Abstract
Purpose
Studies of genitourinary toxicity following radiotherapy for prostate cancer are mainly from high volume single institutions and the incidence and burden of treatment remain uncertain. Hence we determine the cumulative incidence of treatment-related genitourinary toxicity in patients with localised prostate cancer treated with primary external beam radiotherapy (EBRT) at a state population level.
Methods
We analysed data from a prospective population-based cohort, including hospital admission and cancer registry data, for men with localised prostate cancer who underwent primary EBRT without nodal irradiation between 1998 and 2019 in South Australia. The 10-year cumulative incidence of genitourinary toxicity requiring hospitalisation or procedures was determined. Clinical predictors of toxicity and the volume of admissions, non-operative, minor operative and major operative procedures were determined.
Results
All the included patients (n = 3350) had EBRT, with a median (IQR) of 74 Gy (70–78) in 37 fractions (35–39). The 10-year cumulative incidence of was 28.4% (95% CI 26.3–30.6) with a total of 2545 hospital admissions, including 1040 (41%) emergency and 1893 (74%) readmissions. The 10-year cumulative incidence of patients in this cohort requiring a urological operative procedure was 18% (95% CI 16.1–19.9), with a total of 106 (4.2%) non-operative, 1044 (41%) minor operative and 57 (2.2%) major operative urological procedures.
Conclusions
Genitourinary toxicity after radiotherapy for prostate cancer is common. Although there continue to be advancements in radiotherapy techniques, patients and physicians should be aware of the risk of late toxicity when considering EBRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most common form of cancer affecting men worldwide [1]. The majority (94%) of patients with prostate cancer have curable localised disease, for which the treatment options include active surveillance, surgery or radiotherapy [2]. Radiotherapy is a common treatment for localised prostate cancer [3, 4]. However, the incidence of late genitourinary toxicity (GUT) and its associated burden of treatment across a variety of practice settings remains poorly understood. Radiotherapy injuries often present late due to progressive fibrosis and the difficulties in accurately recording these long-term adverse effects are reported in the literature frequently [5,6,7]. The majority of studies on the incidence of genitourinary toxicity after radiotherapy and its associated burden of treatment are studies from specialised high-volume single centres [7,8,9]. There are few multi-institutional studies [10,11,12] and the randomised trials often involve a disproportionately younger and healthier patient demographic when compared to a typical population [13, 14]. An improved understanding of the incidence of late treatment-related genitourinary toxicity following prostate radiotherapy would enhance patient-centred decision making [6].
The primary aim of this study was to determine the cumulative incidence of treatment-related genitourinary toxicity following external beam prostatic radiotherapy in patients with localised prostate cancer at a population level. The secondary aims were to determine clinical factors predictive of genitourinary toxicity and the volume of admissions and procedures required.
Materials and methods
Participants
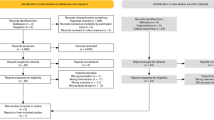
A population-based prospective cohort study of all patients with localised (T1–T3, according to the American Joint Committee on Cancer) biopsy-proven prostate cancer who underwent primary external beam radiotherapy (EBRT) was performed between January 1, 1998, and January 31, 2019, in South Australia. We excluded patients with metastatic prostate cancer and those without a histological tissue diagnosis of prostate cancer. We excluded patients who underwent adjuvant radiotherapy following either radical prostatectomy, or prior radiotherapy treatment (Fig. 1).
The South Australian Prostate Cancer Clinical Outcome Collaborative (SA-PCOCC) registry prospectively recruits > 90% of patients who are diagnosed with prostate cancer in South Australia. We linked patient records from the SA-PCCOC registry with the Integrated South Australian Activity Collection (ISAAC) Hospital Administrative Database to identify patients who presented to any major hospital in South Australia with treatment-related genitourinary toxicity, as defined by a pre-selected list of International Classification Disease 10th Edition (ICD-10-AM)/Australian Classification of Health Interventions (ACHI). Data linkage was performed by matching patient identifiers within Envido (Adelaide, South Australia). The list of admission and procedures codes were selected based on the literature [6], and recommendations from a multidisciplinary panel, including a urologist, radiation oncologist, general surgeon and a clinical epidemiologist (Appendix 1). Baseline characteristics including age, Charlson Comorbidity Index, anticoagulant medication use, and oncological characteristics, including T-stage, Gleason score and baseline Prostate-specific antigen (PSA) level were extracted. Treatment-related factors including dose (Gray), fractionation and date of treatment completion were also extracted.
Primary outcomes
The treatment-related complication categories used were hospital admission and urological procedures associated with genitourinary toxicity. Genitourinary toxicity-related hospital admission or procedures required for each patient were identified using the ISAAC Database (using the relevant hospital admission or procedure code based on the ICD-10 or ACHI codes). The time to the first genitourinary toxicity-related hospital admission, death or censor were analysed to determine the cumulative incidence of genitourinary toxicity. Patients were censored at the last date of the last admission in the ISAAC electronic hospital database.
Secondary outcomes
Demographic factors assessed included age (continuum), Charlson comorbidity score, diabetes (yes/no), hypertension (yes/no), use of anticoagulant (yes/no), smoking history (yes/no), bladder outlet obstruction (yes/no), Transurethral resection of the prostate (TURP) before radiotherapy (yes/no), T stage (T1 vs T2 vs T3), initial prostate-specific antigen level (continuum) and dose (continuum and > 80 Gy vs ≤ 80 Gy). Furthermore, the admission data was separated into patients who received EBRT < 2009 and ≥ 2009, to account for the use of Three-dimensional conformal radiation therapy (3DCRT) and Intensity Modulated Radiotherapy/Volumetric modulated arc therapy (IMRT/VMAT), respectively.
The overall burden of treatment, as defined by the volume of admissions as well as non-operative, minor operative and major operative procedures was determined. Non-operative procedures were defined as ACHI codes involving urethral catheterization or bladder irrigation. Minor operative procedures were defined as ACHI codes involving urethral dilation, cystoscopy, suprapubic catheter insertion, retrograde pyelogram, antegrade or retrograde ureteric stenting. Major operative procedures were defined as ACHI codes involving transurethral resection, ureteroscopy or open surgical procedure.
The outcomes were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [15].
Statistical analysis
The cumulative incidence of hospitalisation for treatment-related genitourinary complications was determined. Patients were considered to be at risk of complications from the end date of their radiotherapy until either the date of their first admission related to genitourinary toxicity, last date of follow-up or date of death, according to the SA-PCCOC registry. The patient-related baseline characteristics and the volume of hospital admissions and procedures were summarised and compared. Categorical variables were compared using the Fischer Exact Test or Pearson’s Chi-square test. Continuous parametric and non-parametric variables were compared using one-way ANOVA or the Kruskal–Wallis Rank Sum test, respectively. p values were calculated for each variable compared and p < 0.05 was considered significant. Relationships between genitourinary toxicity-related hospital admission and patient, tumour or treatment characteristics were analysed using Cox proportional hazard regression at univariate and multivariate levels. The regression analyses’ results are presented as a hazard with a 95% confidence interval. Missing clinical data was replaced using multiple imputations by chained equations before regression analysis (Fig. 1). All statistical analysis was performed using R language, Version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria) [16].
Results
There were 3350 patients with prostate cancer treated with primary external beam radiotherapy in this cohort. We excluded 820 patients who were initially treated surgically, with either robotic-assisted laparoscopic prostatectomy (n = 579) or open radical prostatectomy (n = 241). We also excluded 388 patients who were treated with brachytherapy before external beam radiotherapy and four patients with T4 disease (Fig. 1). All the included patients underwent primary EBRT, with a median (IQR) of 74 Gy (70–78) in 37 fractions (35–39). The median (IQR) age at diagnosis of the included patients was 71 (66–76). Most patients had Stage II (n = 914, 58%) and high-risk disease (n = 1517 [51%]), according to the National Comprehensive Cancer Network (NCCN) 2017 scoring system. Table 1 summarises the patient demographic, oncological and treatment dosimetric characteristics.
The 5- and 10-year cumulative incidence of admission to hospital for treatment-related genitourinary toxicity were 14.8% (95% CI 13.4–16.2) and 28.4% (95% CI 26.3–30.6), respectively (Fig. 2). The 5- and 10-year cumulative incidence of patients in this cohort requiring a urological operative procedure for a treatment-related GUT were 9.9% (95% CI 8.7–11) and 18% (95% CI 16.1–19.9), respectively (Fig. 2). The 5-year cumulative incidence of treatment-related genitourinary toxicity hospital admission were 18% (95% CI 15–20%) and 12% (95 CI 11–14), amongst patients treated before and after 2010, respectively (p < 0.001; Fig. 3).
There were 652 (19.5%) prostate cancer patients who required hospital admission for genitourinary toxicity after primary EBRT, with a total of 2545 hospital admissions, of which 1040 (41%) occurred in the emergency setting. Four-hundred and nine (63%) of these patients had multiple admissions, with a total of 1893 (74%) readmission related to genitourinary toxicity. Haematuria was the most common genitourinary toxicity (n = 386, 59%), and of these patients, 108 (28%) required blood product transfusion, 8 (2%) required HBOT and 4 (1%) required surgical urinary diversion. Table 2 summarises the treatment-related outcomes amongst patients with genitourinary toxicity following primary EBRT. Four-hundred and nine (12%) patients developed genitourinary toxicity which required management with a urological procedure, with a total of 106 (4.2%) non-operative, 1044 (41%) minor and 57 (2.2%) major operative urological procedures (Table 3). The most common procedure was diagnostic cystoscopy (701/1101 [64%] of all procedures).
Patients with BOO without TURP prior to EBRT, had the highest 10-cumulative incidence of admission for genitourinary toxicity (77% [70%, 82%] vs 20% [18%, 22%] p < 0.001; Table 3, Fig. 3). In addition, patients with BOO without TURP prior to EBRT had the most hospital admissions (178/246 [72%] vs 474/3104 [15%], p < 0.001), emergency admissions (136/246 [55%] vs 273/3104 [8.8%], p < 0.001) and readmissions (110/246 [45%] vs 282/3104 [9.1], p < 0.001), for treatment-related genitourinary toxicity (Table 1). Patients with BOO without TURP before EBRT were at the highest risk of developing genitourinary toxicity after adjustment for age, diabetes, smoking, urinary incontinence and EBRT before 2009 (HR 5.87 [95% CI 4.8–7.17], p < 0.001; Table 4).
Discussion
This is one of few studies to evaluate the cumulative incidence of treatment-related genitourinary complications following radiotherapy for prostate cancer at a population level and the first in Australia. The high 10-year cumulative incidence (28.4%) of hospital admission due to treatment-related genitourinary toxicity exceeds previous estimates following primary EBRT [4, 6, 7]. The date of radiotherapy made a minimal difference in the 10-year cumulative incidence of genitourinary toxicity-related admission amongst patients in this cohort, and was not an independent predictor of genitourinary toxicity after adjustment for age, comorbidity, smoking and BOO in multivariable analysis (HR 0.87 [95% CI 0.72, 1.04], p = 0.12; Table 4). This is also the first Australian study to determine the volume of admissions and urological procedures for the management of radiotherapy treatment-related genitourinary complications at a population level. Greater than one-third of genitourinary toxicity-related hospital admissions occurred in the emergency setting. There were a significant number of admissions with a prolonged length of stay of ≥ 3 days. Whilst haematuria was the most common presentation, we are unable to confirm the diagnosis of radiation cystitis due to the limitations associated with administrative coding, we can infer the diagnosis of severe hemorrhagic radiation-induced cystitis occurred in 12/3351 (0.4%) of patients, with 8/3351 (0.2%) and 4/3351 (0.1%) patients requiring HBOT and surgical urinary diversion, respectively. A significant number of patients (18%) required an invasive urological procedure. There were significantly fewer hospital admissions and procedures amongst patients treated with EBRT after 2009, which may reflect improvements in radiotherapy techniques or the shorter follow-up in this group, which likely underestimated late toxicity.
Three large population-based studies have been published in this area with patients from the USA [17], Canada [6], and England [7]. A total of 307,252 patients were described [4, 6, 7]. However, like several other studies [18, 19], these studies did not include patient baseline oncological characteristics [6], or important treatment-related factors, including the dose and fractionation use in the radiation treatment used [6, 7, 17]. The study by Sheets et al., was the first study to demonstrate an increased risk of patients developing genitourinary toxicity following IMRT as compared to conformal radiation therapy, (absolute risk, 5.9 vs 503 per 100 person-years; relative risk, 1.12; 95% CI 1.03–1.20) [17]. Only one of these studies reported 5-year cumulative incidence of treatment-related genitourinary toxicity, which was determined to be 10.7 (95% CI 10.1–11.3) [7]. The estimate determined by the latter study was limited by missing values for the prostate cancer risk group (n = 5753) and radiotherapy treatment region (n = 3793) [7]. The other study reported a 22.2% (95% CI 21.7–22.7) 5-year cumulative incidence of admission for either genitourinary or gastrointestinal treatment-related complication and a 32.0% (95% CI 31.4–32.5) 5-year cumulative incidence of needing a urological procedure [6]. All three studies lacked a 60-month endpoint and this may have led to an underestimation of the late genitourinary toxicity events, as is the case with many other studies [19, 20]. The majority of studies of > 5-year genitourinary toxicity are not population-based, tend to focus on a narrower range of toxicity and have a shorter follow-up duration [9, 21].
Patients with bladder outlet obstruction without TURP before EBRT were at the highest risk of developing genitourinary toxicity after adjustment for age, diabetes, smoking, urinary incontinence and EBRT before 2009 (HR 5.87 [95% CI 4.8–7.17], p < 0.001; Table 4). Similarly, many other studies have also shown that pre-existing urinary symptoms can influence radiotherapy-related genitourinary toxicity [22,23,24]. TURP before radiotherapy demonstrated a protective effect against genitourinary toxicity amongst patients with bladder outlet obstruction in our study (HR 3.6, 95% CI 3.01–4.46, p < 0.001); however, other studies have shown TURP might deteriorate late urinary symptoms [25, 26]. Similarly, several other studies [23, 24, 27] have supported our finding that diabetes is an independent predictor of genitourinary toxicity in patients with prostate cancer treated with radiotherapy (HR 1.25, 95% CI 1.08–1.53, p < 0.004). Furthermore, the role of diabetes may be increasingly important in the era of dose-escalated (≥ 74 Gy) IMRT, as shown by Kalakota et al., who reported diabetes to be an independent predictor of late grade 3 genitourinary toxicity (RR 2.74, p = 0.004) in their multivariate analysis [28]. However, a few studies did not support the impact of diabetes on treatment-related genitourinary toxicity [29,30,31].
Less known is the impact of age on radiation-induced genitourinary toxicity, which may reflect physiological changes and altered clinical decision-making. Whilst we found that increased age was associated with significant lower cumulative 5-, 10- and 15-year EFS rates (p = 0.041, Table 4) in univariate analysis (HR 1.02 95% CI 1.01–1.03, p < 0.001), this did not retain significance in multivariable regression (p = 0.6). However, other studies have shown increased age to be an independent predictor of treatment-related genitourinary toxicity [6, 23, 32], including the study by Nam et al., which reported a higher incidence of hospital admission due to genitourinary toxicity (HR 1.007, 95% CI 1.003–1.010, p < 0.0001) amongst patients with prostate cancer treated with radiotherapy (n = 16,595) in a multivariable analysis performed in Cox proportional hazard modelling, adjusted for age and comorbidity treatment [6].
Similarly, whilst we found an increased risk of genitourinary toxicity amongst patients with a history of anticoagulation medication use on univariable analysis (HR 2.03 95% CI 1.67–2.49, p < 0.001), the significance was not retained in multivariable analysis (p = 0.3). However, in multivariable analysis, other studies have shown an increased risk of haematuria associated with anticoagulant use (RR 2.89, p = 0.01) [23].
Whilst we found that Charlson comorbidity score was not associated with genitourinary toxicity, in univariate analysis (HR 1.06, 95% CI 0.99–1.12, p < 0.091), the study by Nam et al. found that increased comorbidity, as measured by the Johns Hopkins University ACD Case-Mix System, was associated with a higher incidence of hospital admission in multivariate analysis (HR 1·08, 95% CI 1.07–1.09, p < 0.0001) [6].
Similarly, whilst we found no statistically increased risk of toxicity for patients with a history of hypertension (HR 3.91, 95% CI 0.98–15.7, p = 0.12) on univariable analysis, other studies have shown a positive association [30, 33]. Contrastingly, other studies have reported a protective effect of hypertension, suggested to be associated with antihypertensive medication intake [34], with Barnett et al. reporting a correlation with decreased risk of a poor urinary stream (HR 0.25, 95% CI 0.09–0.71, p = 0.009) [30].
Similarly, the data on dose-related genitourinary dysfunction has been controversial, and whilst some studies suggested a correlation between dose to the bladder and genitourinary toxicity [23, 24, 35,36,37,38,39,40,41], this has generally been unconfirmed by other authors [26], including the current study in univariable regression analysis (p = 0.4). This inconsistency may be due to confounding differences in treatment scheme (target volume, position during treatment, bladder volume variation, technique, dose), patient characteristics, grading scale and the length of follow-up [42,43,44].
Similarly, patients who received radiotherapy before 2009 had a higher 10-year cumulative incidence of admission for genitourinary toxicity (29% [26%, 31%] vs 19% [16%, 21%] p < 0.001; Table 3, Fig. 3). In addition, patients with EBRT before 2009 had more hospital readmissions for genitourinary toxicity (1879 [74%] vs 1354 [77%], p < 0.001), urinary retention (757 [43%] vs 287 [38%], p = 0.032) as well as more non-operative (p < 0.001) and minor-operative procedures (p < 0.001) compared with patients who received radiotherapy ≥ 2009 (Table 1). However, date of treatment before 2009 was not an independent predictor of hospitalisation for genitourinary toxicity, after adjustment for age, comorbidity, smoking and BOO (HR 0.87 [95% CI 0.72, 1.04], p = 0.12; Table 4).
Our study has several limitations. First, whilst the use of administrative data coding based on diagnostic and admission codes has been validated in other claims-based studies assessing severe pelvic adverse effects after radiotherapy [45], the number of genitourinary complications has likely been under-reported given the retrospective data-linkage methods used. For example, we would not have captured complications that are non-life-threatening (e.g., lower urinary tract symptoms from urethral stricture or bladder neck contracture) or which do not require further procedures. Furthermore, the sampling methodology used does not account for patients who may have had complications in other states. However, the study benefits from population-level data and longer duration of follow-up. In addition, we are unable to establish a causal link between radiation treatment and the reason for admission. These potential confounding factors may lead to the incorrect attribution of radiation-related toxicity in our data set, especially for late complications given the distant temporal relationship [19, 46]. The work presented here is descriptive and may motivate further investigations focusing on causal pathways, mechanisms of action and preventive strategies. Toxicity grades were unable to be reported, as these were not coded in administrative data. The study does not include radiation-associated secondary malignancy, gastrointestinal or other pelvic treatment-related complications (e.g., rectal and pubic symphysis fistula).
Conclusions
Genitourinary complications after radiotherapy for prostate cancer are common. Although there continue to be significant advancements in radiotherapy techniques, patients and physicians should be aware of the risk of late toxicity when considering treatment options for prostate cancer. Further research is needed to identify predictive factors and develop models predicting late treatment-related genitourinary toxicity to improve pre-treatment counselling and enhance patient-centered decision making.
Abbreviations
- BOO:
-
Bladder Outlet Obstruction
- CTCAE:
-
Genitourinary toxicity is defined by the CTCAE as the presence of any of the following adverse events, as explained in greater detail on the NCI Web site: bladder spasms, cystitis, genitourinary fistula, urinary incontinence, genitourinary leak, genitourinary obstruction, genitourinary perforation, prolapse of stoma, renal failure, stricture/stenosis, urinary electrolyte wasting, urinary frequency/urgency, urinary retention
- GU:
-
Genitourinary
- Gy:
-
Gray
- PSA:
-
Prostate-specific antigen
- RTOG:
-
Radiation Therapy Oncology Group. Grade > / = 3 toxicity is often used a proxy for serious toxicity that would necessitate hospitalisation for management. Published rates from large RCTS for G = / > 3 range from 6 to 13% [38, 47, 48]
- TURBT:
-
Transurethral Resection of Bladder Tumour
- TURP:
-
Transurethral Resection of Prostate
- NCCN:
-
National Comprehensive Cancer Network
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Mohler JL, Armstrong AJ, Bahnson RR, Boston B, Busby JE, D’Amico AV et al (2012) Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 10(9):1081–1087
Cooperberg MR, Broering JM, Carroll PR (2010) Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 28(7):1117–1123
Kramer KM, Bennett CL, Pickard AS, Lyons EA, Wolf MS, McKoy JM et al (2005) Patient preferences in prostate cancer: a clinician’s guide to understanding health utilities. Clin Prostate Cancer 4(1):15–23
Gardner BG, Zietman AL, Shipley WU, Skowronski UE, McManus P (2002) Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. J Urol 167(1):123–126
Nam RK, Cheung P, Herschorn S, Saskin R, Su J, Klotz LH et al (2014) Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. Lancet Oncol 15(2):223–231
Sujenthiran A, Nossiter J, Charman SC, Parry M, Dasgupta P, van der Meulen J et al (2017) National population-based study comparing treatment-related toxicity in men who received intensity modulated versus 3-dimensional conformal radical radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 99(5):1253–1260
Afonso-João D, Pacheco-Figueiredo L, Antunes-Lopes T, Morgado LA, Azevedo V, Vendeira L et al (2018) Cumulative incidence and predictive factors of radiation cystitis in patients with localized prostate cancer. Actas Urológicas Españolas (Engl Edn) 42(4):256–261
Ma JL, Hennessey DB, Newell BP, Bolton DM, Lawrentschuk N (2018) Radiotherapy-related complications presenting to a urology department: a more common problem than previously thought? BJU Int 121:28–32
Jereczek-Fossa BA, Zerini D, Fodor C, Santoro L, Serafini F, Cambria R et al (2010) Correlation between acute and late toxicity in 973 prostate cancer patients treated with three-dimensional conformal external beam radiotherapy. Int J Radiat Oncol Biol Phys 78(1):26–34
Michalski JM, Yan Y, Watkins-Bruner D, Bosch WR, Winter K, Galvin JM et al (2013) Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 Prostate Cancer Trial. Int J Radiat Oncol Biol Phys 87(5):932–938
Sutani S, Ohashi T, Sakayori M, Kaneda T, Yamashita S, Momma T et al (2015) Comparison of genitourinary and gastrointestinal toxicity among four radiotherapy modalities for prostate cancer: conventional radiotherapy, intensity-modulated radiotherapy, and permanent iodine-125 implantation with or without external beam radiotherapy. Radiother Oncol 117(2):270–276
Kimmick GG, Peterson BL, Kornblith AB, Mandelblatt J, Johnson JL, Wheeler J et al (2005) Improving accrual of older persons to cancer treatment trials: a randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol 23(10):2201–2207
Townsley CA, Selby R, Siu LL (2005) Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 23(13):3112–3124
Sharp SJ, Poulaliou M, Thompson SG, White IR, Wood AM (2014) A review of published analyses of case-cohort studies and recommendations for future reporting. PLoS ONE 9(6):e101176
R Core Team (2020) R: a language and environment for statistical computing. R Core Team, Vienna
Sheets NC, Goldin GH, Meyer AM, Wu Y, Chang Y, Sturmer T et al (2012) Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307(15):1611–1620
Zelefsky MJ, Fuks Z, Happersett L, Lee HJ, Ling CC, Burman CM et al (2000) Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol 55(3):241–249
Bekelman JE, Mitra N, Efstathiou J, Liao K, Sunderland R, Yeboa DN et al (2011) Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys 81(4):e325–e334
Hu JC, Gu X, Lipsitz SR, Barry MJ, D’Amico AV, Weinberg AC et al (2009) Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 302(14):1557–1564
Chao M, Ho H, Chan Y, Tan A, Pham T, Bolton D et al (2018) Prospective analysis of hydrogel spacer for patients with prostate cancer undergoing radiotherapy. BJU Int 122(3):427–433
Cozzarini C, Rancati T, Carillo V, Civardi F, Garibaldi E, Franco P et al (2014) Multi-variable models predicting specific patient-reported acute urinary symptoms after radiotherapy for prostate cancer: Ad interim results of a cohort study. Eur Urol Suppl 13(5):114–115
Mathieu R, Arango JD, Beckendorf V, Delobel JB, Messai T, Chira C et al (2014) Nomograms to predict late urinary toxicity after prostate cancer radiotherapy. World J Urol 32(3):743–751
Yahya N, Ebert MA, Bulsara M, Haworth A, Kennedy A, Joseph DJ et al (2015) Dosimetry, clinical factors and medication intake influencing urinary symptoms after prostate radiotherapy: an analysis of data from the RADAR prostate radiotherapy trial. Radiother Oncol 116(1):112–118
Jereczek-Fossa BA, Orecchia R, Bonora M, Scardino E (2011) Response to “Urinary obstruction in prostate cancer patients from the dutch trial (68 Gy vs. 78 Gy): relationships with local dose, acute effects, and baseline characteristics” (Int J Radiat Oncol Biol Phys 2010;78:19–25). Int J Radiat Oncol Biol Phys 79(3):956
Byrne K, Hruby G, Kneebone A, Whalley D, Guo L, McCloud P et al (2017) Late genitourinary toxicity outcomes in 300 prostate cancer patients treated with dose-escalated image-guided intensity-modulated radiotherapy. Clin Oncol 29(9):617–625
Stankovic V, Džamic Z, Pekmezovic T, Tepavcevic DK, Dozic M, Saric M et al (2016) Acute and late genitourinary toxicity after 72 Gy of conventionally fractionated conformal radiotherapy for localised prostate cancer: impact of individual and clinical parameters. Clin Oncol 28(9):577–586
Kalakota K, Liauw SL (2013) Toxicity after external beam radiotherapy for prostate cancer: an analysis of late morbidity in men with diabetes mellitus. Urology 81(6):1196–1201
Heemsbergen WD, Al-Mamgani A, Witte MG, van Herk M, Pos FJ, Lebesque JV (2010) Urinary obstruction in prostate cancer patients from the Dutch trial (68 Gy vs. 78 Gy): relationships with local dose, acute effects, and baseline characteristics. Int J Radiat Oncol Biol Phys 78(1):19–25
Barnett GC, De Meerleer G, Gulliford SL, Sydes MR, Elliott RM, Dearnaley DP (2011) The impact of clinical factors on the development of late radiation toxicity: results from the Medical Research Council RT01 trial (ISRCTN47772397). Clin Oncol 23(9):613–624
Cahlon O, Zelefsky MJ, Shippy A, Chan H, Fuks Z, Yamada Y et al (2008) Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys 71(2):330–337
Ahmed AA, Egleston B, Alcantara P, Li L, Pollack A, Horwitz EM et al (2013) A novel method for predicting late genitourinary toxicity after prostate radiation therapy and the need for age-based risk-adapted dose constraints. Int J Radiat Oncol Biol Phys 86(4):709–715
Cozzarini C, Fiorino C, Da Pozzo LF, Alongi F, Berardi G, Bolognesi A et al (2012) Clinical factors predicting late severe urinary toxicity after postoperative radiotherapy for prostate carcinoma: a single-institute analysis of 742 patients. Int J Radiat Oncol Biol Phys 82(1):191–199
Wedlake LJ, Silia F, Benton B, Lalji A, Thomas K, Dearnaley DP et al (2012) Evaluating the efficacy of statins and ACE-inhibitors in reducing gastrointestinal toxicity in patients receiving radiotherapy for pelvic malignancies. Eur J Cancer 48(14):2117–2124
Ashman JB, Zelefsky MJ, Hunt MS, Leibel SA, Fuks Z (2005) Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 63(3):765–771
Cozzarini C, Fiorino C, Di Muzio N, Alongi F, Broggi S, Cattaneo M et al (2007) Significant reduction of acute toxicity following pelvic irradiation with helical tomotherapy in patients with localized prostate cancer. Radiother Oncol 84(2):164–170
Perez CA, Lee HK, Georgiou A, Lockett MA (1994) Technical factors affecting morbidity in definitive irradiation for localized carcinoma of the prostate. Int J Radiat Oncol Biol Phys 28(4):811–819
Peeters STH, Heemsbergen WD, van Putten WLJ, Slot A, Tabak H, Mens JW et al (2005) Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys 61(4):1019–1034
Acevedo-Henao CM, Lopez Guerra JL, Matute R, Puebla F, Russo M, Rivin E et al (2014) Image-guided radiation therapy based on helical tomotherapy in prostate cancer: minimizing toxicity. Oncol Res Treat 37(6):324–330
Yahya N, Ebert MA, House MJ, Kennedy A, Matthews J, Joseph DJ et al (2017) Modeling urinary dysfunction after external beam radiation therapy of the prostate using bladder dose-surface maps: evidence of spatially variable response of the bladder surface. Int J Radiat Oncol Biol Phys 97(2):420–426
Aluwini S, Lopes AR, Sipkema DJ, Roos MA, Busser WMH, Kolkman-Deurloo IKK (2016) Predictive factors for urinary retention after HDR brachytherapy as monotherapy for prostate cancer. Brachytherapy 15(SUPPL. 1):S193–S194
Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X et al (2012) Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 84(1):125–129
Fiorino C, Alongi F, Perna L, Broggi S, Cattaneo GM, Cozzarini C et al (2009) Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat Oncol Biol Phys 75(1):29–35
Rosewall T, Catton C, Currie G, Bayley A, Chung P, Wheat J et al (2010) The relationship between external beam radiotherapy dose and chronic urinary dysfunction—a methodological critique. Radiother Oncol 97(1):40–47
Sewell JM, Rao A, Elliott SP (2013) Validating a claims-based method for assessing severe rectal and urinary adverse effects of radiotherapy. Urology 82(2):335–340
Chen AB, D’Amico AV, Neville BA, Earle CC (2006) Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol 24(33):5298
Hanks GE (1996) Long-term control of prostate cancer with radiation: past, present, and future. Urol Clin N Am 23(4):605–616
Dearnaley DP, Sydes MR, Langley RE, Graham JD, Huddart RA, Syndikus I et al (2007) The early toxicity of escalated versus standard dose conformal radiotherapy with neo-adjuvant androgen suppression for patients with localised prostate cancer: results from the MRC RT01 trial (ISRCTN47772397). Radiother Oncol 83(1):31–41
Acknowledgements
Scott Walsh, Data Manager, and Matthew Horsfall, Senior Data Management Specialist College of Medicine and Public Health, for performing data linkage using ENVIDO, South Australia.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
RVD: project development, data collection, data analysis and manuscript writing. AAK: data analysis and manuscript editing. JLee: project development and manuscript editing. JLeu: data analysis and manuscript editing. DIW: project development and manuscript editing. MEO: project development, data analysis and manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The SA-PCCOC database has been approved by the Southern Adelaide Clinical Human Research Ethics Committee (SAC HREC). Approval to access the database was granted by the SA-PCCOC steering committee. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
David, R.V., Kahokehr, A.A., Lee, J. et al. Incidence of genitourinary complications following radiation therapy for localised prostate cancer. World J Urol 40, 2411–2422 (2022). https://doi.org/10.1007/s00345-022-04124-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-022-04124-x