Abstract
An equal sex ratio at the population level is the usual, evolutionarily stable condition. However, at the individual level, it may be adaptive for parents to manipulate the sex of their offspring, especially in species with sexual size dimorphism (SSD) when the costs and benefits of producing sons and daughters can vary. In this study, we investigated the hatching sex ratio (HSR) and fledging sex ratio (FSR) in the Whiskered Tern (Chlidonias hybrida). Despite the fact that SSD exists in Whiskered Terns already at the chick stage, HSR and FSR did not deviate from parity at the population level. We tested the dependence of HSR, FSR and the survival probability of males and females on the individual hatching date, average egg volume (in a clutch) and the number of nestlings. None of these factors influenced HSR. Survival probability was negatively correlated with the number of hatchlings. The proportion of females among the fledglings was positively correlated with the average egg volume per clutch. To better explore the effect of egg volume on the sex ratio, we tested the relationship between exact egg volume and hatchling sex or hatching success at the individual level; but despite the quite large sample size, our analyses failed to reveal any relationship. The sex ratio was equal among chicks that were found dead in a nest, mostly due to starvation, but more female than male chicks disappeared from nests (mostly due to predation), primarily in the first week of life. This indicates that females may be easier to predate, very likely by frogs hunting small chicks.
Zusammenfassung
Geschlechterverhältnis und geschlechtsspezifische Kükensterblichkeit bei einer Art mit schwach ausgeprägtem sexuellem Größendimorphismus und Brutverzicht der Weibchen
Ein ausgewogenes Geschlechterverhältnis auf Populationsebene ist der normale, evolutionär stabile Zustand. Aber auf individueller Ebene kann es für Eltern einen Anpassungsvorteil bedeuten, das Geschlecht ihrer Nachkommen zu beeinflussen, insbesondere bei Arten mit Größendimorphismus (SSD) der Geschlechter, bei denen die Kosten und Vorteile der Aufzucht von Söhnen und Töchtern unterschiedlich sein können. In dieser Studie untersuchten wir bei der Weißbart-Seeschwalbe (Chlidonias hybrida) das Geschlechterverhältnis beim Schlüpfen („hatching sex ratio “, HSR) und beim Ausfliegen („fledging sex ratio “, FSR). Trotz der Tatsache, dass der SSD bei den Weißbart-Seeschwalben schon im Kükenstadium besteht, blieben auf Populationsebene HSR und FSR gleichverteilt. Wir untersuchten die Abhängigkeit von HSR, FSR und der Überlebenswahrscheinlichkeit von Männchen und Weibchen vom individuellen Schlüpfdatum, dem durchschnittlichen Eivolumen (innerhalb eines Geleges) und der Anzahl der Jungvögel. Keiner dieser Faktoren beeinflusste das HSR. Die Überlebenswahrscheinlichkeit korrelierte negativ mit der Anzahl der Küken. Der Anteil der weiblichen Küken korrelierte positiv mit dem durchschnittlichen Eivolumen innerhalb eines Geleges. Um eine mögliche Auswirkung vom Eivolumen auf das Geschlechterverhältnis genauer festzustellen, untersuchten wir für einzelne Tiere den Zusammenhang zwischen dem exakten Eivolumen und dem Geschlecht des Kükens und dem Schlüpferfolg; aber trotz der recht großen Stichprobe ergaben unsere Analysen keinen Zusammenhang. Das Geschlechterverhältnis war bei den tot im Nest aufgefundenen Küken ausgeglichen; diese waren zumeist verhungert, aber es verschwanden vor allem in der ersten Lebenswoche mehr weibliche als männliche Küken aus den Nestern (in der Regel als Beute von Raubtieren). Dies deutet darauf hin, dass die weiblichen Küken leichter zu erbeuten sind, höchstwahrscheinlich von Fröschen, die kleine Küken jagen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sex ratio is an important life history trait at both the individual and population levels (West 2009; Guillon 2016; Booksmythe et al. 2017), and the offspring sex ratio can be mediated by both environmental and evolutionary processes (Gowaty 1993; Rosenfeld and Roberts 2004; Rutkowska and Badyaev 2008; Szász et al. 2012). Fisher (1930) attempted to explain the common occurrence of equal sex ratios in nature. He suggested that if both sons and daughters had the same cost (resource allocation) and benefits (fitness return) for parents, they should invest at the same level in both offspring sexes. Selection should, therefore, lead to an equal, evolutionarily stable sex ratio. However, if the cost–benefit ratios of producing sons and daughters are not equal, it may be adaptive for parents to manipulate offspring sex and selection would skew the sex ratio towards that of the sex with reduced costs and/or higher fitness (Trivers and Willard 1973).
In birds, females have the potential to control the sex of eggs because they are heterogametic and because the sex-determining division in avian meiosis occurs prior to ovulation and fertilization (Rutkowska and Badyaev 2008). There is increasing empirical evidence that females can control chromosomal segregation in relation to the social and environmental circumstances experienced at laying (Sheldon et al. 1999; Pike and Petrie 2003; Rutkowska and Badyaev 2008; Gam et al. 2011; Tagirov and Rutkowska 2013). Numerous studies have found several conditions that may influence the female sex allocation in eggs, such as parental condition (Nager et al. 2000; Velando 2002; Weimerskirch et al. 2005), mate attractiveness (Zielińska et al. 2010; Cantarero et al. 2018), clutch size (Lessells et al. 1996; Saino et al. 2002), hatching date (Cordero et al. 2001; Wojczulanis-Jakubas et al. 2013), laying order (Badyaev et al. 2002; Ležalová et al. 2005), food availability (Komdeur et al. 1997; Bukaciński et al. 2020), egg size (Anderson et al. 1997; Cordero et al. 2001; Krist 2011—meta-analysis) and maternal stress hormones (Love et al. 2005).
The sex ratio in young birds is usually studied at three different stages, although the exact boundaries of each stage have not been clearly defined (West 2009; see also the discussion in Kato et al. 2017): a primary stage (at laying), a secondary one (at birth, hatching sex ratio—HSR) and a tertiary one (the fledging stage, fledging sex ratio—FSR). However, the different mortalities of sons and daughters can cause shifts in the sex ratio between these stages (Griffiths 1992; Santoro et al. 2015). Differences in mortality between the sexes were found during both embryonic development (Cichoń et al. 2005; Kato et al. 2017) and the chick-rearing period (Nager et al. 2000; Szczys et al. 2001). This could have been due to the different sensitivities of male and female embryos to environmental pollutants (Fry and Toone 1981; Erikstad et al. 2009), hormonal factors and sex chromosome products (Chandra 1991; Krackow 1999; Pérez et al. 2006), selective provisioning by parents (Teather 1992) or the different vulnerability of the sexes to unfavourable rearing conditions (Nager et al. 2000; Kalmbach et al. 2005).
In birds, sexually size-dimorphic species have been intensively studied in terms of sex allocation and sex-dependent mortality, because a difference in size can lead to sex-specific effects on offspring survival, which might in turn promote the evolution of sex ratio biases (Myers 1978; Weatherhead and Teather 1991; Benito and González‐Solís 2007; Eberhart-Phillips et al. 2017). In sexually size-dimorphic species, the larger sex (usually the male) is commonly assumed to be more costly for parents because of the higher growth and/or metabolic rates, and thus the overall greater energy needs or food intake during development (Slagsvold et al. 1986; Teather and Weatherhead 1988; Stamps 1990; but see Torres and Drummond 1999). In consequence, the sex ratio should be skewed towards the smaller sex because parents are expected to invest more in the cheaper sex (Fisher 1930). In addition, the bigger sex is more susceptible to mortality when food is scarce because of its increased nutrient needs resulting from faster growth rates (Teather and Weatherhead 1989; Griffiths 1992). Indeed, the offspring sex ratio in many bird species is skewed towards the production of the smaller sex in many sexually size-dimorphic species (Teather and Weatherhead 1988; Kalmbach et al. 2001; Benito and González‐Solís 2007). In contrast, differential mortality among the smaller sex has been found in some circumstances. Higher mortality among smaller individuals was found in species where access to food depends on competitive abilities, because of the competitive advantages accruing from a larger size (Edwards and Collopy 1983; Bortolotti 1986; Anderson et al. 1993; but see Drummond et al. 1991). Furthermore, having fewer reserves, the smaller sex may be less able to cope with periods of food scarcity (Kernsten and Brenninkmeijer 1995; Eberhart-Phillips et al. 2017).
To understand the mechanisms leading to a biased offspring sex ratio, it is crucial to investigate successive changes in the sex ratio over different developmental stages based on large data sets. The Whiskered Tern (Chlidonias hybrida) is a sexually dimorphic bird, with moderate size differences between the sexes: adult males are 3–10% larger than females (Ledwoń 2011). This dimorphism is also evident at the nestling stage (Banach et al. 2021). At hatching, males are larger than females only in the total head length, but sexual size dimorphism (SSD) increases during the chicks’ development and, close to fledging, most of the body measurements are larger in males than in females; the greatest differences in size between the sexes is in weight, that of females being only 89% of the male weight. Moreover, the maximum body mass growth rate is higher in males than in females. The occurrence of SSD during the nestling period and the higher maximum growth rate of body mass in males indicates that the costs of raising sons may be higher than of raising daughters. According to the above hypothesis, therefore, this can lead to a female-biased sex ratio. On the other hand, the males being larger are better able to prevail in the competition for food delivered by adults, and this will lead to higher female mortality, especially when food is scarce.
Among gulls and terns, the Whiskered Tern has a unique system of parental care. Almost all adult breeding females in this species desert during both the chick-rearing and post-fledging periods with as much as 52% of nests were deserted prior to fledging (Ledwoń and Neubauer 2017). It has been shown that that parental sex-role reversal may occur in populations that exhibit an extreme male-biased adult sex ratio (Kosztolányi et al. 2011). This situation was found in the related Kentish Plover (Charadrius alexandrines), where, as in the Whiskered Tern, the majority of females desert. Female Kentish Plovers have a higher probability than males of renesting after desertion owing to the male-biased adult sex ratio (Amat et al. 1999; Székely et al. 2004; Eberhart-Phillips et al. 2017, 2018). The unbalanced sex ratio in this species is manifested at fledging and continues during adulthood (Székely et al. 2004; Eberhart-Phillips et al. 2017). In contrast, deserting Whiskered Tern females rarely remate and renest (Ledwoń et al. 2023a), possibly because of the insufficient number of males. The adult sex ratio in the Whiskered Tern is not known. The female biased adult sex ratio is not indicated by the low proportion of super normal clutches that are formed by female–female pairs (Betleja et al. 2007). However biased sex ratio during the pre-fledging period may be indicative of an unbalanced adult sex ratio (Szczys et al. 2001; Kosztolányi et al. 2011; Nisbet et al. 2016; but see Székely et al. 2014a, b).
The above characteristics make the Whiskered Tern a very good and an interesting model for testing some aspects of the sex allocation theory to study the sex ratio at different life stages and sex-specific chick mortality. Here, we investigated whether HSR, FSR and sex-specific chick mortality were biased and correlated with the ecological environment (hatching date, clutch size) and maternal traits (average egg size), over four consecutive years. As SSD in Whiskered Tern is apparent at the nestling stage, we generally expected sex-dependent mortality and in consequence an unbalanced sex ratio (see also above). We expected that as the season progresses—when less experienced parents start their clutches and environmental conditions may deteriorate—HSR and FSR will be female-biased, as parents should invest more in less-expensive offspring. Survival probability should be positively correlated with egg volume, in contrast to brood size with expected negative impact on chick survival—especially of the more sensitive sex.
Methods
Study site and field procedures
The data used in this study were collected during four breeding seasons 2016 to 2019 in nine Whiskered Tern colonies (Table 1) on five carp pond complexes (Spytkowice, Bugaj, Przeręb, Stawy Monowskie, Adolfin) in the Upper Vistula Valley (50°00′ N 19°30′ E), southern Poland (for a detailed description of the study area, see Ledwoń et al. 2013, 2014; Gwiazda and Ledwoń 2015).
Nest monitoring started mostly during the first half of the incubation period and continued until the nestlings fledged. Colony inspections during the egg-laying period were kept to a minimum so as to prevent nest desertion. Eggs were measured with manual callipers to the nearest 0.1 mm and a few days before the expected hatching date nests were enclosed with plastic horticultural mesh to prevent nestlings from escaping from the nest site until they could fly (for a detailed description of the enclosure, see Ledwoń et al. 2015 and Banach et al. 2021). Egg volumes (v) were calculated from the formula v = k × l × b2, where k—volume coefficient, l—length, b—breadth (k = 0.478) (Hoyt 1979; Betleja 2003). First eggs in early colonies were laying around mid-May, last ones in the second half of July. Incubation lasted about 21 days, nestlings usually reached flight ability between 20 and 23 days of age (Betleja 2003; Banach et al. 2021). 85% of the nestlings hatched between 15th June and 20th July (median 30th June). At least one chick hatched in 645 of the monitored nests (Table 1), and chicks hatched from all the eggs in 475 nests. From 2017 onwards, eggs were distinguished with a waterproof marker so that the chick (or unhatched egg) could be linked with the exact egg volume (this was feasible in cases of asynchronous hatching, provided that the inspection fell within the hatching period—the chick usually emerges from the egg within 24 h after the appearance of the hatching star). Colonies were usually visited every 3–4 days (in a few cases more often, even three times a week, or less often, after the hatching period). Each newly hatched chick was ringed with an individually numbered steel ring, and a small sample of blood was taken from the tarsus vein for molecular sexing. The exact hatching date of individuals was determined from the presence of wet feathers after emergence from the egg (the first day of a chick’s life), presence of hatching star during the previous inspection and on the basis of the hatchling’s wing length and body mass (Paillisson et al. 2008; AB—unpublished data). An individual was classified as being alive at fledging if it was absent from the nest at the age of at least c. 20 days (considering the fledging wing length, Banach et al. 2021). Earlier disappearances were put down to predation. Other death causes (disease, starvation, and hypothermia) or drowning if the dead chick’s body was found on the nest or right next to it, on the water (inside the enclosure) were grouped as “dead in the nest”. Whiskered Terns, unlike passerine birds, do not carry their dead chicks outside the nest. The age of the chick at the time of death or disappearance was estimated as the age on the middle day between the last inspection during which the chick was alive and the first day on which the chick was found to be missing or dead. For logistical reasons or damage to the enclosure, we stopped inspecting seven nests before fledging. Moreover, in 51 nests we conducted a brood size manipulation experiment, which changed the conditions of chick development to the extent that they could not be included in the FSR or mortality analysis.
Molecular sex determination
Nestling sex was determined from the CHD gene located on the sex chromosomes (Dubiec and Zagalska-Neubauer 2006). Blood samples were stored in 98% ethanol at − 20 °C. Prior to DNA extraction, c. 20 µl was dried at 42 °C and 80 µl of Tris added. DNA was extracted using a Blood Mini Kit (A&A Biotechnology). PCR was conducted with two sets of primers: F2550 (5′-GTT ACT GAT TCG TCT ACG AGA-3′) and R2718 (5′-ATT GAA ATG ATC CAG TGC TTG-3′) (Fridolfsson and Ellegren 1999), and P2 (5′-TCT GCA TCG CTA AAT CCT TT-3′), P8 (5′-CTC CCA AGG ATG AGR AAY TG-3′) and P0 (5′-ATT GAG TTG GAA CCAGA-ICA-3′) (Griffiths et al. 1998; Han et al. 2009), also used in previous studies of the Whiskered Tern (Ledwoń 2011; Goławski et al. 2016; Banach et al. 2021). PCR products were separated on agarose gel. The presence of one band indicated a male, two bands—a female. Each sample was tested at least twice. If the result was not obvious (usually poor product quality), the PCR was repeated another two or four times (Banach et al. 2021). We were unable to determine the sex of 52 out of 1,694 individuals because of the lack or bad quality of a blood sample.
Statistical analysis
We investigated sex ratios at the population level among hatchlings and fledglings using the Chi2 test. The primary sex ratio (at laying) was tested indirectly in a subsequent analysis by testing whether HSR in broods with full hatching success differed from broods in which at least one egg failed to hatch.
The influence of environmental and maternal factors in all of the following analyses was tested using generalised linear mixed models (GLMM) with binomial distribution. The best random effects structure for each analysis was selected based on the lowest Akaike information criterion (AIC, if AIC comparison did not give a clear answer, we were guided in our choice by the best representation of the relationship between random factors). Model selections were performed using AIC by function dredge in the MuMIn library (Bartoń 2015). The coefficients of the models with ΔAIC < 2 were averaged and used for final inference (Zuur et al. 2009).
In the first GLMM analysis we investigated the relationship between HSR (sex of an individual)—dependent variable and several predefined factors. The global model took the form: sex ~ hatchlings + date + volume + success + nest ID/colony/year. The number of hatchlings in the nest, the date of individual hatching and the average volume of all the eggs in a clutch were continuous predictors, scaled prior to analysis. Nest ID, colony and year were categorical, random factors, nested hierarchically (nest in colony in year). The success of clutch hatching was a categorical fixed factor. Success 1 signifies that all eggs hatched successfully, 0 that at least one egg did not hatch (this predictor enables us to detect the difference between the primary sex ratio and HSR—if this has a statistically significant influence on a hatchling’s sex, we may suspect increased mortality among the embryos of one sex; we were unable to determine the sex of embryos from unhatched eggs, so inferring the sex ratio at the egg-laying stage could only be indirect). The hatching date was the exact hatching date of an individual (measured in days numbered from 1st June each year; 1st June = day 1). The number of successfully hatched hatchlings was from one to four (73.6% of individuals were from nests with three hatchlings, 22.3%—two hatchlings, 2.1%—one hatchling, and 2%—four hatchlings). The exact egg volume was known for only 12% of hatchlings, so the average egg volume was included in this analysis. A complete set of data (sex, hatching date, averaged egg volume, number of nestlings, hatching success) was obtained for 1472 hatchlings from 575 nests (the remaining 170 chick with known sex were from broods for which it was not possible to determine averaged egg volume—mainly broods found near the hatching time). 70 hatchlings included in this analysis (from 43 nests) came from nests where not all chicks had their sex determined.
In the second GLMM analysis, we investigated the relationship between FSR (sex of an individual)—dependent variable and several predefined factors. The global model took the form: sex ~ fledglings + date + volume + nest ID/colony/year. The number of fledglings in the nest, the date of individual hatching and the average volume of all the eggs in a clutch were continuous predictors, scaled prior to analysis. Nest ID, colony and year were categorical, random factors, nested hierarchically (Nest in colony in year). The number of fledgelings varied from one to four (53.8% individuals were from nests with three fledglings, and correspondingly 39.0%—two fledglings, 6.1%—one fledgling, 1.1%—four fledglings). A complete set of data (sex, hatching date, averaged egg volume, number of fledglings) was obtained for 1102 fledglings from 486 nests (the sample of fledglings is smaller than the number of hatchlings due to chick mortality and exclusion from analysis broods subjected to brood-size experiment). 19 fledglings from nine nests (included in analysis) came from nests where not all chicks alive to fledge had their sex determined.
In the third GLMM analysis, we investigated the relationship between nestling mortality (survive)—depended variable and several predefined variables. The global model took the form: survive ~ date*sex + volume*sex + hatchlings*sex + nest ID/colony/year. Survive was whether a chick survived to fledging (c. 20 days; survive 1—alive to fledging, 0—found dead in the nest or disappeared before fledging; 1317 individuals in the analysis). The individual hatching date, average egg volume and the number of hatchlings in the nest were continuous predictors, scaled prior to analysis. The sex of an individual was a categorical fixed factor. The interactions of sex with all the above predictors were also included in the model. nest ID, colony and year were categorical, random factors, nested hierarchically.
Difference in deviation in the sex ratios from parity among all chicks that did not survive to fledge on the population level (n = 230) and divided by specific categories: nestlings that died in the nest (as a results of disease, starvation, hypothermia, drowning: n = 149) and nestlings that were predated (individuals that disappeared from the nest: n = 81) were analysed using the Chi2 test.
Egg numbering allowed us to link some eggs of known volume to certain chicks (it was possible in two circumstances—in part of nests with asynchronous hatching (182 individuals) or when all the chicks in the nest were of the same sex (n = 363)). In the fourth GLMM analysis, we tested the effect of exact egg volume and other predefined factors on the sex of individual chick (dependent variable). The global model took the form: sex ~ exact volume + date + hatchlings + success + nest ID/colony/year. The exact volume of egg, individual hatching date and number of hatchlings were the continuous predictors, scaled prior to analysis. The success of clutch hatching was a categorical fixed factor (see first GLMM analysis). Nest ID, colony and year were categorical, random factors, nested hierarchically. This analysis was similar to that previous of HSR, but took into account the exact egg volume instead of the clutch-averaged egg volume.
Egg numbering also made it possible to control the hatching success of individual eggs. The hatching success of an egg was the dependent variable in the fifth GLMM analysis. The global model took the form: hatching success ~ exact volume + hatch date + eggs + nest ID/colony/year. The exact volume of egg, hatch date of the earliest hatched chick in the clutch and number of eggs in the clutch were the continuous predictors, scaled prior to analysis. Nest ID, colony and year were categorical, random factors, nested hierarchically. The egg was classified as unhatched (hatching success = 0) when it disappeared without signs of hatching (a hatching star was not found during the last inspection) or it demonstrated signs of decay (e.g. unpleasant smell, lower weight). This analysis included only nests with at least one unhatched egg (n = 80 clutches) to test whether there was any variance within the clutch between the hatched (n = 160) and unhatched eggs (n = 86).
All the analyses were conducted in R 4.1.1 (R Core Team 2021). There was no problem with collinearity—the correlation coefficients between the covariates were ≤ 0.1 in all cases. The significance threshold was 0.05.
Results
At the population level, the sex ratio did not deviate from parity in either hatchlings (HSR) or fledglings (FSR, Table 2). Hatch success (on the clutch level) did not affect the hatchlings' sex, which suggests that embryo mortality is not sex-dependent and that the primary sex ratio (at laying) is the same as HSR (Table 3, S1—see electronic supplementary material). The sex of hatchlings was not affected by any of tested factors (hatchlings number, hatching date, averaged egg volume). The sex of fledglings also was not affected by hatching date and the number of fledglings. However, the average volume of eggs had a significant influence on FSR: the greater the average egg volume, the higher the probability of more females than males fledging (Table 4, S2).
The survive probability on individual level was not affected by sex of nestling nor interaction of sex with any of other tested factors (hatching date, averaged egg volume, number of hatchlings). The only factor that influenced the survive probability was number of hatchlings: the higher the number of hatchlings in the nest, the lower the survive probability (Table 5, S3).
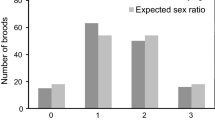
Analysis of the sex ratio at the population level among individuals that died and disappeared during the nestling period showed that more females than males failed to fledge (Table 2). Interestingly, the sex ratio deviated from parity only among nestlings that had disappeared from the nest (most probably killed and taken by a predator); there was no deviation among individuals found dead in the nest (death due to disease, starvation, hypothermia, drowning). Moreover, it was mainly the youngest chicks, in the first week of life, that fell victim to predators (Fig. 1a, b), whereas the number of chicks that died in the nest was independent of their age (Fig. 1c, d).
Despite a quite large sample size, analyses showed that the precise egg volume had no influence on the hatchling's sex (Table 6, S4) or on hatching success (Table 7, S5).
Discussion
This 4-year study allowed us to test for deviations of the offspring sex ratio in a large sample of Whiskered Tern broods. Contrary to our expectations, the primary (at laying, obtained indirectly), secondary (HSR) and fledging (FSR) sex ratios in this population did not differ significantly from parity. Therefore, our research increases the number of studies describing a balanced sex ratio in young birds (review in Hasselquist and Kempenaers 2002; e.g. Ležalová et al. 2005; Dyrcz and Cichoń 2009; Czyż et al. 2012; Bonter et al. 2015; Bartlow et al. 2021). Papers reporting an unbalanced sex ratio are probably overrepresented in the literature, because such a result is considered more interesting and easier to publish. Moreover, publication bias towards significant results may distort our view of adaptive sex ratio manipulation (Hasselquist and Kempenaers 2002). An unbiased sex ratio may actually be more widespread among birds than the literature suggests.
In this population of Whiskered Terns, the primary and HSR did not differ either from each other or from parity. It seems that Whiskered Terns females either did not adjust the sex ratio or did so too weakly for this change to be detectable with our sample size. It is also possible that females manipulate the sex at laying in relation to factors not studied here, e.g. mate attractiveness or food availability. Manipulation of the chick sex ratio by females in this population may not offer benefits that are sufficient to outweigh the costs.
HSR can be adaptively skewed by mothers in response to ecological and environmental factors, as well as other elements like parental quality (reviews in Cockburn et al. 2002; Hasselquist and Kempenaers 2002; Alonso-Alvarez 2006). One of our main aims was to determine whether HSR of a sexually size-dimorphic species (females smaller than males) was influenced by breeding date, brood size and/or average egg volume. We found that none of these factors cause the deviation of HSR from parity. Sex manipulation according to laying date seems to be quite common (Cordero et al. 2001; Andersson et al. 2003; Husby et al. 2006; Bartlow et al. 2021). Although environmental conditions can deteriorate as the season progresses, individuals in poorer condition, and/or younger, and/or less experienced, usually begin laying eggs later. Therefore, females are expected to adjust the sex ratio towards the smaller sex (usually females) in late broods, less expensive to produce and rear, as some studies have shown (Genovart et al. 2003; Bonter et al. 2005; Wojczulanis‐Jakubas et al. 2013; Minias 2016). That females may skew the sex ratio in late broods was found in Whiskered Terns breeding in central Poland (Minias 2016) but not in the closely-related Common Tern (Sterna hirundo) (Benito et al. 2013) or in some other Charadriiformes (Ležalová et al. 2005; Que et al. 2019). We did not assess food availability in relation to breeding season advancement in our population, but the high frequency of female desertion, also late in the breeding season, may be an indication of superabundant food on carp ponds (Ledwoń and Neubauer 2017). This allows males to increase their provisioning rates and to compensate at least partly for the desertion of their mates. In addition, male and female body condition did not appear to decrease during the breeding season (Ledwoń et al. 2023b), neither did brood size influence body mass growth in chicks (Banach et al. 2021). However, nestling growth was negatively correlated with hatching date, which may that condition or parental quality is deteriorating along with breeding season advancement. Overall, the probably good breeding conditions as regards food availability may mean that the costs of raising sons do not significantly exceed those of raising daughters, as a result of which female Whiskered Terns do not adjust the sex in their eggs towards the cheaper sex in relation to season advancement.
Some studies have shown that HSR is skewed towards the cheaper sex (usually females) in relation to clutch size (Lessells et al. 1996; Benito et al. 2013; Bukaciński et al. 2020). Furthermore, the number of the smaller sex increased with increasing egg volume, which may level the playing field in competition with their larger siblings (Anderson et al. 1997; Cordero et al. 2001), but this dependence is not always apparent (Cichoń et al. 2003). We found no connection between brood size, the egg volume averaged within the clutch or the exact egg volume and HSR. However, the latter result should be treated with some caution, as the sample was not fully random owing to the limitations in assigning chicks of known sex to an egg of known volume, while averaging egg volumes within a brood may have hidden potential differences that would only become apparent on a larger scale. Next, it is possible that the fairly constant size of the clutch (85% of hatchlings were from clutches with three eggs) did not give space to highlight the relationship between brood size and sex ratio. Nevertheless, it is difficult to unequivocally explain our results; again, we can only presume that food abundance and/or the similar costs of raising both sexes were acting towards an equal sex ratio.
FSR did not differ from parity in our population of Whiskered Terns, but one of the factors we examined—the average egg volume—did influence FSR: more females than males fledged from clutches with higher average egg volumes. Egg volume could influence individual survival (Krist 2011) by affecting hatchling weight (Arnold et al. 2006). However, our results showed no relationship between average egg volume in clutch and nestling survival. The only factor affecting chick survival was brood size. The chick was less likely to survive in nests with more siblings. Interestingly, there were no sex bias in mortality due to starvation, hypothermia or disease, indicating a shortage in parents’ care abilities for their numerous offspring. Increased mortality was due to predation, with the highest percentage of disappearances observed among chicks in the first week of life. It is possible that the smaller Whiskered Tern females in bigger clutches facing the threat of a predator (such as frogs, which prey on young tern chicks: ML—unpublished data) were more likely to loss in a life-saving competition with their bigger and probably stronger brothers and consequently fall prey to predators.
Even though we found that more females than males disappeared during the chick-rearing period, the difference in mortality between the sexes did not lead to a male-biased FSR. In species with SSD, the larger sex (males in the Whiskered Tern) is more susceptible to mortality under unfavourable conditions because of the greater energy demands or food intake during development (see the relevant references in literature in the Introduction). Males, on the other hand, being larger and probably stronger than females, can prevail in the competition for food or can more easily avoid being caught by a predator. This will lead to female-biased mortality, especially when food is scarce (literature in introduction). It seems that in this population, the above mechanisms lead to sex-biased mortality but not to a statistically significant unbalanced FSR owing to the relatively low overall chick mortality. This could be due to the abundance of food on the carp ponds (see above), but we did not study this in detail. Further studies should focus on food availability in relation to breeding season advancement.
In conclusion, our results suggest that the possible manipulation of the chick sex ratio by females in this Whiskered Tern population may not provide benefits sufficient to outweigh the costs. Hence, HSR did not exhibit any deviation from parity. Nevertheless, the egg volume did influence FSR—in broods with higher average egg volume, more females fledged than males. Despite the fact that SSD is present in Whiskered Terns already at the chick stage and that males may be more costly to raise than females, we found no differences in survival probability between the sexes and balanced FSR. As expected, in larger broods, chicks were less likely to survive (regardless of sex). Moreover, the youngest females (in first week of their live) were easier prey for predators than males. We would encourage sex ratios to be studied in different Whiskered Tern populations, especially in conditions where food is less abundant.
Data availability
Data are available on the Zenodo repository at https://zenodo.org/records/10679167
References
Alonso-Alvarez C (2006) Manipulation of primary sex-ratio: an updated review. Avian Poult Biol Rev 17:1–20. https://doi.org/10.3184/147020606783437930
Amat JA, Fraga RM, Arroyo GM (1999) Brood desertion and polygamous breeding in the Kentish Plover Charadrius alexandrinus. Ibis 141:596–607. https://doi.org/10.1111/j.1474-919X.1999.tb07367.x
Anderson DJ, Budde C, Apanius V, Martinez Gomez JE, Bird D, Weathers W (1993) Prey size influences female competitive dominance in nestling American Kestrels (Falco sparverius). Ecology 74:367–376. https://doi.org/10.2307/1939299
Anderson DJ, Reeve J, Bird DM (1997) Sexually dimorphic eggs, nestling growth and sibling competition in American Kestrels Falco sparverius. Funct Ecol 11:331–335. https://doi.org/10.1046/j.1365-2435.1997.00091.x
Andersson M, Wallander J, Oring L, Akst E, Reed JM, Fleischer RC (2003) Adaptive seasonal trend in brood sex ratio: test in two sister species with contrasting breeding systems. J Evol Biol 16:510–515. https://doi.org/10.1046/j.1420-9101.2003.00533.x
Arnold JM, Hatch JJ, Nisbet IC (2006) Effects of egg size, parental quality and hatch-date on growth and survival of Common Tern Sterna hirundo chicks. Ibis 148:98–105. https://doi.org/10.1111/j.1474-919X.2006.00487.x
Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA (2002) Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 295:316–318. https://doi.org/10.1126/science.1066651
Banach A, Neubauer G, Flis A, Ledwoń M (2021) Sex-specific growth of nestlings of the Whiskered Tern Chlidonias hybrida, a species with sexual size dimorphism and female brood desertion. J Ornithol 162:1035–1047. https://doi.org/10.1007/s10336-021-01911-y
Bartlow AW, Jankowski MD, Hathcock CD, Ryti RT, Reneau SL, Fair JM (2021) Sex ratio of Western Bluebirds (Sialia mexicana) is mediated by phenology and clutch size. Ibis 163:977–989. https://doi.org/10.1111/ibi.12935
Benito MM, González-Solís J (2007) Sex ratio, sex-specific chick mortality and sexual size dimorphism in birds. J Evolution Biol 20:1522–1530. https://doi.org/10.1111/j.1420-9101.2007.01327.x
Benito MM, Schielzeth H, González-Solís J, Becker PH (2013) Sex ratio adjustments in Common Terns: influence of mate condition and maternal experience. J Avian Biol 44:179–188. https://doi.org/10.1111/j.1600-048X.2012.00024.x
Bonter D, Moglia M, DeFisher L (2015) Sons do not take advantage of a head start: parity in Herring Gull offspring sex ratios despite greater initial investment in males. J Avian Biol 47:121–128. https://doi.org/10.1111/jav.00649
Booksmythe I, Mautz B, Davis J, Nakagawa S, Jennions MD (2017) Facultative adjustment of the offspring sex ratio and male attractiveness: a systematic review and meta-analysis. Biol Rev 92:108–134. https://doi.org/10.1111/brv.12220
Bukaciński D, Bukacińska M, Chylarecki P (2020) Effect of food availability on offspring sex ratios in replacement clutches of Mew Gulls (Larus canus) and Black-headed Gulls (Chroicocephalus ridibundus) in the highly unstable environment of the Vistula River. J Ornithol 161:829–847. https://doi.org/10.1007/s10336-020-01761-0
Cantarero A, Pilastro A, Griggio M (2018) Nestling sex ratio is associated with both male and female attractiveness in Rock Sparrows. J Avian Biol 49:e01666. https://doi.org/10.1111/jav.01666
Chandra HS (1991) How do heterogametic females survive without gene dosage compensation? J Genet 70:137–146. https://doi.org/10.1007/BF02927864
Cichoń M, Dubiec A, Stoczko M (2003) Laying order and offspring sex in Blue Tits Parus caeruleus. J Avian Biol 34:355–359. https://doi.org/10.1111/j.0908-8857.2003.03201.x
Cichoń M, Sendecka J, Gustafsson L (2005) Male-biased sex ratio among unhatched eggs in Great Tit Parus major, Blue Tit P. caeruleus and Collared Flycatcher Ficedula albicollis. J Avian Biol 36:386–390. https://doi.org/10.1111/j.0908-8857.2005.03589.x
Cockburn A, Legge S, Double M (2002) Sex ratios in birds and mammals: can the hypotheses be disentangled. In: Hardy ICW (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge, pp 266–286
Cordero PJ, Vinuela J, Aparicio J, Veiga J (2001) Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the Spotless Starling. J Evol Biol 14:829–834. https://doi.org/10.1046/j.1420-9101.2001.00320.x
Czyż B, Rowiński P, Wesołowski T (2012) No evidence for offspring sex ratio adjustment in Marsh Tits Poecile palustris breeding in a primeval forest. Acta Ornithol 47:111–118. https://doi.org/10.3161/000164512X662214
Dubiec A, Zagalska-Neubauer M (2006) Molecular techniques for sex identification in birds. Biol Lett 43:3–12. https://doi.org/10.1038/023240a0
Dyrcz A, Cichoń M (2009) Sex-specific fledgling success and brood sex ratio in the Great Reed Warbler (Acrocephalus arundinaceus). J Ornithol 150:839–844. https://doi.org/10.1007/s10336-009-0404-4
Eberhart-Phillips LJ, Küpper C, Miller TE, Cruz-López M, Maher KH, Dos Remedios N, Stoffel MA, Hoffman JI, Krüger O, Székely T (2017) Sex-specific early survival drives adult sex ratio bias in Snowy Plovers and impacts mating system and population growth. P Natl Acad Sci 114:E5474–E5481. https://doi.org/10.1073/pnas.1620043114
Eberhart-Phillips LJ, Küpper C, Carmona-Isunza MC, Vincze O, Zefania S, Cruz-López M, Kosztolányi A, Miller TEX, Barta Z, Cuthill IC, Burke T, Székely T, Hoffman JI, Krüger O (2018) Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nat Commun 9:1651. https://doi.org/10.1038/s41467-018-03833-5
Edwards TC, Collopy MW (1983) Obligate and facultative brood reduction in eagles: an examination of factors that influence fratricide. Auk 100:630–635. https://doi.org/10.1093/auk/100.3.630
Erikstad KE, Bustnes JO, Lorentsen SH, Reiertsen TK (2009) Sex ratio in Lesser Black-backed Gull in relation to environmental pollutants. Behav Ecol Sociobiol 63:931–938. https://doi.org/10.1007/s00265-009-0736-3
Fisher RA (1930) The genetical theory of natural selection. Clarendon, London
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 20:116–121. https://doi.org/10.2307/3677252
Fry DM, Toone CK (1981) DDT-induced feminization of gull embryos. Science 213:922–924. https://doi.org/10.1126/science.7256288
Gam AE, Mendonça MT, Navara KJ (2011) Acute corticosterone treatment prior to ovulation biases offspring sex ratios towards males in Zebra Finches Taeniopygia guttata. J Avian Biol 42:253–258. https://doi.org/10.1111/j.1600-048X.2010.05251.x
Genovart M, Oro D, Ruiz X, Griffiths R, Monaghan P, Nager RG (2003) Seasonal changes in brood sex composition in Audouin’s Gulls. The Condor 105:783–790. https://doi.org/10.1093/condor/105.4.783
Goławski A, Kasprzykowski Z, Ledwoń M, Mróz E, Morelli F (2016) Brood sex ratio in expansive and non-expansive tern species in east-central Poland. Bird Study 63:31–36. https://doi.org/10.1080/00063657.2015.1122738
Gowaty PA (1993) Differential dispersal, local resource competition, and sex ratio variation in birds. Am Nat 141:263–280. https://doi.org/10.1086/285472
Griffiths R (1992) Sex-biased mortality in the Lesser Black-backed Gull Larus fuscus during the nestling stage. Ibis 134:237–244. https://doi.org/10.1111/j.1474-919X.1992.tb03805.x
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075. https://doi.org/10.1046/j.1365-294x.1998.00389.x
Guillon JM (2016) Sex ratio evolution when fitness and dispersal vary. Evol Ecol 30:1097–1115. https://doi.org/10.1007/s10682-016-9869-9
Gwiazda R, Ledwoń M (2015) Sex-specific foraging behaviour of the Whiskered Tern (Chlidonias hybrida) during the breeding season. Ornis Fennica 92:15–22. https://doi.org/10.51812/of.133864
Han J-I, Kim J-H, Kim S, Park S-R, Na K-J (2009) A simple and improved DNA test for avian sex determination. Auk 126:779–783. https://doi.org/10.1525/auk.2009.08203
Hasselquist D, Kempenaers B (2002) Parental care and adaptive brood sex ratio manipulation in birds. Philos T R Soc B 357:363–372. https://doi.org/10.1098/rstb.2001.0924
Hoyt DF (1979) Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77. https://doi.org/10.1093/auk/96.1.73
Husby A, Sæther B-E, Jensen H, Ringsby TH (2006) Causes and consequences of adaptive seasonal sex ratio variation in House Sparrows. J Anim Ecol 75:1128–1139. https://doi.org/10.1111/j.1365-2656.2006.01132.x
Kalmbach E, Nager RG, Griffiths R, Furness RW (2001) Increased reproductive effort results in male-biased offspring sex ratio: an experimental study in a species with reversed sexual size dimorphism. P R Soc B 268:2175–2179. https://doi.org/10.1098/rspb.2001.1793
Kalmbach E, Furness RW, Griffiths R (2005) Sex-biased environmental sensitivity: natural and experimental evidence from a bird species with larger females. Behav Ecol 16:442–449. https://doi.org/10.1093/beheco/ari018
Kato T, Matsui S, Terai Y, Tanabe H, Hashima S, Kasahara S, Morimoto G, Mikami OK, Ueda K, Kutsukake N (2017) Male-specific mortality biases secondary sex ratio in Eurasian Tree Sparrows Passer montanus. Ecol Evol 7:10675–10682. https://doi.org/10.1002/ece3.3575
Kernsten M, Brenninkmeijer A (1995) Growth, fledging success and post-fledging survival of juvenile Oystercatchers Haematopus ostralegus. Ibis 137:396–404. https://doi.org/10.1111/j.1474-919X.1995.tb08039.x
Komdeur J, Daan S, Tinbergen J, Mateman C (1997) Extreme adaptive modification in sex ratio of the Seychelles Warbler’s eggs. Nature 385:522–525. https://doi.org/10.1038/385522a0
Kosztolányi A, Barta Z, Küpper C, Szekely T (2011) Persistence of an extreme male-biased adult sex ratio in a natural population of polyandrous bird. J Evol Biol 24:1842–1846. https://doi.org/10.1111/j.1420-9101.2011.02305.x
Krackow S (1999) Avian sex ratio distortions: the myth of maternal control. Proc Int Ornithol Congr 22:425–433
Krist M (2011) Egg size and offspring quality: a meta-analysis in birds. Biol Rev 86:692–716. https://doi.org/10.1111/j.1469-185X.2010.00166.x
Ledwoń M (2011) Sexual size dimorphism, assortative mating and sex identification in the Whiskered Tern Chlidonias hybrida. Ardea 99:191–198. https://doi.org/10.5253/078.099.0209
Ledwoń M, Neubauer G (2017) Offspring desertion and parental care in the Whiskered Tern Chlidonias hybrida. Ibis 159:860–872. https://doi.org/10.1111/ibi.12496
Ledwoń M, Neubauer G, Betleja J (2013) Adult and pre-breeding survival estimates of the Whiskered Tern Chlidonias hybrida breeding in southern Poland. J Ornithol 154:633–643. https://doi.org/10.1007/s10336-012-0926-z
Ledwoń M, Betleja J, Stawarczyk T, Neubauer G (2014) The Whiskered Tern Chlidonias hybrida expansion in Poland: the role of immigration. J Ornithol 155:459–470. https://doi.org/10.1007/s10336-013-1027-3
Ledwoń M, Betleja J, Neubauer G (2015) An effective method for trapping both parents and chicks of Whiskered Terns (Chlidonias hybrida) and its impact on breeding success. Waterbirds 38:290–295. https://doi.org/10.1675/063.038.0309
Ledwoń M, Flis A, Banach A, Kusal B, Łożyńska H, Atamas N, Broński S, Betleja J (2023a) Do females of Whiskered Tern Chlidonias hybrida renest after offspring desertion? Eur Zool J 90:237–247. https://doi.org/10.1080/24750263.2023.2184876
Ledwoń M, Neubauer G, Flis A, Banach A (2023b) Female and male body condition in the Whiskered Tern Chlidonias hybrida, a species with female offspring desertion: a test of the differential parental capacity hypothesis. J Ornithol 165:93–103. https://doi.org/10.1007/s10336-023-02099-z
Lessells C, Mateman A, Visser J (1996) Great Tit hatchling sex ratios. J Avian Biol 27:135–142. https://doi.org/10.2307/3677142
Ležalová R, Tkadlec E, Oborník M, Šimek J, Honza M (2005) Should males come first? The relationship between offspring hatching order and sex in the Black-headed Gull Larus ridibundus. J Avian Biol 36:478–483. https://doi.org/10.1111/j.0908-8857.2005.03466.x
Love OP, Chin EH, Wynne-Edwards KE, Williams TD (2005) Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am Nat 166:751–766. https://doi.org/10.1086/497440
Minias P (2016) Seasonal trends in brood sex ratio reflect changes in early-life physiological condition of chicks in the Whiskered Tern. Ethol Ecol Evol 28:385–393. https://doi.org/10.1080/03949370.2015.1062804
Myers JH (1978) Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? Am Nat 112:381–388. https://doi.org/10.1086/283280
Nager RG, Monaghan P, Houston DC, Genovart M (2000) Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus). Behav Ecol Sociobiol 48:452–457. https://doi.org/10.1007/s002650000262
Nisbet IC, Monticelli D, Spendelow JA, Szczys P (2016) Prebreeding survival of Roseate Terns Sterna dougallii varies with sex, hatching order and hatching date. Ibis 158:327–334. https://doi.org/10.1111/ibi.12359
Paillisson J-M, Latraube F, Reeber S (2008) Assessing growth and age of Whiskered Tern Chlidonias hybrida chicks using biometrics. Ardea 96:271–277. https://doi.org/10.5253/078.096.0212
Pérez C, Velando A, Domínguez J (2006) Parental food conditions affect sex-specific embryo mortality in the Yellow-legged Gull (Larus michahellis). J Ornithol 147:513–519. https://doi.org/10.1007/s10336-006-0074-4
Pike TW, Petrie M (2003) Potential mechanisms of avian sex manipulation. Biol Rev 78:553–574. https://doi.org/10.1017/S1464793103006146
Que P, Székely T, Wang P, Lu Q, Lei W, Liu Y, Zhang Z (2019) Offspring sex ratio is unrelated to parental quality and time of breeding in a multiple-breeding shorebird. J Ornithol 160:443–452. https://doi.org/10.1007/s10336-018-1620-6
Rosenfeld CS, Roberts RM (2004) Maternal diet and other factors affecting offspring sex ratio: a review. Biol Reprod 71:1063–1070. https://doi.org/10.1095/biolreprod.104.030890
Rutkowska J, Badyaev AV (2008) Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Philos T R Soc B 363:1675–1686. https://doi.org/10.1098/rstb.2007.0006
Saino N, Ambrosini R, Martinelli R, Calza S, Møller AP, Pilastro A (2002) Offspring sexual dimorphism and sex allocation in relation to parental age and paternal ornamentation in the Barn Swallow. Mol Ecol 11:1533–1544. https://doi.org/10.1046/j.1365-294X.2002.01542.x
Santoro S, Green AJ, Speakman JR, Figuerola J (2015) Facultative and non-facultative sex ratio adjustments in a dimorphic bird species. Oikos 124:1215–1224. https://doi.org/10.1111/oik.01889
Sheldon BC, Andersson S, Griffith SC, Ornborg J, Sendecka J (1999) Ultraviolet colour variation influences Blue Tit sex ratios. Nature 402:874–877. https://doi.org/10.1038/47239
Slagsvold T, Røskaft E, Engen S (1986) Sex ratio, differential cost of rearing young, and differential mortality between the sexes during the period of parental care: Fisher’s theory applied to birds. Ornis Scand 17:117–125. https://doi.org/10.2307/3676860
Szász E, Kiss D, Rosivall B (2012) Sex ratio adjustment in birds. Ornis Hungarica 20:26–36. https://doi.org/10.2478/orhu-2013-0002
Szczys P, Nisbet IC, Hatch JJ, Kesseli RV (2001) Sex ratio bias at hatching and fledging in the roseate tern. The Condor 103:385–389. https://doi.org/10.1093/condor/103.2.385
Székely T, Cuthill IC, Yezerinac S, Griffiths R, Kis J (2004) Brood sex ratio in the Kentish Plover. Behav Ecol 15:58–62. https://doi.org/10.1093/beheco/arg105
Székely T, Liker A, Freckleton RP, Fichtel C, Kappeler PM (2014a) Sex-biased survival predicts adult sex ratio variation in wild birds. P R Soc B 281:20140342. https://doi.org/10.1098/rspb.2014.0342
Székely T, Weissing FJ, Komdeur J (2014b) Adult sex ratio variation: implications for breeding system evolution. J Evolution Biol 27:1500–1512. https://doi.org/10.1111/jeb.12415
Tagirov M, Rutkowska J (2013) Chimeric embryos – potential mechanism of avian offspring sex manipulation. Behav Ecol 24:802–805. https://doi.org/10.1093/beheco/art007
Teather KL (1992) An experimental study of competition for food between male and female nestlings of the Red-winged Blackbird. Behav Ecol Sociobiol 31:81–87. https://doi.org/10.1007/BF00166340
Teather KL, Weatherhead PJ (1988) Sex-specific energy requirements of great-tailed grackle (Quiscalus mexicanus) nestlings. J Anim Ecol 57:659–668. https://doi.org/10.2307/4931
Teather KL, Weatherhead PJ (1989) Sex-specific mortality in nestling Great-tailed Grackles. Ecology 70:1485–1493. https://doi.org/10.2307/1938207
Torres R, Drummond H (1999) Does large size make daughters of the Blue-footed Booby more expensive than sons? J Anim Ecol 68:1133–1141. https://doi.org/10.1046/j.1365-2656.1999.00357.x
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92. https://doi.org/10.1126/science.179.4068.90
Velando A (2002) Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the Blue-footed Booby. Behav Ecol 13:443–449. https://doi.org/10.1093/beheco/13.4.443
Weatherhead PJ, Teather KL (1991) Are skewed fledgling sex ratios in sexually dimorphic birds adaptive? Am Nat 138:1159–1172. https://doi.org/10.1086/285275
Weimerskirch H, Lallemand J, Martin J (2005) Population sex ratio variation in a monogamous long-lived bird, the Wandering Albatross. J Anim Ecol 74:285–291. https://doi.org/10.1111/j.1365-2656.2005.00922.x
West SA (2009) Sex allocation. Princeton University Press, Princeton
Wojczulanis-Jakubas K, Minias P, Kaczmarek K, Janiszewski T (2013) Late-breeding Great Cormorants Phalacrocorax carbo sinensis produce fewer young of the more vulnerable sex. Ibis 155:626–631. https://doi.org/10.1111/ibi.12061
Zielińska M, Dubiec A, Zieliński P (2010) Offspring sex ratio skew in the sexually monomorphic House Martin Delichon urbicum. J Avian Biol 41:591–596. https://doi.org/10.1111/j.1600-048X.2010.04849.x
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Bartoń K (2015) Package ‘MuMIn’. https://cran.rproject.org/web/packages/MuMIn/MuMIn.pdf
Betleja J, Skórka P, Zielińska M (2007) Super-normal clutches and female-female pairs in gulls and terns breeding in Poland. Waterbird 30:624–629. https://www.jstor.org/stable/25148267
Betleja J (2003) Ecological conditions of the expansion of Whiskered Tern. PhD dissertation. University of Wrocław (In Polish)
Bortolotti GR (1986) Influence of sibling competition on nestling sex ratios of sexually dimorphic birds. Am Nat 127:495–507. https://www.jstor.org/stable/2461579
Drummond H, Osorno JL, Torres R, Chavelas CG, Larios HM (1991) Sexual size dimorphism and sibling competition: implications for avian sex ratios. Am Nat 138:623–641. https://www.jstor.org/stable/2462457
Stamps JA (1990) When should avian parents differentially provision sons and daughters? Am Nat 135:671–685. https://www.jstor.org/stable/2462029
Acknowledgements
We greatly appreciate the cooperation of the fish farmers concerned, in particular Jerzy Wojciech Adamek from the Experimental Fish Farm in Zator. We are also grateful to Antonia Łobodzińska, Stanisław Broński and Nataly Atamas for their assistance in the field. We would like to thank Peter Senn for his linguistic guidance.
Funding
This work was supported as part of a project from the National Science Centre (2014/15/B/NZ8/00214). The fieldwork was carried out by permission of the Local Ethical Committee and General Directorate for Environmental Protection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Barbraud.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banach, A., Flis, A., Kusal, B. et al. Sex ratio and sex-specific chick mortality in a species with moderate sexual size dimorphism and female brood desertion. J Ornithol (2024). https://doi.org/10.1007/s10336-024-02182-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10336-024-02182-z