Abstract
Among birds there are considerable interspecific differences in all aspects of visual fields. However, it is hypothesised that the topography of the frontal binocular portion of fields are of only three main types, and their principal functions lie in the degree to which vision is used in the guidance of the bill (or feet) towards food objects or for the provisioning of chicks. In the majority of birds, the width of the frontal binocular field is narrow (20°–30° maximum). It shows a high degree of similarity across species and appears to be independent of phylogeny or ecology. Binocularity appears not to be concerned with higher level visual processing involving the combination of information from the two eyes (as in, for example, stereoscopic vision). Binocularity is concerned with gaining independently, in each eye, information which is derived from the symmetrically expanding optic flow-field, which specifies the direction of travel of the head and its time to contact an object, as in pecking or lunging at food items. Species which do not provision their chicks, and whose foraging is guided by tactile cues or which filter feed, have much smaller binocular overlap (10°) and this seems sufficient to control flight. These birds gain comprehensive visual coverage of the celestial hemisphere and show reduced vigilance behaviour. The visual fields of owls, which combine more extensive binocular overlap (50°) with a large blind area behind the head, may not be primarily associated with nocturnal activity. Visual fields of this type are not found in other nocturnally active birds such as Oilbirds, nightjars and kiwis. The type of visual field found in owls may be a result of large eyes combined with elaborate outer ear structures that are placed within a relatively small skull. Eye movements of significant amplitude do not occur in all birds. However, eye movements of between 14° and 18° occur in species such as herons, hornbills and cormorants and can result in the spontaneous abolition of binocularity. These eye movements are non-conjugate and can produce markedly asymmetric visual fields. The width of any blind area above the head is a function of eye size, with the largest eyes associated with optical adnexa, (eye lashes, brows). These may be associated with avoiding imaging the sun on the retina. However, many small-eyed birds have no optical adnexa and cannot avoid seeing the sun.











Similar content being viewed by others
References
Bugayevskiy LM (1995) Map projections: a reference manual. Taylor & Francis, London
Davies MNO, Green PR (1994) Multiple sources of depth information: an ecological approach. In: Davies MNO, Green PR (eds) Perception and motor control in birds: an ecological approach. Springer, Berlin Heidelberg New York, pp 339–356
Dickinson CM (1991) Optical aids for low vision. In: Charman WN (ed) Vision and visual dysfunction. Macmillan, London, pp 183–228
Frost BJ, Wylie DR, Wang YC (1994) The analysis of motion in the visual systems of birds. In: Davies MNO, Green PR (eds) Perception and motor control in birds: an ecological approach. Springer, Berlin Heidelberg New York, pp 248–269
Gibson JJ (1986) The ecological approach to visual perception. Erlbaum, Hove
Gottschaldt KM (1985) Structure and function of avian somatosensory receptors. In: King AS, Mclelland J (eds) Form and function in birds vol 3. Academic, London, pp 375–461
Guillemain M, Martin GR, Fritz H (2002) Feeding methods, visual fields and vigilance in dabbling ducks (Anatidae). Funct Ecol 16: 522–529
Ho A, Bilton SM (1986) Low contrast charts effectively differentiate between types of blur. Am J Ophthal Physiol Opt 63:202–208
Hughes A (1977) The topography of vision in mammals of contrasting life style: comparative optics and retinal organization. In: Crescitelli F (ed) Handbook of sensory physiology. vol VII/5. Springer, Berlin Heidelberg New York, pp 613–756
Jones R K,Lee D N (1981) Why two eyes are better than one: The two views of binocular vision. J Exp Psychol Hum Percept Perform 7:30–40
Katzir G, Martin GR (1998) Visual fields in the Black-crowned Night Heron Nycticorax nycticorax: nocturnality does not result in owl-like features. Ibis 140:157–162
King AS, King DZ (1980) Avian morphology: general principles. In: King AS, McLelland J (eds) Form and function in birds. Academic, London, pp 10–89
Konishi M (1973) Locatable and non-locatable acoustic signals for barn owls. Am Nat 107:775–785
Kral K (2003) Behavioural-analytical studies of the role of head movements in depth perception in insects, birds and mammals. Behav Processes 64:1–12
Land MF, Nilsson D-E (2002) Animal eyes. Oxford University Press, Oxford
Le Claire J, Nadler M P, Weiss S, Miller D (1982) A new glare tester for clinical testing: results comparing normal subjects and variously corrected aphakic patients. Archiv Ophthal 100:153–158
Lee DN (1980) The optic flow field: the foundation of vision. Philps Trans R Soc Lond B 290:169–179
Lee DN (1994) An eye or ear for flying. In: Davies MNO, Green PR (eds) Perception and motor control in birds: an ecological approach. Springer, Berlin Heidelberg New York, pp 270–291
Lee DN, Reddish PE (1981) Plummeting gannets: a paradigm of ecological optics. Nature 293:293–294
Lee DN, Reddish PE, Rand DT (1991) Aerial docking by Hummingbirds. Naturwissenschaften 78:526–527
Martin GR (1982) An owl’s eye: schematic optics and visual performance in Strix aluco L. J Comp Physiol A 145:341–349
Martin GR (1984) The visual fields of the tawny owl, Strix aluco L. Vision Res 24:1739–1751
Martin GR (1985) Eye. In: King AS, McLelland J (eds) Form and function in birds. vol 3. Academic, London, pp 311–373
Martin GR (1986a) The eye of a passeriform bird, the European starling (Sturnus vulgaris): eye movement amplitude, visual fields and schematic optics. J Comp Physiol A 159:545–557
Martin GR (1986b) Sensory capacities and the nocturnal habit of owls (Strigiformes). Ibis 128:266–277
Martin GR (1986c) Total panoramic vision in the mallard duck, Anas platyrhynchos. Vision Res 26:1303–1306
Martin GR (1994) Visual fields in woodcocks Scolopax rusticola (Scolopacidae; Charadriiformes). J Comp Physiol A 174:787–793
Martin GR (1998) Eye structure and amphibious foraging in albatrosses. Proc R Soc Lond B 265:1–7
Martin GR (1999) Eye structure and foraging in King Penguins Aptenodytes patagonicus. Ibis 141:444–450
Martin GR, Brooke MDL (1991) The eye of a procellariiform seabird, the Manx shearwater, Puffinus puffinus: visual fields and optical structure. Brain Behav Evol 37:65–78
Martin GR, Coetzee HC (2004) Visual fields in Hornbills: precision-grasping and sunshades. Ibis 146:18–26
Martin GR, Katzir G (1994a) Visual fields and eye movements in herons (Ardeidae). Brain Behav Evol 44:74–85
Martin GR, Katzir G (1994b) Visual fields in the stone curlew Burhinus oedicnemus. Ibis 136:448–453
Martin GR, Katzir G (1995) Visual fields in ostriches. Nature 374:19–20
Martin GR, Katzir G (1999) Visual field in Short-toed eagles Circaetus gallicus and the function of binocularity in birds. Brain Behav Evol 53:55–66
Martin GR, Prince PA (2001) Visual fields and foraging in Procellariiform seabirds: sensory aspects of dietary segregation. Brain Behav Evol 57:33–38
Martin GR, Young SR (1983) The retinal binocular field of the pigeon (Columba livia): English racing homer. Vision Res 23:911–915
Martin GR, Young SR (1984) The eye of the Humboldt Penguin, Spheniscus humboldti: visual fields and schematic optics. Proc R Soc Lond B 223:197–222
Martin GR, Rojas L M, Ramirez Y, McNeil R (2004a) The eyes of oilbirds (Steatornis caripensis): pushing at the limits of sensitivity. Naturwissenschaften 91:26–29
Martin GR, Rojas LM, Ramirez Figueroa YM, McNeil R (2004b) Binocular vision and nocturnal activity in Oil birds (Steatornis caripensis) and Pauraques (Nyctidromus albicollis): Caprimulgiformes. Ornithol Neotrop 15(Suppl.):233–242
Martin GR, Jarrett N, Tovey P, White CR (2005) Visual fields in Flamingos: chick-feeding versus filter-feeding. Naturwissenschaften 92:351–354
Martin GR, Jarrett N, Williams M (2007a) Visual fields in Blue Ducks and Pink-eared Ducks: visual and tactile foraging. Ibis 149:112–120
Martin GR, McNeil R, Rojas LM (2007b) Vision and the foraging technique of skimmers (Rynchopidae). Ibis. doi:10.1111/j.1474–919x.2007.00706.x
Martin GR, Wilson KJ, Wild MJ, Parsons S, Kubke MF, Corfield J (2007c) Kiwi forego vision in the guidance of their nocturnal activities. PLoSOne 2(2):e198. doi:10.1371/journal.pone.0000198
Martin GR, White CR, Butler PJ (2007d) Vision and the foraging technique of Great Cormorants Phalacrocorax carbo: pursuit or flush-foraging? Ibis (in press)
McFadden SA (1993) Constructing the three-dimensional image. In: Zeigler HP, Bischof H-J (eds) Vision, brain and behavior in birds. MIT, Cambridge, Mass., pp 47–61
McFadden SA (1994) Binocular depth perception. In: Davies MNO, Green PR (eds) Perception and motor control in birds: an ecological approach. Springer, Berlin Heidelberg New York, pp 54–73
Nebel S, Jackson DL, Elner RW (2005) Functional association of bill morphology and foraging behaviour in calidrid sandpipers. Anim Biol 55:235–243
Nieder A, Wagner H (2000) Horizontal-disparity tuning of neurons in the visual forebrain of the behaving barn owl. J Neurophysiol 83:2967–2979
Nieder A, Wagner H (2001) Hierarchical processing of horizontal disparity information in the visual forebrain of behaving owls. J Neurosci 21:4514–4522
Norberg RA (1968) Physical factors in directional hearing in Aegolius funereus (Strigiformes), with special reference to the significance of the asymmetry of the extrenal ears. Arkive Zool 20:181–204
Norberg RA (1978) Skull asymmetry, ear structure and function and auditory localization in Tengmalm’s Owl, Aegolius funereus. Philos Trans R Soc Lond B 282B:325–410
Payne RS (1971) Acoustic location of prey by barn owls. J Exp Biol 54:535–573
Piersma T, van Aelst R, Kurk K, Berkhoudt H, Maas LRM (1998) A new pressure sensory mechanims for prey detection in birds:the use of principles of seabed dynamics? Proc R Soc Lond B 265:1377–1383
Snyder JP (1993) Flattening the earth: 2000 years of map projections. University of Chicago Press, Chicago
Tansley K (1965) Vision in vertebrates. Chapman & Hall, London
Walls GL (1942) The vertebrate eye and its adaptive radiation. Cranbrook Institute of Science, Michigan
White CR, Day N, Butler PJ, Martin GR (2007) Vision and foraging in cormorants: more like Herons than Hawks? PloSOne. doi:10.1371/journal.pone.0000639
Willigen RFvd, Frost BJ, Wagner H (2003) How owls structure visual information. Anim Cogn 6:39–55
Zusi RL (1996) Family Rynchopidae (Skimmers). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world, vol. 3. Hoatzin to Auks. Lynx Edicions, Barcelona, pp 668–677
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Appendices
Appendix 1
The ophthalmoscopic technique for determining retinal visual fields
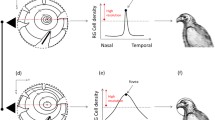
This technique has been used in a range of birds of different phylogeny, ecology and feeding techniques and readily permits interspecific comparisons (Martin and Coetzee 2004; Martin et al. 2005, 2004b; Martin and Prince 2001). For a detailed description of the apparatus and methods see Martin and Katzir (1994a). Briefly, each bird is held in a foam rubber cradle with its head held in position at the centre of a visual perimeter by a holder specially manufactured to hold the bill of each species. The perimeter’s co-ordinate system follows conventional latitude and longitude with the equator aligned vertically in the birds’ median sagittal plane and this co-ordinate system is used for the presentation of visual field data (Fig. 2). The bill is closed and the head positioned so that the tip of the lower mandible projects in the approximate direction adopted spontaneously by the bird when at rest. Head position is also recorded in photographs of birds held in the hand in the open or when at rest in the field. The results are corrected to this spontaneously adopted head position.
The eyes are examined using an ophthalmoscope mounted on the perimeter arm. For each eye, the visual projections of the following are usually determined as a function of elevation in the median sagittal plane at 10° intervals: (1) the limits of the retinal visual field, and (2) the edges of the pecten. From these data (corrected for viewing from an hypothetical viewing point placed at infinity) a topographical map of the visual field and its principal features is constructed. These features are: (1) the monocular fields, the visual field of a single eye; (2) the binocular field, the area where monocular fields overlap; (3) the cyclopean field, the total visual field produced by the combination of both monocular fields; and (4) projection of the pectens. Each pecten creates a blind area within each eye’s visual field. The pecten is a highly vascular and pigmented structure that overlies the exit of the optic nerve and functions as a nutrient organ within the anterior chamber of the eye. Its projection provides a landmark in the field of each eye. Depending upon the species and the time available to hold the birds constranied in this way, it is possible to measure limits of almost the entire frontal visual field from directly below the head through approximetly 270° in the sagittal plane to the horizontal behind the head. Determining the positions of the visual field margins both directly in front and behind the head, i.e. in an approximately horizontal plane, allows determination of monocular field widths and the extent of the cyclopean field in this plane. In some birds, spontaeous eye movements are readily observed and their amplitude as a function of elevation are determined by making light tapping sounds or flashing a small light source in the periphery of the visual field, and then determining the maximum and minimum limits of the retinal margin at each elevation. These procedures are non-invasive and follow guidelines established by the United Kingdom, Animals (Scientific Procedures) Act, 1986.
Appendix 2
Bird species in which visual fields have been determined using the ophthalmoscopic reflex method (14 Orders; 20 families; 32 spp.)


Rights and permissions
About this article
Cite this article
Martin, G.R. Visual fields and their functions in birds. J Ornithol 148 (Suppl 2), 547–562 (2007). https://doi.org/10.1007/s10336-007-0213-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-007-0213-6