Abstract
Purpose
To test the association between external beam radiotherapy (EBRT) after radical prostatectomy (RP) vs RP only on rates of other-cause mortality (OCM) in men with prostate cancer (PCa).
Patients and methods
Within the 2004–2016 Surveillance, Epidemiology, and End Results database, we identified 181,849 localized PCa patients, of whom 168,041 received RP only vs 13,808 who received RP + EBRT. Cumulative incidence plots displayed OCM between RP vs RP + EBRT after propensity score matching for age, PSA, clinical T- and N-stages, and biopsy Gleason scores. Multivariable competing risks regression models addressed OCM, accounting prostate cancer-specific mortality (CSM) as a competing event. Stratifications were made according to low- vs intermediate- vs high-risk groups and additionally according to age groups of ≤ 60, 61–70, and ≥ 71 years, within each risk group.
Results
In low-, intermediate-, and high-risk patients, RP + EBRT rates were 2.7, 5.4 and 17.0%, respectively. After matching, 10-year OCM rates between RP and RP + EBRT were 7.7 vs 16.2% in low-, 9.4 vs 13.6% in intermediate-, and 11.4 vs 13.5% in high-risk patients (all p < 0.001), which, respectively, resulted in multivariable HR of 2.1, 1.3, and 1.2 (all p < 0.001). In subgroup analyses, excess OCM was recorded in low-risk RP + EBRT patients of all age groups (all p ≤ 0.03), but only in the older age group in intermediate-risk patients (61–70 years, p = 0.03) and finally, only in the oldest age group in high-risk patients (≥ 71 years, p = 0.02).
Conclusion
Excess OCM was recorded in patients exposed to RT after RP. Its extent was most pronounced in low-risk patients, decreased in intermediate-risk patients, and was lowest in high-risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adjuvant or salvage external beam radiotherapy therapy (EBRT) after radical prostatectomy (RP) for prostate cancer (PCa), with or without concomitant androgen deprivation therapy (ADT), is regarded as a valuable treatment option in highly select patients with adverse pathology at RP, such as positive surgical margins, Gleason score 8–10, ≥ pT3, or pN1 stage [1,2,3,4,5], and is recommended by the European Association of Urology, as well as by the National Comprehensive Cancer Network [6, 7]. Despite its cancer control advantage, EBRT after RP may cause toxicities. Potentially, some of long-term metabolic toxicities associated with EBRT and/or ADT may result in decreased life expectancy, due to mortality from non-PCa-related causes [8,9,10,11,12,13,14].
Therefore, to address this void, we tested other-cause mortality (OCM) in patients who received RP only vs RP + EBRT. We stratified the analyses according to low- vs intermediate- vs high-risk PCa, as well as according to age groups of ≤ 60, 61–70, and ≥ 70 years, within each risk group. We hypothesized that no OCM difference distinguish RP vs RP + EBRT, regardless of PCa risk group or age [9, 15].
Materials and methods
Study population
Within the Surveillance, Epidemiology, and End Results (SEER) database [16], we identified all patients with biopsy diagnosed, histologically confirmed adenocarcinoma of the prostate [International Classification of Disease for Oncology (ICD-O-3) code 8140 site code C61.9], who received treatment with either RP only or RP followed by EBRT (RP + EBRT), between 2004 and 2016. Autopsy or death certificate only cases were excluded. Eligibility criteria for the analyses were full available information on age at diagnosis, clinical T- and N-stages, PSA values, as well as biopsy Gleason scores. Metastatic PCa or patients aged 85 years or older were excluded. D’Amico low-risk group was defined as clinical T-stage ≤ 2a, Gleason sum ≤ 6, and PSA ≤ 10 ng/ml. D’Amico intermediate-risk group was defined as clinical T-stage 2b, Gleason sum 7, or PSA between 10 and 20 ng/ml. D’Amico high-risk group was defined as clinical T-stage ≥ T2c, Gleason sum 8–10, or PSA ≥ 20 ng/ml [17].
Statistical analyses
Statistical modeling relied on propensity score matching (PSM) as well as competing risks regression models (CRR), ultimately investigating the effect of RP vs RP + EBRT on OCM, which was defined as any death not related to PCa [18]. Statistical analyses consisted of three steps. First, we stratified the population according to D’Amico low- vs intermediate- vs high-risk groups. RP + EBRT patients were matched to RP patients, within each risk group. PSM variables consisted of age at diagnosis (one-year intervals), clinical T-stage (exact), clinical N-stage (exact), PSA (1 ng/ml intervals), and biopsy Gleason score (exact).
Second, cumulative incidence and CRR models addressed OCM, after adjustment for prostate cancer-specific mortality (CSM). OCM rates and 95% confidence intervals (95% CI) were derived from the respective cumulative incidence functions. Within CRR models, further multivariable adjustment for socioeconomic status (distributed in quartiles), pathological Gleason score, and age categories of ≤ 60, 61–70, and ≥ 71 years was performed, ultimately providing hazard ratios (HR) and 95% CI.
Third, within each risk group (low- vs intermediate- vs high), we also stratified OCM analyses after separate PSM within age groups of ≤ 60, 61–70, and ≥ 71 years to test for effect modification according to age strata. Specifically, PSM was applied within each of the resulting nine risk- and age groups combinations: (1) low-risk, ≤ 60 years, (2) low-risk, 61–70 years, (3) low-risk, ≥ 71 years, (4) intermediate-risk, ≤ 60 years, (5) intermediate-risk, 61–70 years, (6) intermediate-risk, ≥ 71 years, (7) high-risk, ≤ 60 years, (8) high-risk, 61–70 years, and (9) high-risk, ≥ 71 years. Finally, CRR analyses were refitted within each of the nine groups. Censoring occurred when the patients were lost to follow-up or in case no event was recorded during study follow-up. All tests were two sided with a level of significance set at p < 0.05 and R software environment for statistical computing and graphics (version 3.4.3) was used for all analyses [19].
Results
Of 181,849 eligible patients, 23.6% were low-risk, 51.8% were intermediate-risk, and 24.5% were high-risk. In general, RP + EBRT patients exhibited more unfavorable patient and tumor characteristics compared to RP patients, as evidenced by higher patient age, higher rates of more aggressive biopsy Gleason score, higher clinical T-stages, and higher rates of cN1 disease (Table 1). Median follow-up was 79 months (IQR 46-112) in the overall cohort, with 11,698 RP patients and 2,110 RP + EBRT patients at risk at 120 months.
RP vs RP + EBRT in low-risk prostate cancer
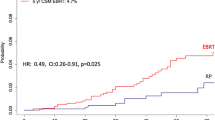
In the 42,951 low-risk patients, the rate of RP + EBRT was 2.7%. PSM focused on 1,121 RP + EBRT patients, who were matched with four RP controls (n = 4,326). After PSM, no residual difference in patient and/or tumor characteristics remained (all p ≥ 0.6). After PSM, 10-year OCM rates (and 95% CI) were 16.2% (13.1–19.6%) vs 7.7% (6.5–8.9%) for RP + EBRT vs RP, respectively (Fig. 1A). In multivariable CRR analyses (Table 2), RP + EBRT was an independent predictor of higher OCM compared to RP (HR 2.1, 95% CI 1.7–2.6, p < 0.001). Within the same multivariable CRR models, compared to the reference of patients aged ≤ 60 years, 61–70-year-old patients as well as ≥ 71-year-old patients also exhibited higher OCM (HR 2.3 for 61–70 years and HR 4.2 for ≥ 71 years, both p < 0.001, (Table 2).
Cumulative incidence plots displaying differences in 10-year other-cause mortality (OCM) rates between D’Amico low-risk prostate cancer patients treated with radical prostatectomy (RP) vs patients treated with RP and external beam radiotherapy (RP + EBRT), after 4:1 propensity score matching for patient and tumor characteristics. Panel A displays low-risk patients of all ages, Panels B–D display low-risk patients according to age groups strata: ≤ 60 years, 61–70 years, and ≥ 71 years
Subsequent stratifications according to age groups identified 409, 489, and 242 RP + EBRT patients in age groups of ≤ 60, 61–70, and ≥ 71 years, respectively. These were matched with four RP controls within each respective age group and yielded cohorts of 405 RP + EBRT vs 1,614 RP patients in the age group ≤ 60 years, 486 RP + EBRT vs 1,938 RP patients in the age group 61–70 years and 233 RP + EBRT vs 824 RP patients in the age group ≥ 71 years. After PSM, no residual differences in patient and/or tumor characteristics in each comparison remained within each age stratum (all p ≥ 0.8). After PSM, OCM rates (and 95% CI) between RP + EBRT vs RP were 8.0% (4.9–12.2%) vs 4.6% (3.1–6.4%) in the age group ≤ 60 years, 14.6% (10.1–19.9%) vs 8.1% (6.4–10.0%) in the age group 61–70 years, and 28.6% (21.1–36.6%) vs 18.5% (15.0–22.3%) in the age group ≥ 71 years, respectively (Fig. 1B, C, D). These OCM rates translated into a multivariable CRR HR of 2.2, 95% CI 1.3–3.6, p < 0.001 for ≤ 60 years, HR of 1.5, 95% CI 1.1–2.2, p = 0.03 for 61–70 years, and HR of 1.9, 95% CI 1.4–2.6, p < 0.001 for ≥ 71 years.
RP vs RP + EBRT in intermediate-risk prostate cancer
In 94,279 intermediate-risk patients, the rate of RP + EBRT was 5.4%. PSM focused on 5,029 RP + EBRT patients, who were matched with four RP controls (n = 19,459). After PSM, no residual difference in patient and/or tumor characteristics remained (all p ≥ 0.6). After PSM, 10-year OCM rates (and 95% CI) were 13.6% (12.2–15.1%) vs 9.4% (8.8–10.0%) for RP + EBRT vs RP, respectively (Fig. 2A). In multivariable CRR analyses (Table 2), RP + EBRT was an independent predictor of higher OCM compared to RP (HR 1.3, 95% CI 1.2–1.5, p < 0.001). Within the same multivariable CRR models, compared to the reference of patients aged ≤ 60 years, both 61–70-year-old patients as well as ≥ 71-year-old patients also exhibited higher OCM (HR 1.9 for 61–70 years and HR 4.1 for ≥ 71 years, both p < 0.001, Table 2).
Cumulative incidence plots displaying differences in 10-year other-cause mortality (OCM) rates between D’Amico intermediate-risk prostate cancer patients treated with radical prostatectomy (RP) vs patients treated with RP and external beam radiotherapy (RP + EBRT), after 4:1 propensity score matching for patient and tumor characteristics. Panel A displays intermediate-risk patients of all ages. Panels B–D display low-risk patients according to age groups strata: ≤ 60 years, 61–70 years, and ≥ 71 years
Subsequent stratifications according to age groups identified 1,969, 2,219, and 896 RP + EBRT patients in age groups of ≤ 60, 61–70, and ≥ 71 years, respectively. These were matched with four RP controls within each respective age group and yielded cohorts of 1,963 RP + EBRT vs 7,663 RP patients in the age group ≤ 60 years, 2,212 RP + EBRT vs 8,652 RP patients in the age group 61–70 years, and 898 RP + EBRT vs 3,370 RP patients in the age group ≥ 71 years. After PSM, no residual differences in patient and/or tumor characteristics in each comparison remained within each age stratum (all p ≥ 0.8). In the matched cohorts, OCM rates (and 95% CI) between RP + EBRT vs RP were 6.1% (4.6–7.7%) vs 4.8% (4.2–5.6%) in the age group ≤ 60 years, 14.6% (12.3–17.0%) vs 10.9% (9.9–12.0%) in the age group 61–70 years, and 29.3% (24.7–34.0%) vs 22.0% (19.8–24.4%) in the age group ≥ 71 years, respectively (Fig. 2B, C, D). These OCM rates translated into a multivariable CRR HR of 1.1, 95% CI 0.9–1.5, p = 0.3 for ≤ 60 years, HR of 1.2, 95% CI 1.0–1.5, p = 0.03 for 61–70 years, and HR of 1.2, 95% CI 1.0–1.4 p = 0.07 for ≥ 71 years.
RP vs RP + EBRT in high-risk prostate cancer
In 44,619 high-risk patients, the rate of RP + EBRT was 17.0%. PSM focused on 7,475 RP + EBRT patients, who were matched with one RP control (n = 7,475). After PSM, no residual difference in patient and/or tumor characteristics remained (all p ≥ 0.7). After PSM, 10-year OCM rates (and 95% CI) were 13.5% (12.4–14.8) vs 11.4% (10.3–12.6) for RP + EBRT vs RP, respectively (Fig. 3A). In multivariable CRR analyses (Table 2), RP + EBRT was an independent predictor of higher OCM compared to RP (HR 1.2, 95% CI 1.1–1.4, p < 0.001). Within the same multivariable CRR models, compared to the reference of patients aged ≤ 60 years, both 61–70-year-old patients as well as ≥ 71-year-old patients also exhibited higher OCM (HR 1.8 for 61–70 years and HR 4.1 for ≥ 71 years, both p < 0.001, Table 2).
Cumulative incidence plots displaying differences in 10-year other-cause mortality (OCM) rates between D’Amico high-risk prostate cancer patients treated with radical prostatectomy (RP) vs patients treated with RP and external beam radiotherapy (RP + EBRT), after 4:1 propensity score matching for patient and tumor characteristics. Panel A displays high-risk patients of all ages. Panels B–D display low-risk patients according to age groups strata: ≤ 60 years, 61–70 years, ≥ 71 years
Subsequent stratifications according to age groups identified 2,735, 3,366, and 1,483 RP + EBRT patients in age groups of ≤ 60, 61–70, and ≥ 71 years, respectively. These were matched with one RP control within each respective age group and yielded cohorts of 2,726 RP + EBRT vs 2,726 RP patients in the age group ≤ 60 years, 3,362 RP + EBRT vs 3,362 RP patients in the age group 61–70 years, and 1,460 RP + EBRT vs 1,460 RP patients in the age group ≥ 71 years. After PSM, no residual differences in patient and tumor characteristics in each comparison remained within each age stratum (all p ≥ 0.8). In the matched cohorts, OCM rates (and 95% CI) between RP + EBRT vs RP were 6.6% (5.3–8.0%) vs 8.4% (6.9–10.0%) for ≤ 60 years, 11.8% (10.2–13.6%) vs 13.3% (11.5–15.3%) for 61–70 years, and 33.3% (29.2–37.4%) vs 23.8% (20.3–27.4%) for ≥ 71 years, respectively (Fig. 3B, C, D). These OCM rates translated into a multivariable CRR HR of 0.8, 95% CI 0.7–1.1, p = 0.18 for ≤ 60 years, HR of 1.0, 95% CI 0.8–1.2, p = 0.9 for 61–70 years, and HR of 1.3, 95% CI 1.0–1.5, p = 0.02 for ≥ 71 years.
Discussion
EBRT after RP is used in up to 50% of patients with adverse pathology [20, 21]. Despite its benefits on cancer control, the effect of EBRT after RP on OCM is unknown. We addressed this void and tested OCM rates between RP only vs matched RP + EBRT patients. We hypothesized that no OCM rate difference should exist between these two groups, since combination therapy candidates have been selected as surgical candidates initially and therefore it may be postulated that their OCM should be very similar to that of their RP only counterparts. Our analyses yielded several noteworthy observations.
First, the rate of RP + EBRT ranged from 2.7% in the low-risk group, 5.4% in the intermediate-risk group, and to 17.0% in the high-risk group. Therefore, non-negligible proportions of intermediate-risk and high-risk patients are exposed to EBRT after RP. Unfortunately, our data did not allow to explain the underlying rationale for EBRT after RP in these individuals, due to insufficiently detailed data regarding RP pathology and/or surgical margins.
Second, important differences in patient, as well as tumor characteristics between RP and RP + EBRT patients were recorded, across all risk strata. Specifically, RP + EBRT patients exhibited higher median patient age, higher median PSA values, higher cT stages, higher biopsy Gleason score, and higher rates of clinically node-positive disease. These differences demonstrate the need for strictest adjustment in the form of PSM and additional multivariable adjustment, as well as adjustment for CSM, to ensure that RP and RP + EBRT populations are comparable regarding patient and tumor characteristics.
Third, in matched competing risks analyses, we invariably recorded higher OCM in RP + EBRT than RP only patients. The excess OCM after RP + EBRT ranged from highest in the low-risk group (+ 8.5%), to intermediate in the intermediate-risk group (+ 4.2%), and to lowest in the high-risk group (+ 2.1%). All of the above OCM rate differences achieved independent predictor status despite the strictest PSM, multivariable adjustment and additional adjustment for CSM and respectively yielded multivariable CRR HR of 2.1 (p < 0.001) in low-risk PCa, HR of 1.3 (p < 0.001) in intermediate-risk PCa, and HR of 1.2 (p < 0.001) in high-risk PCa. Additionally, we tested for effect modification according to age strata. Here, we identified important effect modifications of OCM that increased with age. The effect was most pronounced in oldest patients (≥ 71 years), across all risk strata (HR 4.2 in low-risk, HR 4.1 in intermediate-risk, and HR 4.1 in high-risk, all p < 0.001). Its size was intermediate in the intermediate age category (61–70 years), also across all risk strata (HR of 2.3 in low-risk, HR of 1.9 in intermediate-risk, and HR of 1.8 in high-risk, all p < 0.001).
The above findings indicate that the effect of EBRT after RP is most pronounced in elderly patients (≥ 71 years) and intermediate in the intermediate age strata (61–70 years). Interestingly, this effect is of similar relative magnitude in all risk groups. However, its absolute magnitude, expressed in absolute OCM rate differences, is strongest in low-risk PCa groups. This observation may partly be explained by competing CSM that mostly affects high-risk patients and is least operational in low-risk patients. Based on the highest absolute rate of excess OCM after RP + EBRT in low-risk patients and lowest absolute rate of excess OCM after RP + EBRT in high-risk patients, it is unlikely that ADT may represent an underlying cause, since the opposite association would be expected, if ADT was directly related to OCM rates. Nonetheless, an interplay between EBRT, ADT, and patient characteristics, including age, must be suspected. More detailed, ideally prospective studies, will allow to validate our observations and elucidate the true causative factors. Additionally, our observations question the selection criteria for EBRT after RP that predominantly target elderly individuals. Ideally, intensification of therapy should predominantly focus on younger patients.
Taken together, we recorded excess OCM after EBRT delivered to RP patients, relative to their counterparts treated with RP only. The excess OCM was operational across all risk strata and ranged from + 2.1% (high-risk), + 4.2% (intermediate-risk), to + 8.5% (low-risk). Interestingly, within each PCa risk stratum, intermediate age (61–70 years) predisposed to two-fold OCM increase and oldest age (≥ 71 years) predisposed to four-fold OCM increase. Nonetheless, the absolute increase in OCM was highly statistically significant even in the high-risk group and even despite strictest PSM, multivariable adjustment and accounting for CSM as a competing event in CRR. In consequence, our observations deserve further investigation in other epidemiological and/or institutional databases to validate our findings and to elucidate the underlying causes.
Our study has limitations and should be interpreted in the context of its retrospective and population-based design. First, no distinction could be made according to adjuvant or salvage EBRT after RP. Second, no information on the type or dose of EBRT or the type or dose of concomitant ADT was available. Third, the true cause of death in patients dying of OCM is not incorporated in SEER and therefore a direct association with the toxic effect of radiation and/or ADT can also not be drawn. Finally, no information was available about cancer control outcomes that preceded OCM or CSM. However, since the study was focused on OCM rates that were adjusted for CSM in competing risks analyses, this limitation does not affect its primary outcome.
Conclusion
Excess OCM was recorded in patients exposed to RT after RP. Its extent was most pronounced in low-risk patients, decreased in intermediate-risk patients, and was lowest in high-risk patients.
Data availability
R software environment for statistical computing and graphics (version 3.4.0 for MAC OS X; http://www.r-project.org/) was used for all statistical analyses. Used codes for analyses can be provided. The data that support the findings of this study are available from the Surveillance, Epidemiology, and End results database, (SEER) but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of SEER.
References
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D et al (2009) Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 181:956–962. https://doi.org/10.1016/j.juro.2008.11.032
Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM et al (2012) Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380:2018–2027. https://doi.org/10.1016/S0140-6736(12)61253-7
Hackman G, Taari K, Tammela TL, Matikainen M, Kouri M, Joensuu T et al (2019) Randomised trial of adjuvant radiotherapy following radical prostatectomy versus radical prostatectomy alone in prostate cancer patients with positive margins or extracapsular extension. Eur Urol 76:586–595. https://doi.org/10.1016/j.eururo.2019.07.001
Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A et al (2014) Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96–02/AUO AP 09/95 trial. Eur Urol 66:243–250. https://doi.org/10.1016/j.eururo.2014.03.011
Tilki D, D’Amico AV (2020) Timing of radiotherapy after radical prostatectomy. Lancet 396:1374–1375. https://doi.org/10.1016/S0140-6736(20)31957-7
EAU Guidelines (2021) Edition presented at the EAU Annual Congress Amsterdam ISBN 978-94-92671-07-3. (https://uroweb.org/guideline/prostate-cancer/) Access date 10 May 2021
National Comprehensive Cancer Network, Clinical Practice Guidelines on Prostate Cancer (Version 2.2020 — May 21, 2020)
Saldi S, Bellavita R, Lancellotta V, Palumbo I, Lupattelli M, Chierchini S et al (2019) Acute toxicity profiles of hypofractionated adjuvant and salvage radiation therapy after radical prostatectomy: results of a prospective study. Int J Radiat Oncol 103:105–111. https://doi.org/10.1016/j.ijrobp.2018.08.016
Yamazaki H, Masui K, Suzuki G, Nakamura S, Aibe N, Shimizu D et al (2019) Effect of androgen deprivation therapy on other-cause of mortality in elderly patients with clinically localized prostate cancer treated with modern radiotherapy: is there a negative impact? J Clin Med 8:338. https://doi.org/10.3390/jcm8030338
Yamazaki H, Masui K, Suzuki G, Aibe N, Shimizu D, Kimoto T et al (2020) Potential risk of other-cause mortality due to long-term androgen deprivation therapy in elderly patients with clinically localized prostate cancer treated with radiotherapy—a confirmation study. J Clin Med 9:2296. https://doi.org/10.3390/jcm9072296
Matta R, Chapple CR, Fisch M, Heidenreich A, Herschorn S, Kodama RT et al (2019) Pelvic complications after prostate cancer radiation therapy and their management: an international collaborative narrative review. Eur Urol 75:464–476. https://doi.org/10.1016/j.eururo.2018.12.003
Muniyan S, Xi L, Datta K, Das A, Teply BA, Batra SK et al (2020) Cardiovascular risks and toxicity—the Achilles heel of androgen deprivation therapy in prostate cancer patients. Biochim Biophys Acta—Rev Cancer 1874:188383. https://doi.org/10.1016/j.bbcan.2020.188383
Gandaglia G, Sun M, Popa I, Schiffmann J, Abdollah F, Trinh Q-D et al (2014) The impact of androgen-deprivation therapy (ADT) on the risk of cardiovascular (CV) events in patients with non-metastatic prostate cancer: a population-based study. BJU Int 114:E82–E89. https://doi.org/10.1111/bju.12732
Wenzel M, Würnschimmel C, Chierigo F, Tian Z, Shariat SF, Terrone C et al (2021) Non-cancer mortality in elderly prostate cancer patients treated with combination of radical prostatectomy and external beam radiation therapy. Prostate 81:728–735. https://doi.org/10.1002/pros.24169
Abdollah F, Sammon JD, Reznor G, Sood A, Schmid M, Klett DE et al (2015) Medical androgen deprivation therapy and increased non-cancer mortality in non-metastatic prostate cancer patients aged ≥66 years. Eur J Surg Oncol 41:1529–1539. https://doi.org/10.1016/j.ejso.2015.06.011
Surveillance, Epidemiology, and End Results (SEER) Program (https://www.seer.cancer.gov ; Access date 10 May 2021)
D’Amico AV (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969. https://doi.org/10.1001/jama.280.11.969
Austin PC, Lee DS, Fine JP (2016) Introduction to the analysis of survival data in the presence of competing risks. Circulation 133:601–609. https://doi.org/10.1161/CIRCULATIONAHA.115.017719
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/. (Access date 10 May 2021).
Abdollah F, Suardi N, Cozzarini C, Gallina A, Capitanio U, Bianchi M et al (2013) Selecting the optimal candidate for adjuvant radiotherapy after radical prostatectomy for prostate cancer: a long-term survival analysis. Eur Urol 63:998–1008. https://doi.org/10.1016/j.eururo.2012.10.036
Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N et al (2014) Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol 32:3939–3947. https://doi.org/10.1200/JCO.2013.54.7893
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
CW, FC, MW, and PK: drafting of manuscript, data collection, and statistical analyses. RSF and BH: data collection. ZT: conceived and designed statistical analyses and data collection. FS, AB, SFS, VM, MG, NS, and FKHC: interpretation of data and critical revision. PK and CW: study concept and design, interpretation of data, and critical revision. DT and MG: administrative support and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Würnschimmel, C., Wenzel, M., Chierigo, F. et al. Radiation therapy after radical prostatectomy is associated with higher other-cause mortality. Cancer Causes Control 33, 769–777 (2022). https://doi.org/10.1007/s10552-022-01564-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-022-01564-z