Abstract
Biochar (BC) and plant growth-promoting microbes (PGPR) could represent a suitable agronomical strategy to mitigate the impacts of drought in arid agro-environmental conditions. However, there is currently little understanding of the synergistic benefit of combining BC and PGPR to increase drought tolerance in oilseeds. In this study, the physiological response of two water-stressed canola (Brassica napus L.) plants subjected to the application of BC obtained from waste wood of Morus alba applied solely or in combination with PGPR strains (Pseudomonas sp.) was evaluated. The experiment consists of two genotypes and nine treatments [(C-Control, T1-15 days drought (15DD), T2-30 days drought (30DD), T3-15 days of drought + PG (15DD + PG), T4-30 days of drought + PG (30DD + PG), T5-15 days drought + biochar (15DD + BC), T6-30 days drought + biochar (30DD + BC), T7-15 days drought + biochar + PG (15DD + BC + PG), T8-30 days drought + biochar + PG (30DD + BC + PG)]. Drought stress decreased emergence energy (EE), leaf area index (LAI), leaf area ratio (LAR), root shoot ratio (RSR), moisture content of leaves (MCL), percent moisture content (%MC), moisture content of shoot (MCS) and moisture content of root (MCR), and relative water content (RWC) in both varieties of Brassica napus L., which in contrast, it is increased by the collective application of both biochar and PGPR. In both varieties, N, P, K, Mg, and Ca concentrations were highest in all the biochar and PGPRs separate and combined treatments, while lowest in 15 and 30 days drought treatments. Osmolyte contents like Glycine betaine (GB) and sugar remarkably increased in the stress condition and then reduced due to the synergistic application of biochar and PGPR. Drought stress has a repressive effect on the antioxidant enzymatic system like Peroxidase (POD), Superoxide dismutase (SOD), and glutathione reductase (GR) as well as total flavonoids, phenolics, and protein content. The antioxidant enzymes and phenolic compounds were dramatically increased by the combined action of biochar and PGPRs. A significant increase in EE, LAR, RSR, and RWC under 15 and 30 days drought conditions, evidently highlighting the synergistic effect of BC and PGPR. The results conclude a substantial and positive effect of the combined use of BC and PGPR strains on canola's response to induced drought stress, by regulating the physiological, biochemical, and agronomic traits of the plants.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The world's climate has changed dramatically over the last decades, due to both natural and anthropic factors, even if it has been observed that natural factors determined a smaller impact than anthropogenic factors (Lalay et al. 2021). Climate change is the main responsible for a series of negative events that cause altered precipitation patterns, leading to the rise in sea levels, increased drought and salinity stress, and altered evapotranspiration. In addition to these direct effects, climate change could determine a series of indirect effects such as the possibility of flood overwintering of diseases, enhanced pest and parasite resistance, and decreased plant productivity (Ullah et al. 2021). Biotic and abiotic stresses are directly or indirectly related to climate change having severe impacts on crops (Raza et al. 2019). Drought, heat stress, salinity, cold, and waterlogging are the major stresses faced by crops (Ashraf et al. 2018). Among the climate change causes, drought conditions represent one of the most destructive environmental stressors, which may severely affect crop yield. Drought stress induces a series of physiological changes in plants and metabolic process deficiencies, which leads to the subsequent production of reactive nitrogen species (NOS) and reactive oxygen species (ROS) (Khalvandi et al. 2021). The adverse effects of drought on crop cultivation are much more intensified by the reduction in water supplies and more food demand brought on by the world's population growth. Water shortages do not have a negative consequence on crop yield, but also on product quality (Seleiman et al. 2021).
Biochar is carbon-rich material made from different waste plant materials that are subjected to a pyrolysis process at high temperatures as a process for the production of renewable energy (Ahmad et al. 2012). Biochar could represent a promising soil amendment due to its potential to affect the physical and chemical properties of agricultural soils, through increasing pH, soil nutrients availability, surface area, carbon sequestration due to carbon and ash content and enhanced soil water-holding capacity, and decreased nutrients leaching (Cabeza et al. al. 2018). These properties aid in crop development and production, particularly under stress conditions (Paneque et al. 2016). The increase in soil pH and Electrical conductivity, holds water and nutrients, and, therefore, reduces the risk of nutrient leaching and keeps nutrients at root level available for the plant needs increasing crop yield (Biederman and Harpole 2013).
The effect of biochar is more pronounced under drought scenarios in agricultural fields, where it significantly increased plant water content, stomatal conductance, chlorophyll content, photosynthesis, and water usage efficiency (Safahani Langeoodi et al. 2019; Haider et al. 2020). Plants that grow in soil amended with biochar showed an improved activity of defensive enzymes and electron transport, in addition, biochar has been found to support the defense mechanisms of plant leaves under water-deficit stress, tumbling the antagonistic impacts of induced drought conditions on the photosynthetic apparatus (Lyu et al. 2016). The improved water-holding capacity in agricultural soils could be included as a sustainable agricultural strategy able to mitigate the negative impacts of drought conditions.
Plant growth-promoting rhizobacteria (PGPRs) are soil microorganisms that naturally inhabit the rhizospheric zone of roots and stimulate plant growth directly or indirectly (Kumar et al. 2021). A recent study demonstrates that PGPRs can exert a symbiotic effect on the plant's primary roots and root hairs able to help plants to mitigate environmental stresses such as heavy metal stresses, saline-alkali, drought, as well as nutritional deficiencies (Wang et al. 2021a, b).
The PGPRs affect plant metabolisms by acting on several mechanisms, including the production of exopolysaccharides, plant hormones, and carotenoids, nitrogen fixation, siderophore, amplified light and CO2 fixation rates, improved root and shoot system in response to drought stress, They also help in the development of induced systemic tolerance (IST) (Langeroodi et al. 2020; Ahluwalia et al. 2021). In addition, the PGPRs enhance the activities of antioxidant defense enzymes, including POD, PPO, and CAT, which are widely recognized for their capacity to increase plant stress tolerance and decrease ROS damage (Benidire et al. 2021).
The second-largest oilseed crop in the world is Brassica napus L. commonly identified as canola or rapeseed. The crop is particularly vulnerable to environmental stressors, such as heat and drought, and soil water deficits that, at critical growth stages of the crop, can cause significant damage to crop physiology and consequently determine severe seed yield losses (Iqbal et al. 2022). Canola has a complicated polyploid genome because of its genesis and evolution (Friedt et al. 2018). From an agronomical point of view, the cultivation of canola represents a rotation's key advantage as the well-developed root system positively impacts soil structure and, therefore, the fertility of the agroecosystems. In agreement, tap-rooted plants like canola are often regarded as superior to grasses in their ability to remediate poorly structured soils, and actively expanding root systems have the potential to improve subsoil in poor physical circumstances by “biological drilling” (Burbulis et al. 2013).
The use of biochar in conjunction with PGPRs inoculation might be a valuable and auspicious tactic to mitigate the determinantal effects of water scarcity on agronomic plants (Glodowska et al. 2017). This study hypothesized that appropriate management of plant growth-promoting microbes and biochar could alleviate the adverse effects of drought conditions in canola crops while maintaining agro-physiological responses. Indeed, optimizing the use of bacterial inoculation with biochar might be employed as an auspicious and valuable tactic to alleviate the detrimental effects of water scarcity on agricultural plants supporting agronomic yield, growth, production, and other physiological and water-related characteristics. However, there is currently little understanding of the synergistic benefit of combining BC and PGPR to increase drought tolerance in oilseeds.
Thus, the main objectives of the current study are (i) investigating the impacts of biochar on morphological and physiological attributes of canola plants subjected to drought stress conditions at early vegetative stages, (ii) determining the role of PGPR in taming drought tolerance in canola, and (iii) evaluating the potential effectiveness of combining biochar application with bacterial inoculants to enhance drought resilience in rapeseed.
Materials and Methods
Biochar Preparation and Characterization
Waste wood of Morus alba was collected from the Peshawar area and then the tree cuttings were cleaned from dust and soil, dried in the sun until 10–15% moisture content was left, and finely crushed. The crushed feedstock was pyrolyzed at the temperature of 550–600 °C for 2 h in a specific laboratory-scale stainless furnace with a 15 kg feedstock capacity. After the pyrolysis process, the obtained charcoal was finely crushed into 2-mm particle size when the residence time was completed, and then it was mixed with soil and sand (Fahad et al. 2016). The characteristic of the biochar is reported in previous research by Lalay et al. (2020).
Site Description
The study used wire-house, semi-controlled settings to look examine how biochar and PGPR affected canola. A pot experiment was conducted at the Department of Botany, University of Peshawar, Pakistan 34° 1′ 33.3012" N and 71° 33′ 36.4860" E).
Seeds Collection, Sterilization, PGPRs Collection, and Inoculation
The seeds of Punjab Sarson and Westar canola (Brassica napus L.) genotypes were chosen because of their distinct genotypic characteristics with variations in their genetic makeup. In addition, these are the two varieties for which we have 95% viability rate, and recognized international varieties. According to Barampuram and Krasnyanski (2014), suitable seeds were sterilized in ethanol (70%) for two minutes and then six times in distilled water. Pseudomonas sp., a strain of plant growth-promoting rhizobacteria (PGPR), was supplied by NARC Islamabad, Pakistan. Both canola genotypes’ randomly chosen healthy and physically unblemished seeds were immersed in the inoculum for 30 min before being used for sowing. The rate of inoculation was 5 ml of liquid inoculum per 100 seedlings (Dasgupta et al. 2015).
Experimental Design
The pots were filled with soil, sand, and biochar (2:1:1), and seeds were sown in 5 replicates after PGPR inoculation (Seybold et al. 2002). Properties of soil, including organic matter (0.50%), nitrogen (0.06%), phosphorus (4.7 mg kg−1), potassium (79 mg kg−1), calcium (45 mg kg−1), magnesium (51 mg kg−1), saturation percentage (39%), and water-holding capacity (1.3). The pots were kept away from each other, and the research was carried out in complete randomized block design. To shield the plants from the rain when it rained, a plastic sheet was placed over the experimentation area. The experiment consists of 2 genotypes and 9 treatments including.
No drought stress = Control.
15DD = 15 days of drought.
30DD = 30 days drought.
15DD + PGPR = 15 days drought stress + PGPR.
30DD + PGPR = 30 days drought stress + PGPR.
15DD + BC = 15 days drought stress + biochar.
30DD + BC = 30 days drought stress + biochar.
15DD + BC + PGPR = 15 days drought stress + biochar + PGPR.
30DD + BC + PGPR = 30 days drought stress + biochar + PGPR.
Regular watering was given to control treatments (those without inoculation), but the remaining treatments, in accordance with the experiment's design, were subjected to water-deficit stress for 15 and 30 days at the early vegetative stage, respectively. The plants were removed when the stress period was over to gather information about the plants’ physiological and antioxidant responses.
Elemental Analysis of Leaves
Following the procedures of Seybold et al. (2002), dried samples were dissolved in 70% nitric acid (v/v) at 90 ºC for 4 h in a water bath, and after refrigeration, 1–2 ml of H2O2 was added. Calculations were made on the amounts of various elements using atomic absorption spectrometry.
Germination and Vegetative Attributes
Emergence Energy (EE)
The following equation was used to determine the emergence energy (Basra et al. 2005):
Leaf Area Ratio (LAR)
Leaf area ratio was calculated using the procedure suggested by Shah et al. (2017) by applying the following formula:
Root to Shoot Ratio (RSR)
Root/Shoot ratio was analyzed through the equation of Sainju et al. (2017):
Leaf Area Index (LAI)
Leaf area index was measured by the standard method described by Shah et al. (2017):
Relative Water Content (RWC)
Relative water content was detected by using the procedure (Gonzalez and Gonzalez-Vilar 2001).
Determination of Physiological Attributes
Superoxide Dismutase Activity (SOD)
The standard methodology was used to calculate the SOD activity (Ellouzi et al. 2013). In phosphate buffer, we regimented and then centrifugated plant leaves (0.5 g). After that, the 24 µl of methionine, riboflavin, and nitro-blue tetrazolium were mixed to create a 0.1-ml filter. The final optical density (OD) at 560 nm was recorded after three minutes. Using a typical curve, the SOD absorption was calculated and stated as unit/g F.W.:
Peroxidase (POD) Activity
The activity of POD was assessed using a recognized methodology (Asthir et al. 2009). After centrifuging the morpholine ethane sulphonic acids (MES)-chopped plant samples (0.5 g), the supernatant was obtained. The supernatant was mixed with 30% H2O2, MES, and phenyl diamine. A 485 nm optical density was noted. The absorption of an unidentified sample was calculated and stated in units g−1 fresh weight (f.w.) based on the standard curve.
Dehydroascorbate Reductase (DHAR) Activity
To evaluate the DHAR activity, fresh shoot and root material (0.7 g) was placed in a buffered potassium phosphate solution (pH 7.8) comprising 2 mM mercaptoethanol, 1 mM EDTA, and 8% (v/v) glycerin (Nakano and Asada 1981). The mixture was centrifuged, and the filtrate was collected. The reaction mixture (DHA) contained 90 mM potassium buffer with phosphate (pH 7.0), 12.5 mM glutathione reductase (GSH), 0.178 mM EDTA, 0.1 mM enzymes extract, and 1 mM dehydroascorbate. Notably, the DHAR activity was determined via a measurement at 265 nm. The results are provided using a threshold value of 7.0 mM−1 cm−1. An unknown sample's concentration was determined and given as mol min−1 g−1 of f.w.
Glutathione Reductase (GR) Activity
To determine the GR, fresh leaves and root samples (0.7 g) were homogenized in a mix of potassium phosphate-buffered water and 2 mM EDTA, and the resultant mixture was centrifuged (Lee and Lee 2000). A reaction mixture (GSSG) was made by combining 2 mM EDTA, 0.15 mM NADPH, 50 mM buffer solution of potassium phosphate (pH 7.8), 0.2 ml enzyme extract, and 0.5 mM oxidized glutathione. The reaction was started by the addition of NADPH. Utilizing a decrease in absorption at 340 nm with an attenuation value of 6.2 M m−1 cm−1, the reductase glutathione activity was measured. The enzyme oxidizes one nmol of NADPH once each minute. Using the standard curve, the level of glutathione reductase activity was determined and represented as a unit/ml.
Sugar Content Analysis
A 0.5 g sample of fresh leaves and roots was ground in refined water to quantify the amount of sugar present (Marciska et al. 2013). After adding phenol 80 percent (w/v) to the samples, their absorbance was measured at 420 nm. The absorption of the samples, reported as mM g−1 fresh weight, was calculated using a standard curve using glucose.
Glycine Betaine (GB)
The concentration of GB was found using the accepted protocol (Grieve and Grattan 1983). For 24 h at 25 ºC, mechanically cut root and leaves samples (0.5 g) were combined with deionized water. The filtrate was diluted with 2NHSO4 following filtration (1:1). The rest was then diluted with 2NHSO4 (1:1), the aliquots were kept in centrifuge tubes, refrigerated in ice water for an hour, and then KI-I2 was added and they were vortexed. After 16 h at 4 °C, the filtrate was articulated in a thin glass tube. In 9 mL of 1,2-dichloroethane, the periodide crystals were then liquefied. After two hours, the absorbance was calculated and reported as µg g−1 using GB as a reference.
Protein Extraction
Shoots and roots had their plant protein content assessed using the accepted technique (Wang et al. 2006). Centrifugation was used after homogenizing 0.5 g of plant material in phosphate buffer. CuSO4.5H2O was added to the filtrate, followed by a Folin phenol reagent, which was then incubated for 10 min. The absorbance of each sample was then determined at 650 nm using a spectrophotometer. The protein concentration, reported as mg g−1 F.W.
Total Phenolic Content (TPC)s
According to established procedures, the TPC was identified (Baydar et al. 2004). 2 g of dehydrated root and leaves material were crushed and then stirred for an hour in 90% methanol. After centrifugation, a diluted (4:1) mixture of the folin-Ciocalteu reagent was used. A spectrophotometer was used to measure the filtrate's fluorescence at 760 nm after 10% Na2CO3 was added. Using the standard curve, the number of phenolic compounds in the samples was determined and expressed as mg g−1 dry weight (d.w.).
Total Flavonoid Content (TFC)
Utilizing a colorimetric process with aluminum chloride, the flavonoid concentration was measured (Chang et al. 2002). The dried plant samples (0.1 g) were mixed with 1.5 ml of methanol, 0.1 ml of potassium acetate, 0.1 ml of 10% AlCl3, and 2.8 ml of water. The mixture was then diluted in 0.5 ml of 1:10 g methanol: water. A spectrophotometer was used to measure the density at 415 nm after the solution was left at room temperature for 30 min.
Statistical Analysis
To examine the primary impacts of the canola genotype and stress conditions, as well as their interactions, all gathered data and parameters were put through an analysis of variance (ANOVA) using SPSS statistics 22 and Statistix 10 (SPSS, Inc., Chicago, IL). To ascertain whether the transformation was necessary, the equal variance assumptions were evaluated for each set of data. To homogenize the variance before analysis, percentage data were transformed as an angular transformation (Gomez and Gomez 1984). The information in the tables and figures has undergone a reverse transformation. For the evaluation of vegetative and physiological qualities, a two-way factorial approach was used, with the canola genotype acting as one treatment and the stresses of the environmental conditions acting as the other. Using Fisher's protected LSD test, the main effect and interaction means were evaluated at a 5% level of probability (P = 0.05). The two-dimensional canonical classifier structure plot was examined using a canonical discriminant analysis (CDA). In the CDA analysis, the vector diagrams for each canola genotype were integrated into a single plot based on the total canonical coefficients of each canola feature from the canonical functions. To explain the degree of resemblance in canola features among the water stress circumstances, nine groups were selected in the data about water stress conditions. The group means were calculated and published on axes determined by both the first and second classical functions. The length of the vectors used to represent the canola traits in coordination space (Kenkel et al. 2002) reflects how strongly they are associated with a direction. To find significant relationships between the physiological and vegetative features of canola, Pearson's linear correlation test was computed.
Results
In this study, the synergistic applications of biochar and PGPR to induce drought tolerance in canola crop have been investigated. Physioco-chemical analysis of biochar showed 5.4 cmol kg−1 cation exchange capacity, 6.9 ds/m electrical conductivity, pH of 9.6, 0.50 g cm−3 bulk density, and organic carbon 3.64% (Lalay et al. 2021). Biochar obtained from the waste wood of Morus alba was applied solely or in combination with PGPR strains (Pseudomonas sp.) in water-stressed canola (Brassica napus L.) genotypes under environmental conditions using different parameters like emergence energy (EE), Leaf area ratio (LAR), Relative water content (RWC) and Root/shoot ratio moisture content of soil, leaves, shoot, and roots as represented in Table 1. There was a significant increase in emergence energy (EE), Leaf area ratio (LAR) and root shoot ratio (RSR), and relative water content (RWC) under induced drought stress of 15 and 30 days due to the synergistic effect of BC and PGPR strains (Table 1).
The combined use of BC and PGPRs significantly improved all of the aforementioned traits in both canola genotypes. A reverse reaction was observed in the study of elemental characteristics in plants where nitrogen, phosphorus, potassium, calcium, and magnesium concentrations in both genotypes were at their highest in all BC and PGPRs treatments, either used separately or in combination and at their lowest in the 15- and 30-day drought treatments (Table 2). Drought stress hurts plant internal mechanisms by generating reactive oxygen species (ROS), which cause cell damage. The study of different traits of shoot SOD content was statistically significant between treatment and genotype at the (p < 0.001***) level, while POD, DHAR, sugar, GB, proteins, and phenols were statically significant at the (p < 0.001***) level between treatments and GR and flavonoids at (p < 0.002**) level (Table 3). SOD, POD, DHAR, GR, sugar, GB, proteins, and phenols were statistically significant at (p < 0.001***) level between treatments while flavonoids were at (p < 0.002**) level between treatments.
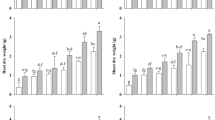
Regarding the canola genotypes subjected to different water stress conditions, the statistical analysis showed that the application of PGPR, BC, and their combined application influenced the response of the physiological attributes compared to the no stressed and stressed canola plants (15DD and 30DD, respectively). The results of superoxide dismutase (SOD) showed a similar trend between the Punjab sarson and Westar genotypes, indeed, in canola shoot, the SOD values tend to decrease from No stressed plants to 15DD and 30DD (335.2 vs. 237.7 units g−1 F.W., respectively, Fig. 1). However, the SOD values in canola shoot tended to increase when PGPR and BC were applied. In general, the SOD values were higher in BC and BC + PGPR compared with PGPR applied alone, regardless of the water stress duration (on average 461.2 vs. 384.9 units g−1 F.W., respectively, Fig. 1), even if, in both canola genotypes, the effect of the PGPR on SOD activity increased from 15 to 30DD (on average 344.3 and 425.4 units g−1 F.W., respectively, Fig. 1), while it tended to decrease under BC treatments (497.4 and 430.9 units g-1 F.W., respectively). The combined application of BC and PGPR resulted in high values of SOD in canola shoot, except for the Punjab sarson genotype which showed a lower value at 30DD of water stress conditions (Fig. 1). The SOD activity of canola root showed a similar trend to that observed in canola shoot (Fig. 1). Also, the peroxidase (POD) activity was significantly affected by the experimental treatments in both shoot and root of canola plants (Fig. 2). In general, under the control treatments, the POD values in canola shoot tended to decrease from no stressed plants to 15DD and 30DD stress levels, regardless the canola genotype (on average 345.0, 223.1, and 111.5 units g−1 F.W., respectively, Fig. 2). However, the POD activity tended to increase with the adoption of PGPR, BC, and their combination. Indeed, higher POD values were observed in BC + PGPR and BC treatments (on average 1177.3 units g−1 F.W., Fig. 2) compared with PGPR applied alone to canola plants (on average 737.6 units g−1 F.W.). It is interesting to note that the application of PGPR differently influenced the POD activity under the different water stress durations in both genotypes it was lower in 15DD than 30DD (on average 633.3 vs. 841.9 units g−1 F.W., respectively, Fig. 2), while under BC and BC + PGPR, the POD activities in canola shoot tended to be similar regardless the water stress duration (Fig. 2). The POD activity on roots was similar among the control treatments (on average 165.0 units g−1 F.W.), while it increased when canola plants were subjected to PGPR (778 units g−1 F.W.), BC (978 units g−1 F.W.) and BC + PGPR (1502 units g−1 F.W., Fig. 2), even if the effects generally were high in Punjab sarson than Westar genotype, respectively.
Impacts of growth-promoting rhizobacteria, biochar application, and their combination on superoxide dismutase (SOD) action in shoot and root of canola plants under tempted 15 and 30 days water-deficit stress, respectively. Means without common alphabets showed substantial variation at the level of 5%, according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected to priming with growth-promoting rhizobacteria; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
Effect of biochar and growth-promoting rhizobacteria, and their combination on peroxidase activity (POD) in shoot and root of canola plants under tempted 15 and 30 days water-deficit stress, respectively. Means without common alphabets represent significant variation at the level of 5% according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected to growth-promoting rhizobacteria; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
The results of the dehydroascorbate reductase (DHAR) activity measured for shoot and root of canola plants affected by different stress conditions of both Punjab sarson and Westar genotypes are reported in Fig. 3. In general, the lower DHAR values were observed in the control treatments (0.21 and 0.26 mol min−1 g−1 F.W. in shoot and root of canola plants, respectively) and tended to increase in PGPR (0.72 and 0.91 mol min−1 g−1 F.W. in shoot and root of canola plants, respectively), BC (1.16 and 1.56 mol min−1 g−1 F.W. in shoot and root of canola plants, respectively), while the high values were observed in the canola plants subjected to BC + PGPR (1.72 and 1.91 mol min−1 g−1 F.W. in shoot and root of canola plants, respectively, Fig. 3). The DHAR values tended to be similar among the canola genotypes and increased from 15 to 30DD water stress duration (Fig. 3).
Impacts of growth-promoting rhizobacteria, biochar application and their combination on dehydroascorbate reductase (DHAR) activity in shoot and root of canola plants tempted 15 and 30 days water-deficit stress, respectively. Means without common alphabets are significantly different at the 5% level according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected to growth-promoting rhizobacteria seeds priming; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
The results of glutathione reductase (GR) activity were also affected by the water stress treatments in both canola genotypes (Fig. 4). Among the control treatments, the GR activity was lower in no stressed plants compared to 15DD and 30DD water stress duration in both shoot and root canola plants (on average 0.63 and 0.70 vs. 1.58 and 1.62 unit ml−1, respectively, Fig. 4). Under the 15DD water stress duration, the application of PGPR inoculated to canola plants tended to not affect the GR activity of canola shoot (on average 1.72 unit ml−1), while at 30DD water stress duration, the values of GR activity increased in both genotypes (1.49 and 2.47 unit ml−1 in Punjab sarson and Westar, respectively, Fig. 4). The application of BC and its combination with PGPR increased the GR activity in canola shoot at both 15DD and 30DD water stress duration (Fig. 4), even if the values were similar among the Punjab sarson genotype (on average 1.99 unit ml−1), while the GR was the highest at 30DD in BC + PGPR in Westar genotype (3.67 unit ml−1, Fig. 4).
Impacts of growth-promoting rhizobacteria, biochar application, and their combination on glutathione reductase (GR) activity in shoot and root of canola plants under tempted 15 and 30 days water-deficit stress. Means without common alphabets are significantly varied at the level of 5% according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected to growth-promoting rhizobacteria biopriming; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
Sugar (Fig. 5) and glycine betaine (GB, Fig. 6) contents showed similar trends in both shoots and roots of canola plants. Under control treatments, both osmolytes generally tended to be higher in 15DD and 30DD water stress duration in both shoot (27.3 mM g−1 of F.W. and 1.3 µg g−1 of sugar and GB, respectively) and root (on average 41.7 mM g−1 of F.W. and 1.5 µg g−1 of sugar and GB, respectively) than no stress conditions (on average 4.5 mM g−1 of F.W. and 0.6 µg g−1 of sugar and GB in canola shoot, respectively, and 7.0 mM g−1 of F.W. and 0.6 µg g−1 of sugar and GB in canola root, respectively). The adoption of PGPR and BC and their interaction contributed to a reduction of both sugar and GB. However, the effects were similar among the PGPR, BC, and BC + PGPR in sugar content in canola shoots and roots (Fig. 5), while the GB showed different trends among the canola genotypes and the treatment applied. GB was lower in the Punjab sarson genotype under PGPR treatments regardless of the water stress duration in shoots and roots (on average 0.54 and 0.66 µg g−1, respectively) compared with BC and BC + PGPR (on average 0.60 and 0.72 µg g−1 in canola shoots and roots, respectively, Fig. 6). Conversely, the GB values observed in shoots and roots of Westar genotype resulted similar and lower in BC and BC + PGPR than PGPR applied alone in canola plants (Fig. 6). Protein content was also affected by the water availability and water stress duration (Fig. 7). In general, the water stress duration determined a reduction of the protein content compared the no stressed canola plants (on average -41% in shoot and -43 in roots, respectively). The application of PGPR, BC, and BC + PGPR as a mitigation strategy for water stress showed an increase in protein content; in particular, the PGPR and BC applied alone showed similar values of protein content in shoots and roots similar to that observed in no stressed plants (Fig. 8), except BC at 15DD and 30DD in Westar genotype, where the protein content was high (16.0 and 19.4 mg g−1 F.W., respectively). The combined application of BC and PGPR increased notably the protein content in shoots and roots of canola plants, regardless of the genotype and the water stress duration (Fig. 7). The total phenolics content in canola shoots decreased among the different water stress conditions, was high in no water stress, intermediate in 15DD and low in 30DD (3.45, 2.43, and 1.59 mg g−1 D.W., respectively, Fig. 8). Similar trend was observed also in the canola roots. The application of PGPR showed an increase of phenols content from 15 to 30DD in both genotypes (on average 3.84 and 5.10 mg g−1 D.W., respectively), even if this effect was observed also in canola shoots (Fig. 8). The BC application and its combination with PGPR resulted in the high values of phenols content (on average 6.70 and 7.74 mg g−1 D.W. in shoots and roots, respectively), even if for these treatments, the phenolics content values were higher in Westar than Punjab sarson genotype (on average 7.56 vs. 5.85 mg g−1 D.W. in shoots and 8.50 vs. 6.98 roots mg g−1 D.W., respectively). Similarly, the flavonoid content decreased by about 31% in both shoots and roots when canola plants were subjected to 15DD and 30DD water stress duration compared to the no stressed plants, regardless of the genotype (Fig. 10). The Punjab sarson genotype showed a high value of flavonoid content at 15DD water stress duration, while Westar showed an opposite trend (Fig. 9). For both genotypes, the application of BC resulted in higher values of flavonoid content at 30DD than 15DD water stress duration in canola shoots (on average 2.32 vs. 1.68 mg g−1 D.W., respectively) and in canola roots (on average 2.77 vs. 1.86 mg g−1 D.W., respectively, Fig. 9).
Impacts of growth-promoting rhizobacteria, biochar application, and their combination on sugar content in shoot and root of canola plants under tempted 15 and 30 days water-deficit stress, respectively. Means without common alphabets represent significant variation at the level of 5% according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected to growth-promoting rhizobacteria seed priming; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
Effect of biochar and growth-promoting rhizobacteria, and their combination on Glycine betaine (GB) in shoot and root of canola plants under tempted 15 and 30 days water-deficit stress, respectively. Means without common alphabets represent significant variation at the level of 5% according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected to growth-promoting rhizobacteria biopriming; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
Impacts of growth-promoting rhizobacteria, biochar application, and their combination on Protein content in shoot and root of canola plants under tempted 15 and 30 days water-deficit stress, respectively. Means without common alphabets showing significant variation at the level of 5% according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected to drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected to drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected to plant growth-promoting rhizobacteria seed biopriming; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
Effect of growth-promoting rhizobacteria, biochar application, and their combination on phenols content in shoot and root of canola plants under tempted 15 and 30 days drought stress, respectively. Means without common alphabets represent significant differences at the level of 5% according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected to growth-promoting rhizobacteria; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
Impacts of growth-promoting rhizobacteria, biochar application and their combination on flavonoid content in shoot and root of canola plants under tempted 15 and 30 days drought stress, respectively. Means without common letters indicate significant variation at the level of 5% according to LSD test. Lower- and upper-case alphabets refer to Punjab sarson and Wastar cultivars, respectively. Bars represent the standard error (n = 5). No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected to drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected to drought stress for 30 days at early vegetative stage; PGPR = Canola plant subjected plant growth-promoting rhizobacteria seed biopriming; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plants subjected to combined application of plant growth-promoting rhizobacteria biopriming and biochar
Measured characteristics of shoots under 15- and 30-day induced drought stress were correlated using the Pearson method. This showed that shoot SOD content has a significant correlation with POD, DHAR, GTR, sugar, GB, proteins, phenols, and flavonoids at (p ≤ 0.05) and (p ≤ 0.01) level whereas shoot POD content has a significant correlation with DHAR, sugar, GB, proteins, phenols, and flavonoids at (p ≤ 0.05) and (p ≤ 0.01) level. DHAR had a significant correlation with GR, sugar, GB, proteins, phenols, and flavonoids; GR had a significant correlation with sugar, GB, proteins, phenols, and flavonoids; sugar had a significant correlation with GB, proteins, phenols, and flavonoids; GB had significant correlation with proteins, phenols, and flavonoids at (p ≤ 0.05) and (p ≤ 0.01) level. Proteins had a significant correlation with phenols at (p ≤ 0.01) level (Table 3).
Pearson correlation coefficient of measured traits of root under induced drought stress of 15 and 30 days in canola plants showed that root SOD content has a significant correlation with POD, DHAR, GR, sugar, GB, proteins, phenols, and flavonoids at (p ≤ 0.05) and (p ≤ 0.01) level while shoot POD content has a significant correlation with DHAR, sugar, GB, proteins, phenols, and flavonoids at (p ≤ 0.05) and (p ≤ 0.01) level. DHAR had a significant correlation with GR, sugar, GB, proteins, phenols, and flavonoids; GR had a significant correlation with sugar, GB, proteins, phenols, and flavonoids; sugar had a significant correlation with GB, proteins, phenols, and flavonoids; GB had a significant correlation with proteins, phenols, and flavonoids at (p ≤ 0.05) and (p ≤ 0.01) level. Proteins had a significant correlation with phenols and flavonoids while phenols have also a significant correlation with flavonoids at (p < 0.05) and (p < 0.01) levels (Table 3). Similarly, a positive and significant correlation was found between SOD, POD, DHAR, GR, sugar, GB, proteins, phenols, and flavonoids of shoot and root as shown in Table 3.
The canonical discriminant analyses (CDA) of the physiological characteristics related to shoots and roots of canola plants for the Punjab sarson and Westar genotypes are reported in Fig. 10. The first two canonical variables explained 48 and 51% of the total variance for shoots and 47 and 45% for roots for water stress treatments in both Punjab sarson and Westar genotypes. Indeed, the CDA analysis showed that sugar and glycine betaine (GB) were generally associated with 15DD and 30DD water stress duration in both canola genotypes. Conversely, the application of biochar (BC) and its combination with PGPR (BC + PGPR) were generally associated with several physiological attributes such as SOD, POD, DHAR, proteins, phenols, and flavonoids, while no stress conditions and 15DD-PGPR are reported in the opposite direction of the physiological vectors (Fig. 10a and b, respectively). A similar trend was observed also for the canola roots where sugar and GB were associated with 15DD and 30DD water stress duration, while the main physiological attributes were associated with BC and BC + PGPR treatments (Fig. 10c and d).
A canonical discriminant analysis (CDA) showing a Biplot of physiological features in the shoot (a and b) and root (c and d) of canola cultivars under tempted 15 and 30 days water = deficit stress, respectively. No stress = Canola plant subjected to regular water availability; 15DD = Canola plant subjected to drought stress for 15 days at early vegetative stage; 30DD = Canola plant subjected to drought stress for 30 days at early vegetative stage; PGPR = Canola seeds subjected to plant growth-promoting rhizobacteria biopriming; BC = Canola plant subjected to biochar application; BC + PGPR = Canola plant subjected to biochar application and seed growth-promoting rhizobacteria biopriming. SOD = superoxide dismutase activity; POD = peroxidase activity; DHAR = dehydroascorbate reductase; GR = glutathione reductase; GB = glycine betaine
Discussion
Drought, heat stress, salinity, cold, and waterlogging are the major stresses faced by crops (Ashraf et al. 2018). Biochar's huge surface area and water-holding capacity impact soil physicochemical characteristics, which aid in crop development and production, particularly under water-stressed circumstances (Paneque et al. 2016). How microbial inoculants increase the production of anti-oxidants, exopolysaccharides, and the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase, they reduce the detrimental effects of drought (Shaffique et al. 2022). Only a few studies have examined the interactions between the application of microbial inoculants and biochar, including Ahmad et al. (2020) in maize (Zea mays L.), Safahani Langeroodi et al. (2021) in sunflower (Helianthus annuus L.), and most recently by Nawaz et al. (2023) in soybean (Glycine max L.).
The adoption of biochar, as a soil amendment, may be a positive effect on the agricultural soil as its porous characteristics help PGPRs' establishment and development (Safahani Langeroodi et al. 2021). In addition, the enhanced water availability due to biochar application may be a favorable factor also for soil biodiversity as could keep alive, healthy, and active soil bacteria and fungi, especially during the dry and hot summer season. Biochar treatment promotes plant growth by improving the microenvironment favorable to the plant (Mihoub et al. 2022). Liu et al. (2021) reported that biochar improves root morphology and growth. They suggested that biochar-mediated increases in root biomass may be attributed to its capacity to reduce the bulk density of topsoil by 10–12%, hence, enhancing soil porosity and allowing for root system extension. Similarly, according to Ducey et al. (2013), biochar in soil can offer living space for microorganisms like PGPR, which eventually enhances soil health and provides an optimal environment for improved germination of seeds and other vegetative characteristics of plants. Biochar acts as a supportive material for increasing the shelf life of soil microorganisms and preserving them from harsh environmental circumstances such as drought stress (Tripti et al. 2017). The current outcomes are in line with the investigation of Hafez et al. (2019) and Malik et al. (2022) who reported that germination and moisture content of soil and plant are increased by the combined use of biochar and PGPR under stress conditions. This increase could be due to an improvement in the soil water-holding capacity, as suggested earlier by Karhu et al. (2011). Ning et al. (2019) reported that using various PGPRs in combination with biochar improved soil quality. The results are similar to the research work of Wang et al. (2021a, b), who investigated that co-application of PGPR and biochar increased the nutrients in the soil as well as in plants under stress conditions. Zhang et al. (2019) claim that biochar can keep roots healthy during the booting phase, ensuring maximum nutrient absorption to increase grain output. The wide surface area and water-holding ability of biochar have a significant impact on soil physicochemical properties that aid in promoting crop nutrient absorption in addition to increasing the amount of organic carbon in the soil (Amin and Mihoub 2021). Our findings are in line with studies on nutrient intake by Ghavami et al. (2017) and Mitra and Long (2004). The porous structure of biochar, which enables greater growth of these bacterial strains through appropriate airflow, ensuring greater accessibility and absorption of nutrients as well as greater strain tolerance against various adverse conditions, including drought stress, is the reason for the positive correlation between rhizobacteria that promote plant growth and biochar (Sangeetha 2012).
Canola plants produce safeguarding osmolytes, proteins, secondary metabolites, and anti-oxidants like catalase (CAT), guaiacol peroxidase (GPx), and superoxide dismutase (SOD) in response to reactive oxygen species (ROS), all of which can scavenge ROS under water stress (Hosseini et al. 2018). In addition, when canola plants were treated with biochar alone or in conjunction with microbial inoculation, antioxidant enzymes increased significantly. According to Wang et al. (2015), adding biochar has a positive impact on the microbiological activity of soil. They found a significant increase in the activity of soil enzymes, which led to a decline in the antioxidant ratio due to the elimination of ROS by the synergistic interaction between bacteria and biochar. Our findings are in line with those of Hafez et al. (2019), who demonstrated that the usage of biochar and PGPR together boosted antioxidant enzymes under stress conditions. Similarly, Ahluwalia et al. (2021) studied that under stress conditions different antioxidant enzymes increased. These results are in line also with previous studies by Reddy et al. (2004), Abbas et al. (2018), Sattar et al. (2019), and Chiappero et al. (2019). The use of growth promoters boosted the activities of the antioxidant enzymes and decreased the negative effects of water deprivation stress, according to Abideen et al. (2020). In contrast to the study's findings, additional research has revealed that plants treated with microorganisms had lower antioxidant enzyme levels than plants untreated, showing an improved ability of microbes to scavenge reactive oxygen species in dry environments (Mahajan et al. 2005).
Our findings are consistent with those of Abbasi et al. (2020), who investigated how the administration of a streptomycin strain reduced the osmolyte contents that had grown under stress. According to research by Gontia-Mishra et al. (2020), plants must modify their metabolism to live under drought stress. This results in the buildup of appropriate osmolytes like proline, glycine betaine (GB), polyamines, sugars (trehalose, polyols), polyhydric alcohols, and dehydrins. It is essential for the maintenance of metabolic activity for the plant to be able to sustain turgor pressure and volume of cells at a low potential for water (Wu et al. 2014). Osmolyte buildup also aids in the restoration of metabolic functions following stress reduction (Abid et al. 2016). Previous studies reported that the application of PGPRs increased the level of secondary metabolites and proteins (Rezazadeh et al. 2019, Abbasi et al. 2020, and Chiappero et al. 2019). This demonstrates how the flavonoids and phenolics that were found when the plant was under stress played a protective role. It is obvious from the results above that applying biochar and PGPR can reduce the suppressive effect of modest levels of water-deficit stress situations.
Conclusions
The development, growth, and physiology of canola were harmfully exaggerated by the drought stress condition. It was observed that the decline of biochemical, physiological, and agronomic properties of plants was accelerated when the water stress was raised. In other words, the application of PGPR and biochar support canola plants to modify their efficiency in water use, which ultimately promotes growth. In addition, the co-application of biochar and growth-promoting rhizobacteria improves the canola plant defense system in terms of different antioxidant enzymes. According to the findings, the Punjab and Sarson varieties showed more resistance to drought stress than the genotype Westar, which was more vulnerable to the condition. The antioxdants levels, which are associated with oxidative stress response decreased from non-stressed plants to 15 and 30 days of drought stress. However, the combined application of BC and PGPR resulted in notably higher SOD values, indicating improved stress resilience. Importantly, the co-application outperformed the individual applications of BC and PGPR, suggesting that the combination offers a more robust approach to improve canola's drought tolerance. Similarly, the physiological attributes and elemental characterization also reveal that this combination consistently outstripped separate applications and proved most effective under drought stress conditions. This co-application approach not only beats individual applications but also positively impacts multiple parameters critical for plant growth and stress resilience.
Limitations
Our study is primarily focused on the vegetative stage of the canola plants and did not extend to the point of maturity (yield stage).
References
Abbas T, Muhammad R, Ali S, Adrees M, Mahmood A, Rehman MZ, Ibrahim M, Arshad M, Qayyum MF (2018) Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol Environ Saf 148:825–833. https://doi.org/10.1016/j.ecoenv.2017.11.063
Abid M, Tian Z, Ata-Ul-Karim ST, Liu Y, Cui Y, Zahoor R, Dai T (2016) Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and-sensitive wheat cultivars. Plant Physiol Biochem 106:218–227. https://doi.org/10.1016/j.plaphy.2016.05.003
Abideen Z, Koyro HW, Huchzermeyer B, Ansari R, Zulfiqar F, Gul B (2020) Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol 22(2):259–266. https://doi.org/10.1111/plb.13054
Ahluwalia O, Singh PC, Bhatia R (2021) A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Res Environ Sustain 5:100032. https://doi.org/10.1016/j.resenv.2021.100032
Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, Yang JE, Ok YS (2012) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol 118:536–544. https://doi.org/10.1016/j.biortech.2012.05.042
Ahmad M, Wang X, Hilger TH, Luqman M, Nazli F, Hussain A, Zahir ZA, Latif M, Saeed Q, Malik HA, Mustafa A (2020) Evaluating biochar-microbe synergies for improved growth, yield of maize, and post-harvest soil characteristics in a semi-arid climate. Agronomy 10:1055. https://doi.org/10.3390/agronomy10071055
Amin AEEAZ, Mihoub A (2021) Effect of sulfur-enriched biochar in combination with sulfur-oxidizing bacterium (Thiobacillus spp.) on release and distribution of phosphorus in high calcareous p-fixing soils. J Soil Sci Plant Nutr 21(3):2041–2047. https://doi.org/10.1007/s42729-021-00500-5
Asthir B, Kaur S, Mann SK (2009) Effect of salicylic and abscisic acid administered through detached tillers on antioxidant system in developing wheat grains under heat stress. Acta Physiol Plant 31:1091–1096. https://doi.org/10.1007/s11738-009-0335-y
Barampuram S, Allen G, Krasnyanski S (2014) Effect of various sterilization procedures on the in vitro germination of cotton seeds. Plant Cell, Tissue and Organ Cult 118:179–185. https://doi.org/10.1007/s11240-014-0472-x
Basra SMA, Farooq M, Tabassum R (2005) Physiological and biochemical aspects of seed vigor enhancement treatments in fine rice (Oryza sativa L.). Seed Sci Technol 33:623–628
Baydar NG, Özkan G, Sağdiç O (2004) Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 15:335–339. https://doi.org/10.1016/S0956-7135(03)00083-5
Benidire L, Madline A, Pereira SIA, Castro PML, Boularbah A (2021) Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 262:127803. https://doi.org/10.1016/j.chemosphere.2020.127803
Burbulis N, Kott LS (2013) Application of doubled haploid technology in breeding of Brassica napus. In: From plant genomics to plant biotechnology, pp 183–203. Woodhead Publishing.
Cabeza I, Waterhouse T, Sohi S, Rooke JA (2018) Effect of biochar produced from different biomass sources and at different process temperatures on methane production and ammonia concentrations in vitro. Anim Feed Sci Technol 237:1–7. https://doi.org/10.1016/j.anifeedsci.2018.01.003
Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10(3):3
Chiappero J, del Rosario CL, Alderete LGS, Palermo TB, Banchio E (2019) Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind Crops Prod 139:111553. https://doi.org/10.1016/j.indcrop.2019.111553
Dasgupta D, Ghati A, Sarkar A, Sengupta C, Paul G (2015) Application of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Sesbania bispinosa on the growth of chickpea (Cicer arietinum L.). Int J Curr Microbiol Appl Sci 4(5):1033–1042
Ducey TF, Ippolito JA, Cantrell KB, Novak JM, Lentz RD (2013) Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl Soil Ecol 65:65–72. https://doi.org/10.1016/j.apsoil.2013.01.006
Ellouzi H, Ben Hamed K, Asensi-Fabado MA, Müller M, Abdelly C, Munné-Bosch S (2013) Drought and cadmium may be as effective as salinity in conferring subsequent salt stress tolerance in Cakile maritima. Planta 237:1311–1323. https://doi.org/10.1007/s00425-013-1847-7
Fahad S, Hussain S, Saud S, Hassan S, Tanveer M, Ihsan MZ et al (2016) A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol Biochem 103:191–198. https://doi.org/10.1016/j.plaphy.2016.03.001
Friedt W, Tu J, Fu T (2018) Academic and economic importance of Brassica napus rapeseed. In The Brassica napus genome (pp. 1–20). Springer, Cham.
Ghavami N, Alikhani HA, Pourbabaei AA, Besharati H (2017) Effects of two new siderophore-producing rhizobacteria on growth and iron content of maize and canola plants. J Plant Nutr 40:736–746. https://doi.org/10.1080/01904167.2016.1262409
Glodowska M, Schwinghamer T, Husk B, Smith D (2017) Biochar based inoculants improve soybean growth and nodulation. Agric Sci 8(9):1048–1064. https://doi.org/10.4236/as.2017.89076
Gontia-Mishra I, Sapre S, Deshmukh R, Sikdar S, Tiwari S (2020) Microbe-mediated drought tolerance in plants: current developments and future challenges. In: Yadav AN, Singh J, Rastegari AA, Yadav N (eds) Plant microbiomes for sustainable agriculture. Springer, Cham, pp 351–379
Gonzalez L, Gonzalez-Vilar M (2001) Determination of relative water content. Handbook of plant ecophysiology techniques. Springer, Dordrecht, pp 207–212
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Hafez EM, Alsohim AS, Farig M, Omara AED, Rashwan E, Kamara MM (2019) Synergistic effect of biochar and plant growth promoting rhizobacteria on alleviation of water deficit in rice plants under salt-affected soil. Agronomy 9(12):847. https://doi.org/10.3390/agronomy9120847
Haider I, Raza MAS, Iqbal R, Aslam MU, Habib-ur-Rahman M, Raja S, Khan MT, Aslam MM, Waqas M, Ahmad S (2020) Potential effects of biochar application on mitigating the drought stress implications on wheat (Triticum aestivum L.) under various growth stages. J Saudi Chem Soc 24(12):974–981. https://doi.org/10.1016/j.jscs.2020.10.005
Hosseini MS, Samsampour D, Ebrahimi M, Abadía J, Khanahmadi M (2018) Effect of drought stress on growth parameters, osmolyte contents, antioxidant enzymes and glycyrrhizin synthesis in licorice (Glycyrrhiza glabra L.) grown in the field. Phytochemistry 156:124–134. https://doi.org/10.1016/j.phytochem.2018.08.018
Iqbal W, Afridi MZ, Jamal A, Mihoub A, Saeed MF, Székely Á, Pompelli MF (2022) Canola seed priming and its effect on gas exchange, chlorophyll photobleaching, and enzymatic activities in response to salt stress. Sustainability 14(15):9377. https://doi.org/10.3390/su14159377
Karhu K, Mattila T, Bergström I, Regina K (2011) Biochar addition to agricultural soil increased CH4 uptake and water holding capacity–Results from a short-term pilot field study. Agric Ecosyst Environ 140(1–2):309–313. https://doi.org/10.1016/j.agee.2010.12.005
Khalvandi M, Siosemardeh A, Roohi E, Keramati S (2021) Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 7(1):e05908. https://doi.org/10.1016/j.heliyon.2021.e05908
Kumar A, Usmani Z, Kumar V (2017) Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J Environ Manag 190:20–27. https://doi.org/10.1016/j.jenvman.2016.11.060
Kumar M, Giri VP, Pandey S, Gupta A, Patel MK, Bajpai AB, Jenkins S, Siddique KH (2021) Plant-growth-promoting rhizobacteria emerging as an effective bioinoculant to improve the growth, production and stress tolerance of vegetable crops. Int J Mol Sci 22(22):12245. https://doi.org/10.3390/ijms222212245
Lalay G, Ullah S, Ahmed I (2021) Physiological and biochemical responses of Brassica napus L. to drought-induced stress by the application of biochar and Plant Growth Promoting Rhizobacteria. Microsc Res Tech 85(4):1267–1281. https://doi.org/10.1002/jemt.23993
Lee DH, Lee CB (2000) Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci 159:75–85
Liu B, Li H, Li H, Zhang A, Rengel Z (2021) Long-term biochar application promotes rice productivity by regulating root dynamic development and reducing nitrogen leaching. GCB Bioenergy 13(1):257–268. https://doi.org/10.1111/gcbb.12766
Lyu S, Du G, Liu Z, Zhao L, Lyu D (2016) Effects of biochar on photosystem function and activities of protective enzymes in Pyrus ussuriensisi. Maxim under drought stress. Acta Physiol Plant 38(9):1–10. https://doi.org/10.1007/s11738-016-2236-1
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444(2):139–158. https://doi.org/10.1016/j.abb.2005.10.018
Malik L, Sanaullah M, Mahmood F, Hussain S, Siddique MH, Anwar F, Shahzad T (2022) Unlocking the potential of co-applied biochar and plant growth-promoting rhizobacteria (PGPR) for sustainable agriculture under stress conditions. Chem Biol Technol Agric 9(1):1–29
Marcińska I, Czyczyło-Mysza I, Skrzypek E, Filek M, Grzesiak S, Grzesiak MT, Janowiak F, Hura T, Dziurka M, Dziurka K (2013) Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol Plant 35:451–461. https://doi.org/10.1007/s11738-012-1088-6
Mihoub A, Amin AE, Motaghian HR, Saeed MF, Naeem A (2022) Citric acid (CA)–modified biochar improved available phosphorus concentration and its half-life in a P-fertilized calcareous sandy soil. J Soil Sci Plant Nutr 22:465–474. https://doi.org/10.1007/s42729-021-00662-2
Mitra RM, Long SR (2004) Plant and bacterial symbiotic mutants define three transcriptionally distinct stages in the development of the Medicago truncatula/Sinorhizobium meliloti symbiosis. Plant Physiol 134:595–604. https://doi.org/10.1104/pp.103.031518
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 97:30–39. https://doi.org/10.1016/j.envexpbot.2013.09.014
Nawaz F, Rafeeq R, Majeed S, Ismail MS, Ahsan M, Ahmad KS, Akram A, Haider G (2023) Biochar amendment in combination with endophytic bacteria stimulates photosynthetic activity and antioxidant enzymes to improve soybean yield under drought stress. J Soil Sci Plant Nutr 23(1):746–760. https://doi.org/10.1007/s42729-022-01079-1
Ning Y, Xiao Z, Weinmann M, Li Z (2019) Phosphate uptake is correlated with the root length of celery plants following the association between arbuscular mycorrhizal fungi, Pseudomonas sp. and biochar with different phosphate fertilization levels. Agronomy 9(12):824. https://doi.org/10.3390/agronomy9120824
Paneque M, Jose MJ, Franco-Navarro JD, Colmenero-Flores JM, Knicker H (2016) Effect of biochar amendment on morphology, productivity and water relations of sunflower plants under non-irrigation conditions. CATENA 147:280–287. https://doi.org/10.1016/j.catena.2016.07.037
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Sainju UM, Allen BL, Lenssen AW, Ghimire RP (2017) Root biomass, root/shoot ratio, and soil water content under perennial grasses with different nitrogen rates. Field Crops Res 210:183–191. https://doi.org/10.1016/j.fcr.2017.05.029
Sangeetha D (2012) Survival of plant growth promoting bacterial inoculants in different carrier materials. Int J Pharm Biol Arch 3(1):170–178
Sattar A, Sher A, Ijaz M, Irfan M, Butt M, Abbas T, Hussain S, Abbas A, Ullah MS, Cheema MA (2019) Biochar application improves the drought tolerance in maize seedlings. Phyton 88(4):379
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-Wajid HH, Battaglia ML (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10(2):259. https://doi.org/10.3390/plants10020259
Seybold CA, Mersie W, Huang J, McNamee C (2002) Soil redox, pH, temperature, and water-table patterns of a freshwater tidal wetland. Wetlands 22(1):149–158. https://doi.org/10.1672/0277-5212(2002)022[0149:SRPTAW]2.0.CO;2
Shaffique S, Khan MA, Imran M, Kang SM, Park YS, Wani SH, Lee IJ (2022) Research progress in the field of microbial mitigation of drought stress in plants. Front Plant Sci 13:870626. https://doi.org/10.3389/fpls.2022.870626
Shah AN, Yang G, Tanveer M, Iqbal J (2017) Leaf gas exchange, source–sink relationship, and growth response of cotton to the interactive effects of nitrogen rate and planting density. Acta Physiol Plant 39:119. https://doi.org/10.1007/s11738-017-2402-0
Ullah A, Bano A, Khan N (2021) Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2021.618092
Wang Y, Li W, Du B, Li H (2021a) Effect of biochar applied with plant growth-promoting rhizobacteria (PGPR) on soil microbial community composition and nitrogen utilization in tomato. Pedosphere 31(6):872–881. https://doi.org/10.1016/S1002-0160(21)60030-9
Wang C, Wang H, Li Y, Li Q, Yan W, Zhang Y, Wu Z, Zhou Q (2021b) Plant growth-promoting rhizobacteria isolation from rhizosphere of submerged macrophytes and their growth-promoting effect on Vallisneria natans under high sediment organic matter load. Microb Biotechnol 14(2):726–736. https://doi.org/10.1111/1751-7915.13756
Wang W, Vignani R, Scali M, Cresti M (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. J Electrophor 27:2782–2786. https://doi.org/10.1002/elps.200500722
Wang X, Song D, Liang G, Zhang Q, Ai C, Zhou W (2015) Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl Soil Ecol 96:265–272. https://doi.org/10.1016/j.apsoil.2015.08.018
Wu S, Hu C, Tan Q, Nie Z, Sun X (2014) Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (Triticum aestivum) under drought stress. Plant Physiol Biochem 83:365–374. https://doi.org/10.1016/j.plaphy.2014.08.022
Zhang D, Wang Y, Tang X, Zhang A, Li H, Rengel Z (2019) Early priority effects of occupying a nutrient patch do not influence final maize growth in intensive cropping systems. Plant Soil 442(1):285–298. https://doi.org/10.1007/s11104-019-04155-1
Acknowledgements
We are thankful to the Researchers Supporting Project Number (RSPD2024R751), King Saud University, Riyadh, Saudi Arabia.
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement. Researchers Supporting Project number (RSPD2024R751), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
G-Lalay: contributed to Data curation, Formal analysis, Investigation, Visualization, and Writing and original draft preparation. SU: contributed to Conceptualization, Methodology, Visualization, Supervision, and Writing, reviewing, and editing of the manuscript. SS: contributed to Conceptualization, Investigation, and Formal analysis. AJ: contributed to Conceptualization, Data curation, Formal analysis, Supervision, and Writing, reviewing, and editing of the manuscript. MFS: contributed to Conceptualization, Data curation, Formal analysis, Data curation, Visualization, Supervision, and Writing, reviewing, and editing of the manuscript. AM: contributed to Data curation, Formal analysis, and Writing, reviewing, and editing of the manuscript. AZ: Investigation, Methodology, Visualization, and Writing and original draft preparation. IA: Data curation, Formal analysis, Investigation, Supervision, and Writing, reviewing, and editing of the manuscript. MF (Seleiman): Data curation, Formal analysis, Supervision, and Writing, reviewing, and editing of the manuscript. RM: Visualization, Supervision, and Writing, reviewing, and editing of the manuscript. ER: Data curation, Formal analysis, Supervision, and Writing, reviewing, and editing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Ravinder Kumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gul-Lalay, Ullah, S., Shah, S. et al. Combined Effect of Biochar and Plant Growth-Promoting Rhizbacteria on Physiological Responses of Canola (Brassica napus L.) Subjected to Drought Stress. J Plant Growth Regul 43, 1814–1832 (2024). https://doi.org/10.1007/s00344-023-11219-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11219-1