rates of all birds in the dataset, one for growth rates of Passerines, and one for growth rates of Non-Passerines. Phylogenetic-independent contrast was run in R version 3.0.2 (R Development Core Team 2013) using the package ape v2.6 (Paradis et al. 2004). There was a phylogenetic signal in growth rates of tropical and temperate species for all birds in the dataset (Bloomberg’s K = 1.47). When phylogenetically informed analysis was used, tropical birds had slower growth rates than temperate birds (PIC, F = 18.34, p < 0.001), consistent with the results from conventional analysis. There was no phylogenetic signal in growth rates of tropical and temperate species for Passeriformes (Bloomberg’s K = 0.4). For our colleagues that would insist that we include phylogeny in the analysis, growth rates of tropical passerines were significantly lower than temperate passerines when we used statistics that were phylogenetically informed (PIC, F = 3.9, p = 0.01), consistent with our results from conventional ANOVAs. There was no phylogenetic signal in growth rates of tropical and temperate species for Passeriformes (Bloomberg’s K = 0.8), and phylogenetically informed analysis did not change the results (PIC, F = 2.93, p = 0.06).
Survival
We tested for phylogenetic signal in our comparisons of survival rate in tropical and temperate birds. We first sampled 1,000 trees from birdtree.org, and used BEAST TreeAnnotator v1.8.0 (Drummond et al. 2012) to identify a consensus tree. Phylogenetic-independent contrast was run in R version 3.0.2 (R Development Core Team 2013) using the package ape v2.6 (Paradis et al. 2004). There was no phylogenetic signal in survival rates of tropical and temperate species (Bloomberg’s K = 0.2). When we ran phylogenetically informed analysis, survival rates of tropical species were significantly higher than temperate birds (PIC, F = 3.9, p = 0.01), consistent with our results from conventional analysis.
All consensus trees used in this study are available from cooper-mullin.1@buckeyemail.osu.edu.
Rights and permissions
About this article
Cite this article
Jimenez, A.G., Cooper-Mullin, C., Calhoon, E.A. et al. Physiological underpinnings associated with differences in pace of life and metabolic rate in north temperate and neotropical birds. J Comp Physiol B 184, 545–561 (2014). https://doi.org/10.1007/s00360-014-0825-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-014-0825-0