Abstract
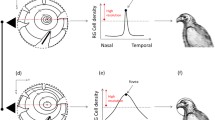
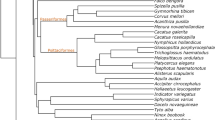
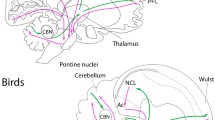
Eye movements are a critical component of visually guided behaviours, allowing organisms to scan the environment and bring stimuli of interest to regions of acuity in the retina. Although the control and modulation of eye movements by cranial nerve nuclei are highly conserved across vertebrates, species variation in visually guided behaviour and eye morphology could lead to variation in the size of oculomotor nuclei. Here, we test for differences in the size and neuron numbers of the oculomotor nuclei among birds that vary in behaviour and eye morphology. Using unbiased stereology, we measured the volumes and numbers of neurons of the oculomotor (nIII), trochlear (nIV), abducens (nVI), and Edinger-Westphal (EW) nuclei across 71 bird species and analysed these with phylogeny-informed statistics. Owls had relatively smaller nIII, nIV, nVI and EW nuclei than other birds, which reflects their limited degrees of eye movements. In contrast, nVI was relatively larger in falcons and hawks, likely reflecting how these predatory species must shift focus between the central and temporal foveae during foraging and prey capture. Unexpectedly, songbirds had an enlarged EW and relatively more nVI neurons, which might reflect accommodation and horizontal eye movements. Finally, the one merganser we measured also has an enlarged EW, which is associated with the high accommodative power needed for pursuit diving. Overall, these differences reflect species and clade level variation in behaviour, but more data are needed on eye movements in birds across species to better understand the relationships among behaviour, retinal anatomy, and brain anatomy.





Similar content being viewed by others
Data availability statement
This article has no additional data.
References
Bringmann A (2019) Structure and function of the bird fovea. Anatom Histol Embryol 48:177–200
Büttner-Ennever JA (2006) The extraocular motor nuclei: organization and functional neuroanatomy. Prog Brain Res 151:95–125
Collewijn H, Martins A, Steinman R (1983) Compensatory eye movements during active and passive head movements: fast adaptation to changes in visual magnification. J Physiol 340:259–286
Corfield JR, Long B, Krilow JM, Wylie DR, Iwaniuk AN (2016) A unique cellular scaling rule in the avian auditory system. Brain Struct Funct 221:2675–2693
Cunha F, Racicot K, Nahirney J, Heuston C, Wylie DR, Iwaniuk AN (2020) Allometric scaling rules of the cerebellum in galliform birds. Brain Behav Evol 95:78–92
De Groot S, Gebhard J (1952) Pupil size as determined by adapting luminance. J Optical Soc Amer 42:492–495
Donaldson I, Knox PC (1991) Afferent signals from pigeon extraocular muscles modify the vestibular responses of units in the abducens nucleus. Proc R Soc Lond Ser B: Biol Sci 244:233–239
Ericson PG, Anderson CL, Britton T, Elzanowski A, Johansson US, Källersjö M, Ohlson JI, Parsons TJ, Zuccon D, Mayr G (2006) Diversification of Neoaves: integration of molecular sequence data and fossils. Biol Lett 2:543–547
Fite KV, Rosenfield-Wessels S (1975) A comparative study of deep avian foveas. Brain Behav Evol 12:97–115
Fitzgerald M, Vana BA, Reiner A (1990) Control of choroidal blood flow by the nucleus of Edinger-Westphal in pigeons: a laser doppler study. Invest Ophthalmol vis Sci 31:2483–2492
Fox R, Lehmkuhle SW, Bush RC (1977) Stereopsis in the falcon. Science 197:79–81
Gamlin PD, Reiner A (1991) The Edinger-Westphal nucleus: sources of input influencing accommodation, pupilloconstriction, and choroidal blood flow. J Comp Neurol 306:425–438
Garamszegi LZ (2014) Modern phylogenetic comparative methods and their application in evolutionary biology: concepts and practice. Springer, Heidelberg
Gardella D, Hatton WJ, Rind HB, Rosen GD, von Bartheld CS (2003) Differential tissue shrinkage and compression in the z-axis: implications for optical disector counting in vibratome-, plastic-and cryosections. J Neurosci Methods 124:45–59
Van Gils J, Wiersma P, Kirwan GM (2020) Eurasian woodcock (Scolopax rusticola), version 1.0. In Birds of the World (del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E, Eds). Cornell Lab of Ornithology, Ithaca, NY, USA
Glaeser G, Paulus HF (2015) The evolution of the eye. Springer, Heidelberg
Glasser A, Howland HC (1996) A history of studies of visual accommodation in birds. Quart Rev Biol 71:475–509
Glasser A, Murphy CJ, Troilo D, Howland HC (1995) The mechanism of lenticular accommodation in chicks. Vision Res 35:1525–1540
Glasser A, Pardue MT, Andison ME, Sivak JG (1997) A behavioral study of refraction, corneal curvature, and accommodation in raptor eyes. Can J Zool 75:2010–2020
Gundersen H, Jensen E, Kiêu K, Nielsen J (1999) The efficiency of systematic sampling in stereology—reconsidered. J Microscopy 193:199–211
Gutiérrez-Ibáñez C, Iwaniuk AN, Lisney TJ, Faunes M, Marín GJ, Wylie DR (2012) Functional implications of species differences in the size and morphology of the isthmo optic nucleus (ION) in birds. PLoS One 7:e37816
Hall M, Ross C (2007) Eye shape and activity pattern in birds. J Zool 271:437–444
Heaton MB, Wayne DB (1983) Patterns of extraocular innervation by the oculomotor complex in the chick. J Comp Neurol 216:245–252
Herculano-Houzel S, Manger PR, Kaas JH (2014) Brain scaling in mammalian evolution as a consequence of concerted and mosaic changes in numbers of neurons and average neuronal cell size. Front Neuroanat 8:77
Herculano-Houzel S, Messeder DJ, Fonseca-Azevedo K, Pantoja NA (2015) When larger brains do not have more neurons: increased numbers of cells are compensated by decreased average cell size across mouse individuals. Front Neuroanat 9:64
Howland HC, Rowland M, Schmid K, Pettigrew JD (1991) Restricted range of ocular accommodation in barn owls (Aves: Tytonidae). J Comp Physiol A 168:299–303
Inzunza O, Bravo H, Smith RL, Angel M (1991) Topography and morphology of retinal ganglion cells in Falconiforms: a study on predatory and carrion-eating birds. Anat Rec 229:271–277
Iwaniuk AN, Hurd PL (2005) The evolution of cerebrotypes in birds. Brain Behav Evol 65:215–230
Iwaniuk AN, Wylie DRW (2006) The evolution of stereopsis and the Wulst in caprimulgiform birds: a comparative analysis. J Comp Physiol A 192:1313–1326
Iwaniuk AN, Wylie DR (2020) Sensory systems in birds: What we have learned from studying sensory specialists. J Comp Neurol 528:2902–2918
Jerison H (1973) Evolution of the brain and intelligence. Academic Press, New York
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 491:444–448
Katzir G, Howland HC (2003) Corneal power and underwater accommodation in great cormorants (Phalacrocorax carbo sinensis). J Exp Biol 206:833–841
Knudsen EI (1982) Auditory and visual maps of space in the optic tectum of the owl. J Neurosci 2:1177–1194
Krabichler Q, Vega-Zuniga T, Carrasco D, Fernandez M, Gutierrez-Ibanez C, Marin G, Luksch H (2017) The centrifugal visual system of a palaeognathous bird, the Chilean Tinamou (Nothoprocta perdicaria). J Comp Neurol 525:2514–2534
Labandeira-Garcia J, Guerra-Seijas M, Segade L, Suarez-Nuñez J (1987) Identification of abducens motoneurons, accessory abducens motoneurons, and abducens internuclear neurons in the chick by retrograde transport of horseradish peroxidase. J Comp Neurol 259:140–149
Land MF (2015) Eye movements of vertebrates and their relation to eye form and function. J Comp Physiol A 201:195–214
Land M (2019) Eye movements in man and other animals. Vision Res 162:1–7
Land M, Mennie N, Rusted J (1999) The roles of vision and eye movements in the control of activities of daily living. Perception 28:1311–1328
Levy B, Sivak J (1980) Mechanisms of accommodation in the bird eye. J Comp Physiol 137:267–272
Lind OE, Kelber A, Kröger RH (2008) Multifocal optical systems and pupil dynamics in birds. J Exp Biol 211:2752–2758
Lisney TJ, Iwaniuk AN, Bandet MV, Wylie DR (2012) Eye shape and retinal topography in owls (Aves: Strigiformes). Brain Behav Evol 79:218–236
Lord RD Jr (1956) A comparative study of the eyes of some falconiform and passeriform birds. Am Midl Nat 56:325–344
Machovsky-Capuska GE, Howland HC, Raubenheimer D, Vaughn-Hirshorn R, Würsig B, Hauber ME, Katzir G (2012) Visual accommodation and active pursuit of prey underwater in a plunge-diving bird: the Australasian gannet. Proc R Soc B: Biol Sci 279:4118–4125
Martin GR (1984) The visual fields of the tawny owl, Strix aluco L. Vision Res 24:1739–1751
Martin GR (1986) The eye of a passeriform bird, the European starling (Sturnus vulgaris): eye movement amplitude, visual fields and schematic optics. J Comp Physiol A 159:545–557
Martin GR (1994) Visual fields in woodcocks Scolopax rusticola (Scolopacidae; Charadriiformes). J Comp Physiol A 174:787–793
Martin GR (1999) Eye structure and foraging in King Penguins Aptenodytes patagonicus. Ibis 141:444–450
Martin GR (2007) Visual fields and their functions in birds. J Ornith 148:547–562
Martin GR (2009) What is binocular vision for? A Birds’ Eye View. J Vision 9:14
Martin GR (2017) The sensory ecology of birds. Oxford University Press, Oxford
Martin GR, Katzir G (1994) Visual fields and eye movements in herons (Ardeidae). Brain Behav Evol 44:74–85
Marwitt R, Pilar G, Weakly J (1971) Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res 25:317–334
Moore BA, Doppler M, Young JE, Fernández-Juricic E (2013) Interspecific differences in the visual system and scanning behavior of three forest passerines that form heterospecific flocks. J Comp Physiol A 199:263–277
Moore BA, Pita D, Tyrrell LP, Fernández-Juricic E (2015) Vision in avian emberizid foragers: maximizing both binocular vision and fronto-lateral visual acuity. J Exp Biol 218:1347–1358
Moore BA, Tyrrell LP, Pita D, Bininda-Emonds OR, Fernández-Juricic E (2017) Does retinal configuration make the head and eyes of foveated birds move? Sci Rep 7:38406
Moroney MK, Pettigrew JD (1987) Some observations on the visual optics of kingfishers (Aves, Coraciformes, Alcedinidae). J Comp Physiol A 160:137–149
Murphy C, Howland HC (1983) Owl eyes: accommodation, corneal curvature and refractive state. J Comp Physiol 151:277–284
Nath T, Mathis A, Chen AC, Patel A, Bethge M, Mathis MW (2019) Using DeepLabCut for 3D markerless pose estimatation across species and behaviors. Nat Prot 14:2152–2176
O’Rourke CT, Hall MI, Pitlik T, Fernández-Juricic E (2010a) Hawk eyes I: diurnal raptors differ in visual fields and degree of eye movement. PLoS One 5:e12802
O’Rourke CT, Pitlik T, Hoover M, Fernández-Juricic E (2010b) Hawk eyes II: diurnal raptors differ in head movement strategies when scanning from perches. PLoS One 5:e12169
Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W (2013) The caper package: comparative analysis of phylogenetics and evolution in R. https://cran.r-project.org/web/packages/caper/index.html
Ott M (2006) Visual accommodation in vertebrates: mechanisms, physiological response and stimuli. J Comp Physiol A 192:97
Pagel M (1999) The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst Biol 48:612–622
Pettigrew JD (1986) The evolution of binocular vision. In: Pettigrew JD, Sanderson KJ, Levick WR (eds) Visual neuroscience. Cambridge University Press, Cambridge, pp 208–222
Pettigrew JD, Collin SP, Ott M (1999) Convergence of specialised behaviour, eye movements and visual optics in the sandlance (Teleostei) and the chameleon (Reptilia). Curr Biol 9:421–424
Potier S, Duriez O, Cunningham GB, Bonhomme V, O’Rourke C, Fernández-Juricic E, Bonadonna F (2018) Visual field shape and foraging ecology in diurnal raptors. J Exp Biol 221:jeb177295
Potier S, Mitkus M, Kelber A (2020) Visual adaptations of diurnal and nocturnal raptors. Sem Cell Develop Biol 106:116–126
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reiner A, Karten HJ, Gamlin PDR, Erichsen JT (1983) Parasympathetic ocular control–functional subdivisions and circuitry of the avian nucleus of Edinger-Westphal. Trend Neurosci 6:140–145
Reiner A, Erichsen JT, Cabot JB, Evinger C, Fitzgerald ME, Karten HJ (1991) Neurotransmitter organization of the nucleus of Edinger-Westphal and its projection to the avian ciliary ganglion. Visual Neurosci 6:451–472
Schaeffel F, Wagner H (1992) Barn owls have symmetrical accommodation in both eyes, but independent pupillary responses to light. Vision Res 32:1149–1155
Schliep KP (2011) phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593
Sivak JG (1980) Avian mechanisms for vision in air and water. Trend Neurosci 3:314–317
Sivak JG, Hildebrand T, Lebert C (1985) Magnitude and rate of accommodation in diving and nondiving birds. Vision Res 25:925–933
Sohal G, Holt R (1978) Identification of the trochlear motoneurons by retrograde transport of horseradish peroxidase. Exp Neurol 59:509–514
Steinbach MJ, Money K (1972) Eye movements of the owl. Vision Res 13:889–891
Steinbach MJ, Angus RG, Money K (1974) Torsional eye movements of the owl. Vision Res 14:745–746
Strod T, Arad Z, Izhaki I, Katzir G (2004) Cormorants keep their power: visual resolution in a pursuit-diving bird under amphibious and turbid conditions. Curr Biol 14:R376–R377
Wagner H, Schaeffel F (1991) Barn owls (Tyto alba) use accommodation as a distance cue. J Comp Physiol A 169:515–521
Wallman J, Pettigrew JD (1985) Conjugate and disjunctive saccades in two avian species with contrasting oculomotor strategies. J Neurosci 5:1418–1428
Walls GL (1942) The vertebrate eye and its adaptive radiation. Cranbrook Institute of Science, Bloomfield Hills
Walls GL (1962) The evolutionary history of eye movements. Vision Res 2:69–80
Wathey JC, Pettigrew JD (1989) Quantitative analysis of the retinal ganglion cell layer and optic nerve of the barn owl Tyto alba. Brain Behav Evol 33:279–292
Wood CA (1917) The fundus oculi of birds: especially as viewed by the ophthalmoscope; a study in the comparative anatomy and physiology. Lakeside Press, Chicago
Wylie DR, Frost BJ (1996) The pigeon optokinetic system: visual input in extraocular muscle coordinates. Visual Neurosci 13:945–954
Wylie DR, Shaver SW, Frost BJ (1994) The visual response properties of neurons in the nucleus of the basal optic root of the northern saw-whet owl (Aegolius acadicus). Brain Behav Evol 43:15–25
Wylie DR, Gutiérrez-Ibáñez C, Iwaniuk AN (2015) Integrating brain, behavior, and phylogeny to understand the evolution of sensory systems in birds. Front Neurosci 9:281
Yorzinski JL, Patricelli GL, Babcock JS, Pearson JM, Platt ML (2013) Through their eyes: selective attention in peahens during courtship. J Exp Biol 216:3035–3046
Acknowledgements
We wish to thank Maurice Needham for assistance with microscopy and Michelle Martin for assistance in acquiring some of our hawk and falcon brains from wildlife rehabilitation centres.
Funding
Funding to support this study was provided by scholarships to FC from the University of Lethbridge, NSERC Discovery grants to DRW and ANI and the Canada Foundation for Innovation and Canada Research Chairs Program to ANI.
Author information
Authors and Affiliations
Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. FC, CGI, DRW, and ANI: study concept and design. FC, CGI, and BB: acquisition of the data. FC, CGI, DRW, and ANI: analysis and interpretation of the data. FC, CGI, DRW, and ANI: drafting of the manuscript. CGI, DRW, and ANI: critical revision of the manuscript for important intellectual content, administrative, technical, and material support.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Handling editor: Kentaro Arikawa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cunha, F., Gutiérrez-Ibáñez, C., Brinkman, B. et al. The relative sizes of nuclei in the oculomotor complex vary by order and behaviour in birds. J Comp Physiol A 209, 341–360 (2023). https://doi.org/10.1007/s00359-022-01598-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-022-01598-3