Abstract
Purpose
The risk of treatment-related toxicity is important for patients with localised prostate cancer to consider when deciding between treatment options. We developed a model to predict hospitalisation for radiation-induced genitourinary toxicity based on patient characteristics.
Methods
The prospective South Australian Prostate Cancer Clinical Outcomes registry was used to identify men with localised prostate cancer who underwent curative intent external beam radiotherapy (EBRT) between 1998 and 2019. Multivariable Cox proportional regression was performed. Model discrimination, calibration, internal validation and utility were assessed using C-statistics and area under ROC, calibration plots, bootstrapping, and decision curve analysis, respectively.
Results
There were 3,243 patients treated with EBRT included, of which 644 (20%) patients had a treated-related admission. In multivariable analysis, diabetes (HR 1.35, 95% CI 1.13–1.60, p < 0.001), smoking (HR 1.78, 95% CI 1.40–2.12, p < 0.001), and bladder outlet obstruction (BOO) without transurethral resection of prostate (TURP) (HR 7.49, 95% CI 6.18–9.08 p < 0.001) followed by BOO with TURP (HR 4.96, 95% CI 4.10–5.99 p < 0.001) were strong independent predictors of hospitalisation (censor-adjusted c-statistic = 0.80). The model was well-calibrated (AUC = 0.76). The global proportional hazards were met. In internal validation through bootstrapping, the model was reasonably discriminate at five (AUC 0.75) years after radiotherapy.
Conclusions
This is the first study to develop a predictive model for genitourinary toxicity requiring hospitalisation amongst men with prostate cancer treated with EBRT. Patients with localised prostate cancer and concurrent BOO may benefit from TURP before EBRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most common malignancy amongst men worldwide, and the number of long-term prostate cancer survivors continues to increase. [1] Prostate cancer is often treated with radiotherapy or surgery, with similar local control outcomes but different treatment-related toxicity profiles and side effect profiles. [2] However, there are limited high-quality data identifying predictive factors for genitourinary toxicity after radiotherapy.
The development of genitourinary toxicity following EBRT has been demonstrated to be influenced by a range of factors other than dosimetric variables alone [3, 4] and include baseline urinary symptoms [5, 6] and comorbidities such as diabetes [7]. However, predictive models classically have been limited to mechanistic analysis of dose-volume metrics [8, 9], which are often already incorporated into radiotherapy delivery planning systems.
This study used pre-treatment clinical factors to develop and validate a novel predictive model for radiotherapy-related genitourinary toxicity requiring hospital admission and then determined the clinical utility of the model by using decision curve analysis.
Material and methods
Participants
The prospective South Australian Prostate Cancer Clinical Outcome Collaborative (SA-PCCOC) registry was used to identify men with localised prostate cancer who underwent local curative intent external beam radiotherapy between January 1, 1998, and January 31, 2019. The SA-PCCOC registry prospectively recruits > 90% of patients who are diagnosed with prostate cancer in the State of South Australia. We linked patient records from the SA-PCCOC registry with the Integrated South Australian Activity Collection (ISAAC) Hospital Administrative Database to identify patients who presented to any major hospital in South Australia with treatment-related genitourinary toxicity, as defined by a pre-selected list of International Classification Disease 10th Edition (ICD-10-AM)/ Australian Classification of Health Interventions (ACHI). Data linkage was performed by matching patient identifiers within ENVIDO, South Australia. The list of admission and procedures codes were selected based on the literature [10] and recommendations from a multidisciplinary panel, including a urologist, radiation oncologist, general surgeon and a clinical epidemiologist (Supplementary Table 1). A range of genitourinary toxicity events were analysed, including haematuria, irradiation cystitis, urethral stricture, urinary incontinence and urinary retention (Supplementary Table 2).
Primary outcomes
Of the identified patients with prostate cancer, baseline characteristics, including age (continuum), Charlson Comorbidity Index (continuum, 0/1–2/3–4/ > 4), diabetes mellitus (present/absent), hypertension (present/absent), smoking history (present/absent), bladder outlet obstruction (yes/ no), transurethral resection of the prostate (TURP) before radiotherapy (yes/ no), were extracted. Patients were further categorised as having bladder outlet obstruction (BOO) with or without TURP prior to EBRT. Genitourinary toxicity event-free survival (EFS) rates were then determined and compared between patient groups at increased risk of treatment-related GU toxicity.
Secondary outcomes
Treatment-related factors, including dose (grey; continuum and > 80 Gy vs ≤ 80 Gy), fractionation and date of treatment completion (< 2009 vs ≥ 2009), were extracted. Oncological characteristics, including T-stage (T1 vs T2 vs T3), Gleason score (< 7 vs 3 + 4 vs 4 + 3 vs > 7 and continuum) and baseline prostate-specific antigen (PSA; continuum) level, were also extracted. The admission data were separated into patients who received EBRT < 2009 and ≥ 2009 to account for the use of three-dimensional conformal radiation therapy (3DCRT) and intensity-modulated radiotherapy/volumetric-modulated arc therapy (IMRT/VMAT), respectively.
Statistical analysis
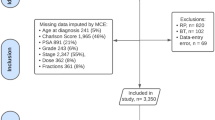
Relationships between genitourinary toxicity-related hospital admission and patient, tumour or treatment characteristics were analysed using multivariable Cox proportional hazard regression analysis. Regression analysis results are presented as a hazard with a 95% confidence interval. Missing clinical data were replaced using multiple imputations by chained equations before regression analysis (Supplementary Fig. 1).
The model development process was conducted following the TRIPOD checklist. [11] Multivariable model development used a backward elimination variable selection process with two-sided alpha = 0.05. [12] Collinearity among the variables was assessed using correlation coefficients. Diabetes was selected rather than the Charlson comorbidity score to reduce multicollinearity in the multivariable analysis. Model validation was performed by the ABCD approach put forward by Steyerberg et al. [13] The proportional hazard hypotheses were tested by Schoenfeld's residual method. The global proportional hazards assumption would not be met if we record significant associations (p < 0·05) for all correlation coefficients. Model discrimination was determined using a censor-adjusted c-statistic. Model calibration was demonstrated with a calibration plot generated using bootstrap resampling (n = 10,000), and the area under the receiver operating characteristic curve (AUC) was determined. Internal validation was performed using a penalised Cox model by adaptive elastic-net regularisation, which can outperform Lasso on data with highly correlated predictors. [14] Tenfold repeated cross-validation was used, which is a more robust internal validation method than bootstrapping (Supplementary Fig. 2). [15] The model utility was assessed using decision curve analysis [16]. A nomogram was developed, which incorporated the clinical predictive factors included in the final model. All statistical analyses were performed using R language, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).[17].
Results
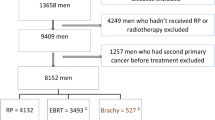
There were 3,243 patients with localised prostate cancer treated with curative intent radiotherapy included in the modelling dataset (Fig. 1). Table 1 outlines the patient baseline characteristics. Patients with BOO without TURP had the lowest 10 year EFS rates (20% [95% CI 15–27%], p < 0.001; Supplementary Table 3, Supplementary Fig. 3).
After adjusting for age, multivariable analysis revealed diabetes (HR 1.35, 95% CI 1.13–1.60, p < 0.001), smoking (HR 1.78, 95% CI 1.40–2.12, p < 0.001), and BOO without TURP (HR 7.49, 95% CI 6.18–9.08 p < 0.001) followed by BOO with TURP ((HR 4.96, 95% CI 4.10–5.99 p < 0.001), to be strong independent predictors of hospitalisation for treatment-related genitourinary toxicity (Fig. 2). Baseline stress urinary incontinence was a strong independent predictor in multivariable analysis (HR 3.95, 95% CI 3.28–4.75, p < 0.001) but failed to meet the Schoenfeld proportional hazards test (p < 0.0001), with suspected multicollinearity with BOO and TURP and was therefore removed from the final model.
The final model met the proportional hazards with a global Schoenfeld test p = 0.1762.
The predictive model performed well with a censor-adjusted c-statistic of 0.80. The model was reasonably discriminate at 1 (AUC 0.765) and five years (AUC 0.75), (Supplementary Fig. 3) and was internally validated (Supplementary Fig. 4). The decision curve analysis determined the model's utility, with a consistently greater net benefit to patients with prostate cancer at risk of radiation-induced genitourinary from threshold probability > 5% (Fig. 3). A nomogram was developed to predict 5-year overall genitourinary toxicity event-free survival (Fig. 4).
Discussion
This is the first study to develop a data-driven predictive model for treatment-related genitourinary toxicity requiring hospitalisation using pre-treatment clinical characteristics amongst patients with localised prostate cancer treated by curative intent EBRT. This involved the analysis of a prospectively state population-level cohort of patients (n = 3243) with an adequate median length of follow-up (5 years), which provided valuable information regarding predictive factors for the development of treatment-related genitourinary toxicity. With the selection of hospitalisation for treatment-related toxicity as an endpoint, the model can be compared with grade 3 RTOG/ CTCAE toxicity reported in the literature. The model performed strongly in calibration at one (AUC 0.765) and five years (AUC 0.75) (Supplementary Fig. 3). In addition, the model was discriminate (concordance index = 0.67, censor-adjusted c-statistic = 0.80) and is consistent with the most robust models in the literature, including the study by Yahya 2015 (concordance index 0.548–0.780). [5].
This was also the first predictive study for genitourinary toxicity requiring hospitalisation to include decision curve analysis (Fig. 3) and a nomogram (Fig. 4). The decision curve analysis consistently demonstrated net benefit in using the model compared to the treat-all approach above 5% threshold probability. The reliable prediction of radiotherapy-related toxicity amongst patients with prostate cancer has been valued by numerous other authors because it could guide the allocation of patients into treatment groups based on their probability of severe toxicity and improve the therapeutic ratio [18,19,20]. Patients at high risk of radiotherapy-related toxicity could be counselled about treatment alternatives, modifications (e.g. advanced planning corrections or dose-reduction) or deferrals. Although not statistically framed to address the question, our analysis also revealed that TURP prior to radiotherapy in patients with BOO might reduce the hazards of GU toxicity requiring admission [HR 7.49 (95% CI 6.18–9.08) vs HR 4.96 (95% CI 4.10–5.99)].
Few other models utilise the clinical characteristics of patients with prostate cancer treated with radiotherapy to predict post-treatment toxicity. Most other models were developed in small cohorts with few toxicity-related events (n < 500) [19, 21, 22]. In addition, given the plethora of complex biophysical manifestations of genitourinary toxicity that can develop, other investigators focus on different toxicity outcomes: early [23] vs late [5, 6, 19] toxicity, mild or severe toxicity (based upon variable grading systems [24], RTOG/EORTC [25, 26], CTCAE [21, 26], LENT-SOMA [5, 6, 9]) or specific symptoms [6, 26, 27] including haematuria [19, 21, 26], nocturia [19], IPSS [20, 23] and erectile dysfunction [28]. These perhaps have less observable impacts on the health system than hospital admissions, the outcome we have used.
Furthermore, very few predictive studies meet the TRIPOD criteria for reporting. There was inconsistent reporting of concordance index, with some reporting concordance probability estimates [9] and others AUC [5, 19, 26], creating difficulties comparing model calibration across studies. Other models were also less discriminative [5, 19, 26]. The calibration plot included in the current study appears as well calibrated as others presented in the literature. [6, 20] No other studies reported a c-statistic. Only one predictive model was externally validated [28]. Whilst other models often failed to report optimism [21, 26], we used a penalised Cox model by adaptive elastic-net regularisation.
Our study has several limitations. Firstly, we did not analyse radiotherapy delivery technique (i.e. 3D-CRT IMRT, VMAT, IGRT), field size or dose-volume effect, as these data were unavailable in the current study and have already been described [8, 9]. However, the majority of included patients were treated with EBRT ≥ 2009 (62%), indicating mostly contemporary treatment techniques. Furthermore, the included clinical predictive factors remained significant in multivariable analysis adjusted for year of treatment, as demonstrated in our recently published article [29]. In addition, we do not have information regarding baseline IPSS, prostate volume or 5-ARI or alpha-blocker medication use before EBRT. Similarly, we do not have information about whether patients received androgen deprivation therapy; however, we acknowledge that the impact of hormone therapy cannot accurately be determined given the bias to treat more unfavourable patients with hormone therapy. Finally, whilst the lack of external validation limits the generalisability of the study results, this is mitigated by using a prospectively captured state-population level dataset.
Conclusion
This study demonstrates the feasibility of predicting radiotherapy-related genitourinary toxicity requiring hospitalisation utilising pre-treatment clinical characteristics for men with localised prostate cancer. Clinicians in the pre-operative counselling setting could use our nomogram to inform patient selection and treatment-related toxicity. TURP before EBRT partially reduces the risk of genitourinary toxicity for men with prostate cancer and bladder outlet obstruction, and this relationship requires further prospective scrutiny.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Rawla P (2019) Epidemiology of prostate cancer. World J Oncol 10(2):63–89
D’Amico AV, Whittington R, Malkowicz SB, Cote K, Loffredo M, Schultz D et al (2002) Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer 95(2):281–286
Heemsbergen WD, Al-Mamgani A, Witte MG, van Herk M, Pos FJ, Lebesque JV. Urinary obstruction in prostate cancer patients from the Dutch trial (68 Gy vs. 78 Gy): relationships with local dose, acute effects, and baseline characteristics. International Journal of Radiation Oncology, Biology, Physics. 2010;78(1):19–25.
Carillo V, Cozzarini C, Rancati T, Avuzzi B, Botti A, Borca VC et al (2014) Relationships between bladder dose-volume/surface histograms and acute urinary toxicity after radiotherapy for prostate cancer. Radiother Oncol 111(1):100–105
Yahya N, Ebert MA, Bulsara M, Haworth A, Kennedy A, Joseph DJ et al (2015) Dosimetry, clinical factors and medication intake influencing urinary symptoms after prostate radiotherapy: an analysis of data from the RADAR prostate radiotherapy trial. Radiother Oncol 116(1):112–118
Mathieu R, Arango JD, Beckendorf V, Delobel JB, Messai T, Chira C et al (2014) Nomograms to predict late urinary toxicity after prostate cancer radiotherapy. World J Urol 32(3):743–751
Herold DM, Hanlon AL, Hanks GE. Diabetes mellitus: a predictor for late radiation morbidity. International Journal of Radiation Oncology*Biology*Physics. 1999;43(3):475–9.
Mavroidis P, Pearlstein KA, Dooley J, Sun J, Saripalli S, Das SK et al (2018) Fitting NTCP models to bladder doses and acute urinary symptoms during post-prostatectomy radiotherapy. Radiat 13(1):17
Ahmed AA, Egleston B, Alcantara P, Li L, Pollack A, Horwitz EM et al (2013) A novel method for predicting late genitourinary toxicity after prostate radiation therapy and the need for age-based risk-adapted dose constraints. Int J Radiat Oncol Biol Phys 86(4):709–715
Nam RK, Cheung P, Herschorn S, Saskin R, Su J, Klotz LH et al (2014) Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. Lancet Oncol 15(2):223–231
Collins GS, Reitsma JB, Altman DG, Moons KGM (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med 13(1):1
Hong HG, Zheng Q, Li Y (2019) Forward regression for Cox models with high-dimensional covariates. J Multivar Anal 173:268–290
Steyerberg EW, Vergouwe Y (2014) Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J 35(29):1925–1931
Zou H, Zhang HH (2009) On the adaptive elastic-net with a diverging number of parameters. Ann Stat 37(4):1733–1751
Jung Y (2018) Multiple predicting K-fold cross-validation for model selection. J Nonparametric Stat 30(1):197–215
Zhang Z, Rousson V, Lee WC, Ferdynus C, Chen M, Qian X et al (2018) Decision curve analysis: a technical note. Ann Transl Med 6(15):308
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria2020.
Fiorino C, Rancati T, Valdagni R (2009) Predictive models of toxicity in external radiotherapy: dosimetric issues. Cancer 115(SUPPL 13):3135–3140
De Langhe S, De Meerleer G, De Ruyck K, Ost P, Fonteyne V, De Neve W et al (2014) Integrated models for the prediction of late genitourinary complaints after high-dose intensity modulated radiotherapy for prostate cancer: making informed decisions. Radiother Oncol 112(1):95–99
Cozzarini C, Rancati T, Carillo V, Civardi F, Garibaldi E, Franco P et al (2015) Multi-variable models predicting specific patient-reported acute urinary symptoms after radiotherapy for prostate cancer: results of a cohort study. Radiother Oncol 116(2):185–191
Sanguineti G, Arcidiacono F, Landoni V, Saracino BM, Farneti A, Arcangeli S et al (2016) Macroscopic hematuria after conventional or hypofractionated radiation therapy: results from a prospective phase 3 study. Int J Radiat Oncol Biol Phys 96(2):304–312
Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X et al (2012) Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 84(1):125–129
Carillo V, Botti A, Casanova Borca V, Cattari G, Civardi F, Cozzarini C et al (2014) Modelling acute urinary toxicity after radiotherapy for prostate cancer. Radiother Oncol 110(SUPPL. 1):S20–S21
Kalakota K, Liauw SL (2013) Toxicity after external beam radiotherapy for prostate cancer: an analysis of late morbidity in men with diabetes mellitus. Urology 81(6):1196–1201
Byrne K, Hruby G, Kneebone A, Whalley D, Guo L, McCloud P et al (2017) Late genitourinary toxicity outcomes in 300 prostate cancer patients treated with dose-escalated image-guided intensity-modulated radiotherapy. Clin Oncol 29(9):617–625
Inokuchi H, Mizowaki T, Norihisa Y, Takayama K, Ikeda I, Nakamura K, et al. Correlation between urinary dose and delayed radiation cystitis after 78 Gy intensity-modulated radiotherapy for high-risk prostate cancer: A 10-year follow-up study of genitourinary toxicity in clinical practice. 2017;6:31–6.
Yahya N, Ebert MA, Bulsara M, House MJ, Kennedy A, Joseph DJ et al (2015) Urinary symptoms following external beam radiotherapy of the prostate: Dose-symptom correlates with multiple-event and event-count models. Radiother Oncol 117(2):277–282
Alemozaffar M, Regan MM, Cooperberg MR, Wei JT, Michalski JM, Sandler HM et al (2011) Prediction of erectile function following treatment for prostate cancer. JAMA 306(11):1205–1214
David RV, Kahokehr AA, Lee J, Watson DI, Leung J, O’Callaghan ME. Incidence of genitourinary complications following radiation therapy for localised prostate cancer. World journal of urology. 2022.
Acknowledgements
Scott Walsh, Data Manager, and Matthew Horsfall, Senior Data Management Specialist, College of Medicine and Public Health, for performing data linkage using ENVIDO, South Australia.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
RVD did project development, data collection, data analysis and manuscript writing. MH performed data analysis and manuscript writing. AAK done data analysis and manuscript editing. JL was involved in project development and manuscript editing. JL contributed to data analysis and manuscript editing. DI Watson done project development and manuscript editing. MEO' did project development, data analysis and manuscript editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
The SA-PCCOC database has been approved by the Southern Adelaide Clinical Human Research Ethics Committee (SAC HREC). Approval to access the database was granted by the SA-PCCOC steering committee. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
David, R., Hiwase, M., Kahokehr, A.A. et al. Predicting post-radiation genitourinary hospital admissions in patients with localised prostate cancer. World J Urol 40, 2911–2918 (2022). https://doi.org/10.1007/s00345-022-04212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-022-04212-y