Abstract
Drought is a major abiotic stress, affecting the metabolism, growth, and productivity of plants worldwide. Therefore, this study aimed/hypothesized to investigate the ameliorative effects of biochar and rhizobacteria in drought-damaged Brassica napus L. genotypes. The plants were divided into two groups based on the drought stress employment (15 days and 30 days). Both groups were then treated with PGPR, biochar, and their co-application, and the samples were taken from shoots and roots of both genotypes. Our results revealed that the drought resulted in a substantial decline in total flavonoids, phenolics, proteins, peroxidase (POD), superoxide dismutase (SOD), glutathione reductase (GR) as well as nutrient uptake in shoots and roots of both the studied genotypes. Contrarily, sugar and glycine betaine (GB) contents increased in both shoots and roots under drought stress conditions. However, the plants with co-application of biochar and PGPR showed better improvement of nutrient uptake, leaf relative water content (RWC), and growth parameters compared to drought-stressed, control, and the plants with sole biochar and PGPR application. In addition, the co-application of PGPR and biochar produced higher levels of sugar, proteins, flavonoids, phenolic compounds, and enzymatic activities (POD, SOD, GR, and dehydroascorbate reductase (DHAR)) than those that were not treated with biochar and PGPR or treated solely. Relative to Westar genotype, the Punjab sarson showed higher drought tolerance. In conclusion, we suggest that the co-application of biochar and PGPR can be an effective strategy for overcoming the drought-induced damage in plants.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plants experience several environmental stresses which can affect their physiological function at any developmental stage. These stress conditions could be salinity, drought, cold, or contamination of toxic compounds due to natural or anthropogenic activities (Ozturk et al., 2021). Water deficit or drought is among the most severe abiotic stresses which hinder the metabolism, growth, and development, as well as productivity of a wide range of economic plants, particularly in arid and semi-arid regions (Kour et al., 2020; Zhang et al., 2022). The depletion of already present water resources and the food demand of an exponentially growing population have gained serious attention globally. Further, industrialization and urbanization also resulted in land shrinkage for growing more cereal crops to feed the growing population (Seleiman et al., 2021). Interestingly, around 80–95% of water is present in the fresh biomass of a plant body required for various metabolic processes (Okorie et al., 2019). Climate change can lead to more frequent and severe droughts shortly converting agricultural lands into barren lands. In addition, the excessive use of fertilizers and improper agricultural practices can cause soil degradation and contamination of available water resources (Shivaraj et al., 2021). The rapid changes in climatic conditions increase the severity of abiotic stress including drought leading to hindrance in germination, seedlings establishment, growth, and metabolism of plants (Grant, 2012; Jaleel et al., 2009). The effect of climate change is also very influential in the region where the studied was designed since the impact of drought stress was also investigated in the earlier studies as well, where the physiological and biochemical processes involved in photosynthesis, chlorophyll synthesis, nutrient metabolism, ion uptake and translocation, respiration, and carbohydrates metabolism are all impacted by drought stress, which inhibits plant growth (Jaleel et al., 2009). Moreover, drought stress results in approximately 9–10% reduction in cereal crop production and acts as a major challenge to world food security to enhance crop productivity and yield worldwide (Lesk et al., 2016). Drought stress is a global problem, but scant attention was paid to this alarming challenge to lessen its impacts on the agriculture sector.
Drought stress can result in the dysfunction and modification of many physiological and biochemical processes in plants such as photosynthesis, transpiration, respiration, enzymatic activities, and hormonal signaling (Gao et al., 2019). Further, drought stress causes stomatal closure and a decrease in CO2 concentrations and photosynthesis, which may result in death and low productivity under severe stress conditions (Rashwan et al., 2020; Siddiqui et al., 2015). Additionally, water deficits negatively affect water uptake, ion transport, carbohydrate digestion, and nutrient absorption (Abdelaal, 2015). Additionally, the production of reactive oxygen species (ROS) including superoxide (O2.−) and hydrogen peroxide (H2O2) is considered one of the early plant responses. The damage caused by hyperaccumulated ROS to membrane and chlorophyll structure adversely affects the optimum metabolism of the plant (Oraee and Tehranifar, 2020). Drought-induced ROS are eliminated by plants’ antioxidative defense system comprising of enzymatic and non-enzymatic antioxidants. These potent antioxidants such as catalase (CAT), ascorbate peroxidase (APX), and superoxide dismutase (SOD) constitute a few of the major enzymes capable of detoxifying ROS (Begum et al., 2020). Currently, the application of genetic engineering, traditional breeding, and the use of water-saving management, are some of the strategies used to increase plant tolerance to drought stress. Due to their highly technical and laborious nature, these methods are difficult to implement in practice. Plant growth-promoting rhizobacteria (PGPR) can be used as an alternative for growing plants under dry conditions.
Abiotic stresses, such as drought, can be successfully mitigated in plants by plant growth-promoting bacteria (PGPB), which are naturally occurring bacteria in the rhizospheric soils. In the rhizosphere, PGPR microorganisms are found as an endophyte as well as at the root surface, contributing to plant growth under normal, or stress conditions (Bashan, 1998; Cassan et al., 2009). Common plant growth-promoting (PGP) genera/species are Bacillus, Rhizobium, Azotobacter, Staphylococcus, Azospirillum, Pseudomonas, Paenibacillus, Klebsiella, and Serratia. They are best known for their ability to increase growth characteristics and productivity under natural environments through nitrogen fixation, amino acid production, and phytohormone production as well as nutrient availability to plants under stressful conditions (García et al., 2017). PGP microbes, for instance, have been reported to ameliorate drought stress in several crops, such as maize (García et al., 2017) and rice (Gusain et al., 2014), primarily through increased nutrient mobilization. Therefore, PGPBs are considered to play a key role in facilitating sustainable agriculture. They are a cheap and eco-friendly alternative method for enhancing the metabolism, development, and overall growth of plants under drought conditions. Additionally, improving the soil moisture-holding capacity is another key factor that can enhance crop productivity during drought conditions. A soil’s ability to hold nutrients and water is determined by its management strategy (Baronti et al., 2014). However, the knowledge about the use of PGPR in the alleviation of drought stress was not fully explored.
In addition to the use of PGPR, biochar application plays a significant role in organic amendment by reducing the effects of drought stress on plants by increasing the availability of nutrients and the soil’s ability to store water (Qayyum et al., 2015). Biochar is a carbon-rich organic compound produced through the process of pyrolysis (Singh et al., 2010). Although biochar could alter soil properties, its ability to do so depends greatly on several factors, such as its physical and chemical characteristics, surface area, feedstock type, pyrolysis conditions, as well as rate and method of application (Baronti et al., 2014). According to a recent study, applying biochar to cowpea plants can reduce the effects of drought stress by increasing biomass output, nutrient uptake, and antioxidant responses (Farooq et al., 2021). Nevertheless, a little is known about the combined effects and underlying mechanisms of PGPR and biochar application to remediate drought stress in plants.
Brassica napus L. (B. napus) also known as Rapeseed, or canola, is one of the most important oil crops in the world. It ranks second among all oil crops with a value of US $41 billion annually. The plants are used as a food crop for livestock and as oil source (Elahi et al., 2015). Pakistan grows this crop over 62.02 thousand hectares with a total annual production of 102.0 thousand tons. Yet, rapeseed requires a greater amount of water to grow and develop than other crops (corn, wheat). The shortage of water at the seedling level will hinder the growth of leaves and roots, resulting in reduced crop yields. Therefore, we aimed to examine whether biochar amendment and PGPR application could potentially improve the drought tolerance of B. napus by regulating its morphological and physio-biochemical attributes and nutritional uptake in a time course experiment.
2 Materials and methods
2.1 Preparation of biochar
A specially designed pyrolyzer was used to prepare biochar from Morus alba L. wood. As mentioned in our previous article, the wood was air-dried before being pyrolyzed at high temperatures (550–600 °C) in a pyrolyzer under partial anaerobic conditions (Lalay et al., 2022). It was continued until the desired level (50 wt.%) of biochar was achieved, following which the biochar was powdered and mixed (25% v/v) at a 1:3 ratio with sand and soil (Fahad et al., 2016). In our previous article, we have studied the SEM, EDX, and physicochemical characteristics of biochar (Lalay et al., 2022).
2.2 Site description
During the growing season of canola (2019), the experiment was conducted in a natural habitat at the Department of Botany of the University of Peshawar, Pakistan (34° 1′ 33.3012″ N and 71° 33′ 36.4860″ E) (Lalay et al., 2022).
2.3 Seeds and PGPRs Collection and seed sterilization and inoculation with strains
Seeds of two genotypes of the canola plant, Punjab sarson, and westar were obtained from the National Agricultural Center Islamabad, Pakistan (NARC). The surface sterilization of vigorous seeds was achieved by using ethanol (70%) for two minutes followed by washing with distilled water (Barampuram et al., 2014). In this study, one strain of PGPRs Staphylococcus sp was obtained from National Agricultural Research Center Islamabad, Pakistan. The sterilized seeds of canola were inoculated for 30 min in broth cultures (> 1*107 cells/mL) of PGPR (Dasgupta et al., 2015).
2.4 Experimental design
A 3:1 mixture of soil and sand was used in the pots, along with farmyard manure. The following soil properties were analyzed in soil elemental analysis: organic matter (0.50%), nitrogen (N) (0.060%), phosphorus (P) (4.7 mg/kg), potassium (K) (79 mg/kg), calcium (Ca) (45 mg/kg), magnesium (Mg) (51 mg/kg), saturation percentage (39%), and water-holding capacity (1.3). Five replicates of inoculated seeds of both canola genotypes were planted (Seybold et al., 2002). A complete block design experiment was conducted, with pots kept at a short distance from one another for rain protection and better ventilation. This experiment was consisted of 5 treatments such as control (C), drought stress (D), drought stress + PGPR (D + P), drought stress + biochar (D + B), and drought stress + combined biochar and PGPR (D + B + P) and was conducted for 15 and 30 days, respectively. At the vegetative stage, the plants were exposed to drought stress for 15 and 30 days following germination. All the plants were normally irrigated up to a proper germination period. Afterward control plants were irrigated normally, while the counting of drought stress was measured from that day when normal plants were irrigated. At the end, plants were harvested to measure the growth characteristics and fresh root and shoot material were stored in the refrigerator at -20℃ for the determination of physio-biochemical attributes.
2.5 Plant and soil elemental analysis
For the determination of elemental analysis, dried samples were integrated into nitric acid (70%-vol/vol) for four hours at 90 °C in a water bath and, after cooling, added with 1–2 ml of hydrogen peroxide, as reported (Seybold et al., 2002). The concentration of different elements was calculated using atomic absorption spectrophotometry.
2.6 Morphology of B. napus leaves trichomes through SEM
Freshly hydrated leaves were sampled and cut into 3-8-mm pieces. To prevent unnecessary dehydration, the leaves pieces were fixed in epoxy glue, cooled up to 5 ºC, and then fixed on a scanning electron microscope (SEM). These samples were subjected to leaf studies following standard protocols (Ensikat et al., 2010).
2.7 Germination and vegetative attributes
2.7.1 Emergence energy (EE)
Emergence energy was determined according to the following equation (Basra et al., 2005).
2.7.2 Leaf area ratio (LAR)
LAR was calculated according to the following equation (Shah et al., 2017).
2.7.3 Leaf area index (LAI)
LAI was calculated using the following equation (Shah et al., 2017).
2.7.4 Percent moisture content of plants and soil (%MC)
Percent MC of plants and soil was detected using the following formula (Basra et al., 2005).
2.7.5 Percent relative water content (RWC)
Percent RWC was detected by using the following equation (González & González-Vilar, 2001).
2.8 Determination of physiological attributes
2.8.1 Protein extraction
Plant protein content was determined for shoots and roots according to the standard method (Wang et al., 2006). 0.5 g plant material was homogenized in phosphate buffer and centrifuged. In the filtrate, CuSO4.5H2O was added, followed by a Folin phenol reagent, which was incubated for 10 min, before the absorbance of each sample was measured at 650 nm on a spectrophotometer. A standard curve was used to determine the protein concentration and was expressed as mg/g F.W.
2.8.2 Total phenolics content (TPC)
The TPC was determined following standard procedure (Baydar et al., 2004). Two grams of dried root and shoot samples was crushed in 90% methanol and subsequently shaken for one hour. Following centrifugation, a diluted solution of Folin–Ciocalteu reagent (4:1) was added. After adding 10% Na2CO3, the mixture was kept for one hour in the dark, and then the absorbance was measured using a spectrophotometer at 760 nm. Using the standard curve, the phenolic content of the samples was determined and expressed as mg/g DW.
2.8.3 Sugar content analysis
The measurement of sugar was taken by grinding 0.5 g sample of fresh shoots and roots in distilled water and centrifuging the samples (Marcińska et al., 2013). Phenol 80% (w/v) was then added to the samples, and after incubation, the samples’ absorbance was measured at 420 nm. A standard curve was constructed using glucose to determine the concentration of the samples, which was expressed as mM/g fresh weight.
2.8.4 Total flavonoid content
Flavonoid content was determined according to aluminum chloride colorimetric method (Chang et al., 2002). The dried plant samples (0.1 g) diluted in 0.5ml of 1:10 g ml− l methanol were mixed with 1.5ml of methanol, 0.1ml of potassium acetate, and 0.1 ml of 10% AlCl3, and 2.8 ml of H2O. The solution was kept at room temperature for 30 min, and the density was determined at 415 nm using a spectrophotometer.
2.8.5 Superoxide dismutase (SOD) activity
The SOD activity was calculated according to the standard methodology (Ellouzi et al., 2013). We homogenized and centrifuged plant material (0.5 g) in phosphate buffer. Then, the 24 µl of nitro-blue-tetrazolium, riboflavin, and methionine was combined to obtain a filtrate of 0.1 ml. After 3 min, the final optical density (OD) was noted at 560 nm. SOD concentration was determined using a standard curve and represented as unit/g F.W.
2.8.6 Peroxidase (POD) activity
The POD activity was determined following a standard method (Asthir et al., 2009). Plant samples (0.5 g) were chopped in morpholine ethane sulfonic acids (MES), and the supernatant was collected after centrifugation. Phenyl diamine, MES, and 30% H2O2 were added to the supernatant. An optical density of 485 nm was recorded. Based on the standard curve, the concentration of an unknown sample was determined and expressed in units/g F.W.
2.8.7 Estimation of glycine betaine (GB)
The GB was measured following the standard procedure (Grieve and Grattan, 1983). Root and shoot samples (0.5 g) were mechanically chopped and mixed with deionized water for 24 h at 25 °C. After filtration, the filtrate was diluted with 2NHSO4 (1:1). Then, the filtrate was diluted with 2NHSO4 (1:1) and stored the aliquots in centrifuge tubes and chilled them in ice water for one hour before adding KI-I2 and agitating them with a vortex. The filtrate was articulated in a fine glass tube by keeping the tubes at 4 °C for 16 h. Thereafter, the periodide crystals were liquefied in 9 mL of 1,2-dichloroethane. Using GB as a reference, the absorbance was measured after two hours and expressed as µg/g.
2.8.8 Dehydroascorbate reductase (DHAR) activity
For the determination of DHAR activity (Nakano and Asada, 1981), fresh shoot and root material (0.7 g) were quantified in potassium phosphate buffer (pH 7.8) containing 2 mM mercaptoethanol, 1 mM EDTA, and 8% (v/v) glycerol. The mixture was centrifuged, and the filtrate was collected. The reaction mixture contained 90 mM potassium phosphate buffer (pH 7.0), 12.5 mM glutathione reductase (GSH), 0.178 mM EDTA, 0.1 mM enzyme extract, and 1 mM dehydroascorbate (DHA). We measured DHAR activity using the rise in absorbance at 265 nm. The results were recorded using an extinction coefficient of 7.0 mM− 1 cm− 1. According to the enzyme assay, one unit is defined as one nmol DHA reduced per minute. Based on the standard curve, the concentration of an unknown sample was determined and expressed as mol/min/g F.W.
2.8.9 Glutathione reductase (GR) activity
For the determination of GR fresh shoot and root samples (0.7 g) were homogenized in a solution of potassium phosphate buffer solution and 2mM EDTA, and the mixture was centrifuged (Lee and Lee, 2000). A reaction mixture (GSSG) was prepared by adding 2 mM EDTA, 0.15 mM NADPH, 50 mM potassium phosphate buffer (pH 7.8), 0.2 ml enzyme extract, and 0.5 mM oxidized glutathione. NADPH was added to start the reaction. A reduction in absorbance at 340 nm was used to determine glutathione reductase activity with an extinction value of 6.2 Mm− 1 cm− 1. The unit of enzyme activity is 1 nmol NADPH oxidized per minute. Using the standard curve, the glutathione reductase concentration was determined and expressed as a unit/ml.
2.9 Statistical analysis
The experiment was repeated three times, and each treatment was consisted of minimum three replicates. The statistical analysis was performed using SigmaPlot 14.5 software. A one-way ANOVA (analysis of variance) was conducted to find significant difference among different treatments. The data values were presented as mean ± standard error (SE), and significant differences among treatments were detected using Tukey’s test at P ≤ 0.05.
3 Results
3.1 Interactive effect of PGPR and biochar on growth attributes of drought-stressed Brassica napus L. seedlings
The growth attributes including emergence energy (EE), percent moisture content (%MC), leaf area ratio (LAR), relative water content (RWC), and leaf area index (LAI) were minimum under both water-deficit conditions (15 and 30 days). However, the studied growth parameters were positively impacted using both biochar and PGPR in both canola genotypes (Table S1). Briefly, Punjab sarson plants showed 1.4- and 1-fold higher EE in combined treatment of biochar and PGPR as compared to drought-induced plants after relevant 15 and 30 days, respectively, while plants with co-application of biochar and PGPR exhibited 1- and 1.7-fold in %MC for Punjab sarson and 1.3- and 2.3-fold increase in wester plants as compared to control plants after respective time points. Concurrently, other vegetative attributes were recorded such as LAR 2.7- & 3-fold higher in Punjab sarson and 1-fold higher in wester genotype, RWC 2.5- 3-fold higher in Punjab sarson and 1.7- and 1.3-fold higher in wester plants, and LAI 5.5 & 10-fold higher in Punjab sarson and 1.3 & 16-fold higher in wester plants in combined treatment of both biochar and PGPR as compared to their respective drought-induced plants after 15 and 30 days, respectively (Table S1).
3.2 SEM micrograph of trichome in drought-stressed Brassica napus L.
Results indicated that micrographs of trichomes obtained from scanning electron microscopy (SEM) revealed the simple structure of trichomes (Fig. 1). According to these findings, drought improved the length and density of both genotypes to conserve water under stress conditions, which were then positively improved by applying the co-application of biochar and PGPR.
3.3 Ameliorative effects of PGPR and biochar on the nutritional profile of drought-stressed Brassica napus L
The elemental analysis of both genotypes exhibited obvious differences in response to biochar and PGPR treatments after drought stress. The elements such as N, P, K, Ca, and Mg were maximum in both genotypes under co-application of biochar and PGPR, while they were lowest in the 15- and 30-day drought treatments in both rapeseed genotypes (Table 1). More specifically, the Punjab sarson plants treated with both biochar and PGPR showed an increase in N (4- and 6-fold), P (2- and 3.6-fold), K (1.4 and 1.7-fold), Ca (1- and 3-fold), and Mg (1.6- and 2.6-fold) as compared to drought-induced plants after 15 and 30 days, respectively. Similarly, wester plants treated with combined biochar and PGPR exhibited an increase of (4- and 15-fold) in N, (2- and 4.5-fold) in P, (1- and 2-fold) in K, (1.4- and 3.4-fold) in Ca, and (1.4- and 2.5-fold) in Mg than drought-induced plants after exposure to 15 and 30 days, respectively. In parallel, sole application of PGPR and biochar amendment also displayed significant improvement of these essential nutrients in both canola genotypes as compared to drought-induced plants (Table 1).
3.4 Combined application of PGPR and biochar on protein and sugar contents of Brassica napus L. subjected to drought stress
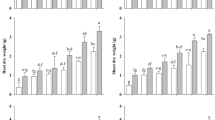
The protein content of the shoot was significantly reduced (46% and 67%) under drought stress as compared to control plants after 15 and 30 days, respectively. However, the highest protein content (2.7-fold) was observed co-application of biochar and PGPR. Similarly, the sole application of biochar and PGPR also enhanced protein content and remarkably recovered plants from drought stress (Fig. 2a). Likewise, the same trend was shown in the case of protein content in the roots. The biochar amendment and PGPR inoculum significantly increased the protein content (2.2- and 3.2-fold) of root as compared to drought-stressed plants (15 and 30 days), respectively (Fig. 2b). In addition, 27% and 100% reduction in protein contents was recorded under drought stress as compared to control plants after respective 15 and 30 days. Nevertheless, no significant difference was observed between both Punjab sarson and wester varieties.
Effects of PGPR application and biochar amendment on the protein and sugar contents in the shoots and roots of both Punjab sarson and wester varieties of B. napus plants under induced drought stress in 15- and 30-day treatments. a Protein content in shoot. b Protein content in root. c Sugar content in shoot, and d sugar content in root. Data are presented as mean ± SE. Different small letters denote the significant difference using a one-way ANOVA based on Tukey’s test if P ≤ 0.05 for respective time points
On the contrary, the sugar content in the shoot of B. napus exhibited a significant increase (2.5- and 3.2-fold) under drought stress than control plants after relevant 15 and 30 days of treatments. Interestingly, no significant difference was found among all other treatments (Fig. 2c). Similarly, the sugar content in the root also displayed a significant rise (2.6- and 3.2-fold) under drought as compared to control plants after 15 and 30 days, respectively. Surprisingly, the PGPR inoculation and biochar amendment did not show any increase in the sugar content neither in individual treatments nor in combined treatments (Fig. 2d). Nonetheless, in wester variety, the higher sugar content was observed after 15 days as compared to Punjab sarson genotype, but after 30 days, no significant difference was reported between both B. napus genotypes.
3.5 PGPR- and biochar-induced changes in phenolics, flavonoids, and GB contents of drought-stressed Brassica napus L.
The phenolic content was determined in the shoots of both B. napus genotypes (more than 2-fold) and was found higher in wester plants as compared to Punjab sarson plants with combined treatment of biochar and PGPR applications after 15 days (Fig. 3a). However, plants under drought stress reduced phenolic contents under both time points (15 and 30 days) as compared to other treatments. In parallel, the phenolic content in the roots was significantly enhanced (more than 2-fold) in all treated plants of both genotypes except plants under drought stress followed by 15 and 30 days, respectively, while plants under drought stress displayed 49% and 85% reduction in phenolic contents in Punjab sarson and wester plants as compared to their respective control plants after 15 days (Fig. 3b).
Effects of PGPR application and biochar amendment on the phenolic, flavonoid, and glycine betaine (GB) contents in the shoots and roots of both Punjab sarson and wester varieties of B. napus plants under induced drought stress in 15- and 30-day treatments. a Phenolic content in shoot. b Phenolic content in root. c Flavonoid content in shoot. d Flavonoid content in root. e GB content in shoot, and f GB content in root. Data are presented as mean ± SE. Different small letters denote the significant difference using a one-way ANOVA based on Tukey’s test if P ≤ 0.05 for respective time points
Similarly, the flavonoid content also exhibited a similar trend to phenolic compounds. Higher flavonoid contents (1.8- and 2.5-fold) were recorded in shoot under combined treatment of biochar and PGPR application as compared to drought treatments at both time points, respectively (Fig. 3c). Nevertheless, no significant difference was found between both genotypes under both time points. Likewise, the flavonoid content was observed significantly higher (more than 2-fold) in roots treated with both biochar and PGPR as compared to plants under drought stress after the proposed time periods. Moreover, drought-stressed plants showed 53% and 76% inhibition in flavonoid contents than control plants after 15 and 30 days, respectively (Fig. 3d).
Comparably, the GB contents were significantly elevated (3-fold) under drought stress as compared to other treatments, especially after 30 days (Fig. 3e). Intriguingly, Punjab sarson plants showed substantially higher GB levels as compared to wester plants after 30 days. But both genotypes did not show any significant difference among all other treatments except drought treatment. At the same time, the roots of B. napus exhibited the highest GB content (2.3-fold) under drought stress in both genotypes, especially after 30 days (Fig. 3f). Nonetheless, no significant difference was found between both canola genotypes.
3.6 Modifications of antioxidant activities in drought-stressed Brassica napus L. by combined application of PGPR and biochar
The drought stress significantly decreased SOD activity (2.5- and 1.5-fold) in the roots of Punjab sarson and wester plants as compared to relevant control plants after 15 days. In contrast, the application of PGPR and biochar significantly enhanced (2-fold) SOD activity in their sole treatments and combined treatments than drought-stressed plants in both time points (Fig. 4a). Concurrently, the SOD activity in the roots of rapeseed was observed higher in PGPR-supplied treatments (2- and 1.5-fold), biochar-amended treatments (2.6- and 1.6-fold), and co-application (3.4- and 3.1-fold) as compared to drought-stressed treatments (15 and 30 days), respectively, while drought-stressed Punjab sarson and wester genotypes showed 56% and 36% decline in SOD activity as compared to their respective control plants. However, no significant difference in SOD activity was noticed between Punjab sarson and wester varieties (Fig. 4b).
Effects of PGPR application and biochar amendment on the SOD and POD activities in the shoots and roots of both Punjab sarson and wester varieties of B. napus plants under induced drought stress in 15- and 30-day treatments. a SOD activity in shoot, b SOD activity in root, c POD activity in shoot, and d POD activity in root. Data are presented as mean ± SE. Different small letters denote the significant difference using a one-way ANOVA based on Tukey’s test if P ≤ 0.05 for respective time points
On the other hand, the POD activity in the shoot was observed 2- and 1.3-fold lower in drought treatments as compared to relevant control treatments after 15 and 30 days, respectively. Nevertheless, the POD activity was significantly elevated the plants supplemented with PGPR and amended with biochar as compared to the plants under drought stress followed by respective 15- and 30-day treatments (Fig. 4c). Besides, the combined treatment of both PGPR and biochar significantly enhanced POD activity (7.6- and 4-fold) in both canola varieties at both proposed time periods. Additionally, Punjab sarson variety also showed higher POD activity in shoots as compared to wester plants. Comparably, the POD activity in the roots also displayed a similar trend. The combined treatment of biochar and PGPR exhibited 5.7- and 6.6-fold increment in POD activity in the roots of canola plants than drought-stressed plants after respective 15 and 30 days (Fig. 4d). Further, plants under drought stress showed 52% reduction in POD activity than control plants after 15 days. However, in the case of POD activity in roots, no significant difference was observed between both canola genotypes.
The GR activity in the shoots of B. napus was found higher when biochar amendment or PGPR was applied separately as compared to control plants and plants under drought stress on both varieties under 15- and 30-day drought treatments (Fig. 5a). Intriguingly, the combined application of biochar and PGPR significantly increased GR activity in wester plants as compared Punjab sarson plants. Moreover, wester plants with combined treatment of biochar and PGPR showed 2.15- and 1.89-fold higher GR activity than drought-stressed treatments at 15 and 30 days, respectively. Similarly, the GR activity in the canola roots was found higher in all applications than drought-stressed plants at both time intervals (Fig. 5b). In particular, wester plants treated with co-application of biochar and PGPR exhibited 3.3-fold more GR activity in roots as compared to drought-stressed plants.
Effects of PGPR application and biochar amendment on the GR and DHAR activities in the shoots and roots of both Punjab sarson and wester varieties of B. napus plants under induced drought stress in 15- and 30-day treatments. a GR activity in shoot. b GR activity in root. c DHAR activity in shoot, and D). DHAR activity in root. Data are presented as mean ± SE. Different small letters denote the significant difference using a one-way ANOVA based on Tukey’s test if P ≤ 0.05 for respective time points
Likewise, the activity of DHAR enzyme was observed to be significantly greater (4-fold) in the shoots of wester plants as compared to control and drought-stressed plants under 15- and 30-day treatments (Fig. 5c). However, sole applications of PGPR and biochar did not exhibit any remarkable difference in both genotypes or among the treatments. In parallel, the DHAR activity of roots was significantly greater in combined treatments of biochar and PGPR compared to untreated and drought-treated plants (15 days), while the application of biochar and PGPR separately and together showed a significant increase (6-fold) in DHAR activity as compared to respective drought and control treatments after 15 and 30 days (Fig. 5d). Nevertheless, no significant difference was recorded between Punjab sarson and wester canola varieties.
3.7 Relationship between the studied parameters
The correlation revealed a positive correlation between RWC and protein, SOD, POD, N, P, and Ca in shoots which were employed under 15 and 30 days of drought stress, while the negative correlation was observed between RWC and sugar and GB under 15 days of drought stress. In the case of roots, the protein showed a strong positive relationship with phenol, flavonoid, SOD, POD, GTR, DHAR, P, and K and almost the similar results were seen regarding phenols under 15 days of drought stress, while a negative correlation was exhibited by GB with all the other studied parameters except sugar. In addition, almost the similar results were shown under 30 days of drought with some exceptions (Fig. 6).
Relationship between the studied parameters. Red represents positive correlation, and blue represents negative correlation (*P ≤ 0.05, 0.01, 0.001). The intensity of color represents the significance of a variable. The SOD, POD, GB, GTR, DHAR, N, P, K, Ca, and Mg refer to superoxide dismutase, peroxidase, glycine betaine, glutathione reductase, dehydroascorbate reductase, nitrogen, phosphorus, calcium, and magnesium, respectively
4 Discussion
Drought stress is one of the most challenging issues worldwide in terms of crop productivity. It limits both crop cultivation area and productivity (Rawat et al., 2015). Plant growth-promoting rhizobacterial (PGPR) microbes have been used for a long time in agricultural production systems, and growing evidence has shown that these microbes can improve plants’ resistance to adverse environmental stresses (Lizana et al., 2006; Vile et al., 2005). Furthermore, improving soil moisture-holding capacity by using biochar is another option available to enhance crop productivity during drought stress (Qayyum et al., 2015). In the selected genotypes of B. napus, however, the physiological significance of biochar and PGPR in terms of drought tolerance has not yet been explored. Therefore, this study investigated the ameliorative effects of PGPR and biochar amendment on drought-stressed two B. napus L. genotypes.
In the current study, the drought stress declined the growth characteristics of both genotypes (Table 1). Generally, drought stress caused a reduction in tissue water content and turgor pressure within the cell, which ultimately reduces the ability of the cell to grow and divide (Shao et al., 2007). However, the growth attributes were significantly enhanced by using PGPR and biochar in drought conditions, which is consistent with the previous findings (Khan & Bano, 2019; Naseem et al., 2018; Nawaz et al., 2021). However, it is a great challenge for plants under drought conditions to maintain a high-water potential since it is required for normal growth and development. The RWC is regarded as a good indicator of plant hydration capacity and community diversity (Parker et al., 1982). The relation between leaf water availability and stomatal conductance is well understood, and even in drought stress conditions. The reduction in RWC is known to lead to stomatal closure, which limits photosynthesis (Lawlor, 2002). We found that drought stress significantly reduced the leaf RWC in both genotypes, in comparison with the control (Oliveira et al., 2014). However, the application of PGPR and biochar significantly improved the leaf RWC as an adaptive strategy to maintain a higher RWC to cope with the stress condition (Agami et al., 2016; Keshavarz Afshar et al., 2016).
Our results revealed that the higher concentrations of N, P, K+, Ca2+, and Mg2+ were found in biochar and PGPR treatments relative to drought-stressed plants. There is a strong relationship between the availability of P and plant metabolism, and plant photosynthesis is considerably impaired in P-starved plants due to a low concentration of compounds containing P in the primary metabolism (Warren, 2011). In addition, P nutrition plays an important role in nitrogen uptake: low availability of P can impair the uptake of nitrate (Lambers et al., 2015). Through mineralization, mass flow, and diffusion, drought reduces mineral nutrient supply, thereby reducing plant growth (Cramer et al., 2009; Sardans and Peñuelas, 2012). Moreover, P is available in soil in the insoluble forms of phosphotriester and phosphomonoester, which are inaccessible to plants. The PGPRs have been shown to increase N-fixation, soil phosphate solubility, and other nutrient mobility and absorption (Bennett et al., 2003; Glick, 2012). Our results regarding the improvement of soil nutrients by PGPR application are in line with the previous findings (Ghavami et al., 2017; Khan & Bano, 2019). Besides P, other nutrients such as, K+, Ca2+, and Mg2+ were improved upon application of biochar and PGPR in wheat plants and soil as well (Çığ et al., 2021). Similarly N utilization was also enhanced due to the abundance of PGPR in the rhizospheric soil (Yuan et al., 2021).
Trichome is a specialized structure within plant epidermal cells and is generally long and dense in desert-adapted plants (Gianoli and González-Teuber, 2005). There have been many previous studies that have examined trichome characteristics in response to water deficits to determine whether (and to what extent) the trichome enhances drought resistance in plants (Gianoli and González-Teuber, 2005; Meng et al., 2014). Trichomes density offers a plastic adaptive response to drought by influencing the CO2 and H2O exchanges, hence decreasing the risk of excessive water loss and photoinhibition (Fu et al., 2013; Gianoli and González-Teuber, 2005; Palliotti et al., 1994). By increasing the boundary layer on the leaf surface, trichomes can also reduce the plant’s solar radiation absorption and decrease its temperature, thereby mitigating the risk of drought (Schreuder et al., 2001). In the current study, morphological structures of trichomes showed greater density in drought stress conditions and under the application of biochar and PGPR which are in line with findings reported previously (Liu and Liu, 2016; Shahzad et al., 2021), who found that density and length of trichomes increased under drought stress, and further improvement was attributed to the microorganisms used (PGPR).
Moreover, protein content was consistently reduced during drought stress, while augmented with the addition of biochar and PGPR, which plays a key role in plans under drought stress (Khan et al., 2020; Khan & Bano, 2019). Interestingly, another study reported the reduction in protein content under drought conditions in lentil (Lens culinaris Medikus) and decreased its nutritional quality (Choukri et al., 2020). The sugar concentration was maximum in drought-stressed plants in comparison with the control (Agami et al., 2016). It is also documented the effect of drought on sugar accumulation in many plants which in turn disturbed many cellular processes and hindered the transport between sink and source (Kour et al., 2020). In contrast, the application of PGPR and biochar decreased the concentration of sugar under drought stress for proper functioning of plants (Agami et al., 2016). The conversion of sugar into fructose and glucose plays a key role in the regulation of osmotic adjustment under drought stress. It has been reported that plants under drought stress accumulated the sugar for osmotic adjustment and the application of growth regulators decreased the sugar content (Wang et al., 2016).
Drought stress also resulted in the significant accumulation of GB in the shoot and root. However, the GB was reduced under the application of growth regulators. GB acts as a compatible solute and enables plants to adapt more readily to abiotic stresses by improving the water status of tissues and protecting them from ROS under drought stress (Tahira et al., 2018; Zohaib et al., 2018). Moreover, the concentration of total phenolics and total flavonoids was significantly reduced under drought stress but enhanced under the application of biochar and PGPR. Our results coincide with the previous findings (Rezazadeh et al., 2019). Conversely, drought stress induced by polyethylene glycol (PEG) increased phenolic and flavonoid contents at 15% and 5% PEG in RGS003 and Sarigo cultivars of canola (Rezayian et al., 2018). However, the treatment of both biochar and PGPR applications significantly improved phenolic and flavonoid contents in Vicia faba under drought stress, suggesting the important role of biochar and PGPR treatments to improve biochemical properties of plants under stress conditions (Nafees et al., 2022).
In drought-stressed plants with biochar and PGPR showed maximum activities of POD and SOD in comparison with the control, to counter-balance the over-production of ROS and protect the plants from the damaging effects of drought (Agami et al., 2016; Batool et al., 2020). In parallel, our findings coincide with another study in which biochar amendment and silicon application significantly enhanced SOD and POD activities in maize plants to lessen the adverse impacts of drought stress (Sattar et al., 2020). Similarly, (Yuan et al., 2021) reported the synergistic effects of PGPR and biochar on tomato plants showing elevated levels of both SOD and POD activities and improving microbial community and nitrogen utilization; however, alone treatments neither biochar nor PGPR showed any significant increase in enzymatic activities.
Moreover, the activity of DHAR was also higher in plants subjected to biochar and PGPR. The activity of DHAR generally increases under abiotic stress (Hasanuzzaman et al., 2021). In the present study, the activity of GR was significantly lower under drought stress, whereas the application of PGPR and biochar increased the GR activity. Likewise, another report documented the efficient use of PGPR with zinc oxide nanoparticle in increasing GR and DHAR activities in wheat plants under heat and drought stress conditions (Azmat et al., 2022). Additionally, biochar amendment with chitosan significantly enhanced GR and DHAR activities under salinity stress in jute (Corchorus olitorius L.), indicating the crucial role of biochar to enhance crop defense system under stress conditions (Hasanuzzaman et al., 2021). Therefore, our findings match with previous studies (Agami et al., 2016; Batool et al., 2020), in which higher antioxidant activities were observed to play a significant role in protecting plants from the damaging effects of drought stress. Additionally, the combined use of PGPR and biochar can significantly promote plant growth and development by improving plant growth parameters and antioxidant response to alleviate the adverse impacts of drought.
5 Conclusion
Drought stress adversely affected the growth, physio-biochemical characteristics, and nutrient uptake of both genotypes. However, the application of biochar and PGPR (Staphylococcus sp.) significantly improved several physiological characteristics and nutrient uptake and thereby reduced the negative effects of drought on the growth of both genotypes of B. napus L. The synergistic effect of biochar and PGPR has been demonstrated to be a promising strategy for improving B. napus production in drought-prone areas. In addition, the genotype Punjab sarson exhibited higher drought tolerance than westar. Therefore, the integrated approach of PGPR and biochar could be recommended for other plants as well to cope with various environmental stresses crop productivity and yield enhancement of economically important agricultural crops.
Available of data and material (data transparency)
Not applicable.
Code Availability
Not applicable.
References
Abdelaal, K. A. (2015). Effect of salicylic acid and abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. Journal of Plant Production, 6, 1771–1788.
Agami, R. A., Medani, R. A., Abd El-Mola, I. A., & Taha, R. S. (2016). Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. International Journal of Environmental & Agriculture Research, 2, 78.
Agami, R. A., Medani, R. A., El-Mola, A., & Taha, I. A. (2016). Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. International Journal of Environmental & Agriculture Research, 2, 78.
Asthir, B., Kaur, S., & Mann, S. K. (2009). Effect of salicylic and abscisic acid administered through detached tillers on antioxidant system in developing wheat grains under heat stress. Acta Physiologiae Plantarum, 31, 1091–1096.
Azmat, A., Tanveer, Y., Yasmin, H., Hassan, M. N., Shahzad, A., Reddy, M., & Ahmad, A. (2022). Coactive role of zinc oxide nanoparticles and plant growth promoting rhizobacteria for mitigation of synchronized effects of heat and drought stress in wheat plants. Chemosphere, 297, 133982.
Barampuram, S., Allen, G., & Krasnyanski, S. (2014). Effect of various sterilization procedures on the in vitro germination of cotton seeds. Plant Cell Tissue and Organ Culture (PCTOC), 118, 179–185.
Baronti, S., Vaccari, F. P., Miglietta, F., Calzolari, C., Lugato, E., Orlandini, S., Pini, R., Zulian, C., & Genesio, L. (2014). Impact of biochar application on plant water relations in Vitis vinifera (L.). European Journal of Agronomy, 53, 38–44.
Bashan, Y. (1998). Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant-growth-promoting bacteria) and PGPB. Soil Biology & Biochemistry, 30, 1225–1228.
Basra, S. M. A., Farooq, M., Tabassam, R., & Ahmad, N. (2005). Physiological and biochemical aspects of pre-sowing seed treatments in fine rice (Oryza sativa L.). Seed Science and Technology, 33, 623–628.
Batool, T., Ali, S., Seleiman, M. F., Naveed, N. H., Ali, A., Ahmed, K., Abid, M., Rizwan, M., Shahid, M. R., & Alotaibi, M. (2020). Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Scientific Reports, 10, 1–19.
Baydar, N. G., Özkan, G., & Sağdiç, O. (2004). Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control, 15, 335–339.
Begum, N., Ahanger, M. A., & Zhang, L. (2020). AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environmental and Experimental Botany, 176, 104088.
Bennett, J. O., Krishnan, A. H., Wiebold, W. J., & Krishnan, H. B. (2003). Positional effect on protein and oil content and composition of soybeans. Journal Of Agriculture And Food Chemistry, 51, 6882–6886. https://doi.org/10.1021/jf034371l
Cassan, F., Maiale, S., Masciarelli, O., Vidal, A., Luna, V., & Ruiz, O. (2009). Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. european Journal of Soil Biology, 45, 12–19.
Chang, C. C., Yang, M. H., Wen, H. M., & Chern, J. C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10, 178–182.
Choukri, H., Hejjaoui, K., El-Baouchi, A., El Haddad, N., Smouni, A., Maalouf, F., Thavarajah, D., & Kumar, S. (2020). Heat and drought stress impact on phenology, grain yield, and nutritional quality of lentil (Lens culinaris Medikus). Frontiers in Nutrition, 7, 596307.
Cramer, M. D., Hawkins, H. J., & Verboom, G. A. (2009). The importance of nutritional regulation of plant water flux. Oecologia, 161, 15–24.
Dasgupta, D., Ghati, A., Sarkar, A., Sengupta, C., & Paul, G. (2015). Application of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Sesbania bispinosa on the growth of chickpea (Cicer arietinum L.). International Journal of Current Microbiology and Applied Science, 4, 1033–1042.
Elahi, N., Duncan, R. W., & Stasolla, C. (2015). Decreased seed oil production in FUSCA3 Brassica napus mutant plants. Plant Physiology and Biochemistry, 96, 222–230.
Ellouzi, H., Ben Hamed, K., Asensi-Fabado, M. A., Müller, M., Abdelly, C., & Munné-Bosch, S. (2013). Drought and cadmium may be as effective as salinity in conferring subsequent salt stress tolerance in Cakile maritima. Planta, 237, 1311–1323.
Ensikat, H. J., Ditsche-Kuru, P., Barthlott, W., & Méndez-Vilas, A. (2010). Scanning electron microscopy of plant surfaces: Simple but sophisticated methods for preparation and examination. Microscopy: Science, Technology, Applications and Education, 1, 248–255.
Fahad, S., Hussain, S., Saud, S., Hassan, S., Tanveer, M., Ihsan, M. Z., Shah, A. N., Ullah, A., Khan, F., & Ullah, S. (2016). A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiology and Biochemistry, 103, 191–198.
Farooq, M., Romdhane, L., Rehman, A., Al-Alawi, A. K., Al-Busaidi, W. M., Asad, S. A., & Lee, D. J. (2021). Integration of seed priming and biochar application improves drought tolerance in cowpea. Journal of Plant Growth Regulation, 40, 1972–1980.
Fu, Q. S., Yang, R. C., Wang, H. S., Zhao, B., Zhou, C. L., Ren, S. X., & Guo, Y. D. (2013). Leaf morphological and ultrastructural performance of eggplant (Solanum melongena L.) in response to water stress. Photosynthetica, 51, 109–114.
Gao, J., Yang, X., Zheng, B., Liu, Z., Zhao, J., Sun, S., Li, K., & Dong, C. (2019). Effects of climate change on the extension of the potential double cropping region and crop water requirements in Northern China. Agricultural and Forest Meteorology, 268, 146–155.
García, J. E., Maroniche, G., Creus, C., Suárez-Rodríguez, R., Ramirez-Trujillo, J. A., & Groppa, M. D. (2017). In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiological Research, 202, 21–29.
Ghavami, N., Alikhani, H. A., Pourbabaei, A. A., & Besharati, H. (2017). Effects of two new siderophore-producing rhizobacteria on growth and iron content of maize and canola plants. Journal of Plant Nutrition, 40, 736–746.
Gianoli, E., & González-Teuber, M. (2005). Environmental heterogeneity and population differentiation in plasticity to drought in Convolvulus chilensis (Convolvulaceae). Evolutionary Ecology, 19, 603–613.
Glick, B. R. (2012). Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012.
González, L., & González-Vilar, M. (2001). Handbook of plant ecophysiology techniques. Handbook of plant ecophysiology techniques.
Grant, O. M. (2012). Understanding and exploiting the impact of drought stress on plant physiology. In Abiotic stress responses in plants (pp. 89–104). Springer.
Grieve, C. M., & Grattan, S. R. (1983). Rapid assay for determination of water soluble quaternary ammonium compounds. Plant and soil, 70, 303–307.
Gusain, Y.S., Singh, U.S., & Sharma, A.K. (2014). Enhance activity of stress related enzymes in rice (Oryza sativa L.) induced by plant growth promoting
Hasanuzzaman, M., Raihan, M., Hossain, R., Khojah, E., Samra, B. N., Fujita, M., & Nahar, K. (2021). Biochar and chitosan regulate antioxidant defense and methylglyoxal detoxification systems and enhance salt tolerance in jute (Corchorus olitorius L.). Antioxidants, 10, 2017.
Jaleel, C. A., Manivannan, P., Wahid, A., Farooq, M., Al-Juburi, H. J., Somasundaram, R., & Panneerselvam, R. (2009). Drought stress in plants: A review on morphological characteristics and pigments composition. International Journal of Agriculture and Biology, 11, 100–105.
Keshavarz Afshar, R., Hashemi, M., DaCosta, M., Spargo, J., & Sadeghpour, A. (2016). Biochar application and drought stress effects on physiological characteristics of Silybum marianum. Communications in Soil Science and Plant Analysis, 47, 743–752.
Khan, N., & Bano, A. (2019). Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE, 14, e0222302.
Khan, N., Bano, A. M., & Babar, A. (2020). Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS ONE, 15, e0231426.
Kour, D., Rana, K. L., Yadav, A. N., Sheikh, I., Kumar, V., Dhaliwal, H. S., & Saxena, A. K. (2020). Amelioration of drought stress in Foxtail millet (Setaria italica L.) by P-solubilizing drought-tolerant microbes with multifarious plant growth promoting attributes. Environmental Sustainability, 3, 23–34.
Lalay, G., Ullah, S., & Ahmed, I. (2022). Physiological and biochemical responses of Brassica napus L. to drought-induced stress by the application of biochar and plant growth promoting Rhizobacteria. Microscopy Research and Technique, 85, 1267–1281.
Lambers, H., Finnegan, P. M., Jost, R., Plaxton, W. C., Shane, M. W., & Stitt, M. (2015). Phosphorus nutrition in Proteaceae and beyond. Nature Plants, 1, 1–9.
Lawlor, D. W. (2002). Limitation to photosynthesis in water-stressed leaves: Stomata versus metabolism and the role of ATP. Annals of Botany, 89, 871–885.
Lee, D. H., & Lee, C. B. (2000). Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: In gel enzyme activity assays. Plant Science, 159, 75–85.
Lesk, C., Rowhani, P., & Ramankutty, N. (2016). Influence of extreme weather disasters on global crop production. Nature, 529, 84–87. https://doi.org/10.1038/nature16467.
Liu, X., & Liu, C. (2016). Effects of drought-stress on Fusarium crown rot development in Barley. PLoS ONE, 11, e0167304.
Lizana, C., Wentworth, M., Martinez, J. P., Villegas, D., Meneses, R., Murchie, E. H., Pastenes, C., Lercari, B., Vernieri, P., & Horton, P. (2006). Differential adaptation of two varieties of common bean to abiotic stress: I. Effects of drought on yield and photosynthesis. Journal of Experimental Botany, 57, 685–697.
Marcińska, I., Czyczyło-Mysza, I., Skrzypek, E., Filek, M., Grzesiak, S., Grzesiak, M. T., Janowiak, F., Hura, T., Dziurka, M., & Dziurka, K. (2013). Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiologiae Plantarum, 35, 451–461.
Meng, F., Peng, M., Pang, H., & Huang, F. (2014). Comparison of photosynthesis and leaf ultrastructure on two black locust (Robinia pseudoacacia L.). Biochemical Systematics and Ecology, 55, 170–175.
Nafees, M., Ullah, S., & Ahmed, I. (2022). Modulation of drought adversities in Vicia faba by the application of plant growth promoting rhizobacteria and biochar. Microscopy Research and Technique.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880.
Naseem, H., Ahsan, M., Shahid, M. A., & Khan, N. (2018). Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. Journal of Basic Microbiology, 58, 1009–1022.
Nawaz, M., Wang, X., Saleem, M. H., Khan, M. H. U., Afzal, J., Fiaz, S., Ali, S., Ishaq, H., Khan, A. H., & Rehman, N. (2021). Deciphering plantago ovata forsk leaf extract mediated distinct germination, growth and physio-biochemical improvements under water stress in maize (Zea mays L.) at early growth stage. Agronomy, 11, 1404.
Okorie, V. O., Mphambukeli, T. N., & Amusan, S. O. (2019). Exploring the political economy of water and food security nexus in BRICS. Africa Insight, 48, 21–38.
Oliveira, M. T., Medeiros, C. D., Frosi, G., & Santos, M. G. (2014). Different mechanisms drive the performance of native and invasive woody species in response to leaf phosphorus supply during periods of drought stress and recovery. Plant Physiology and Biochemistry, 82, 66–75.
Oraee, A., & Tehranifar, A. (2020). Evaluating the potential drought tolerance of pansy through its physiological and biochemical responses to drought and recovery periods. Scientia Horticulturae, 265, 109225.
Ozturk, M., Turkyilmaz Unal, B., García-Caparrós, P., Khursheed, A., Gul, A., & Hasanuzzaman, M. (2021). Osmoregulation and its actions during the drought stress in plants. Physiologia Plantarum, 172, 1321–1335.
Palliotti, A., Bongi, G., & Rocchi, P. (1994). Peltate trichomes effects on photosynthetic gas exchange of Olea. Plant Physiology (Life Science Advances), 13, 35–44.
Parker, W. C., Pallardy, S. G., Hinckley, T. M., & Teskey, R. O. (1982). Seasonal changes in tissue water relations of three woody species of the Quercus-Carya forest type. Ecology, 63, 1259–1267.
Qayyum, M. F., Abid, M., Danish, S., Saeed, M. K., & Ali, M. A. (2015). Effects of various biochars on seed germination and carbon mineralization in an alkaline soil. Pakistan Journal of Agricultural Sciences, 51, 977–982.
Rashwan, E., Alsohim, A. S., El-Gammaal, A., Hafez, Y., & Abdelaal, K. A. (2020). Foliar application of nano zinc-oxide can alleviate the harmful effects of water deficit on some flax cultivars under drought conditions. Fresenius Environmental Bulletin, 29, 8889–8904.
Rawat, M., Arunachalam, K., & Arunachalam, A. (2015). Plant functional traits and carbon accumulation in forest. Climate Change and Environmental Sustainability, 3, 1–12.
Rezayian, M., Niknam, V., & Ebrahimzadeh, H. (2018). Differential responses of phenolic compounds of Brassica napus under drought stress. Iranian Journal of Plant Physiology, 8, 2417–2425.
Rezazadeh, S., Ilkaee, M., Aghayari, F., Paknejad, F., & Rezaee, M. (2019). The physiological and biochemical responses of directly seeded and transplanted maize (Zea mays L.) supplied with plant growth-promoting rhizobacteria (PGPR) under water stress. Iranian Journal of Plant Physiology, 10, 3009–3021.
Sardans, J., & Peñuelas, J. (2012). The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiology, 160, 1741–1761.
Sattar, A., Sher, A., Ijaz, M., Ul-Allah, S., Butt, M., Irfan, M., Rizwan, M. S., Ali, H., & Cheema, M. A. (2020). Interactive effect of biochar and silicon on improving morpho-physiological and biochemical attributes of maize by reducing drought hazards. Journal of Soil Science and Plant Nutrition, 20, 1819–1826.
Schreuder, M. D., Brewer, C. A., & Heine, C. (2001). Modelled influences of non-exchanging trichomes on leaf boundary layers and gas exchange. Journal of Theoretical Biology, 210, 23–32.
Seleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M., Refay, Y., Dindaroglu, T., Abdul-Wajid, H. H., & Battaglia, M. L. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants, 10, 259.
Seybold, C. A., Mersie, W., Huang, J., & McNamee, C. (2002). Soil redox, pH, temperature, and water-table patterns of a freshwater tidal wetland. Wetlands, 22, 149–158.
Shah, A. N., Yang, G., Tanveer, M., & Iqbal, J. (2017). Leaf gas exchange, source–sink relationship, and growth response of cotton to the interactive effects of nitrogen rate and planting density. Acta Physiologiae Plantarum, 39, 1–10.
Shahzad, M., Khan, Z., Nazeer, W., Arshad, S. F., Ahmad, F., Farid, B., Shahid, M. R., & Riaz, H. (2021). Effect of drought on trichome density and length in cotton (Gossypium hirsutum). Journal of Bioresource Management, 8, 15.
Shao, H. B., Jiang, S. Y., Li, F. M., Chu, L. Y., Zhao, C. X., Shao, M. A., Zhao, X. N., & Li, F. (2007). Some advances in plant stress physiology and their implications in the systems biology era. Colloids and surfaces B: Biointerfaces, 54, 33–36.
Shivaraj, S. M., Sharma, Y., Chaudhary, J., Rajora, N., Sharma, S., Thakral, V., Ram, H., Sonah, H., Singla-Pareek, S. L., & Sharma, T. R. (2021). Dynamic role of aquaporin transport system under drought stress in plants. Environmental and Experimental Botany, 184, 104367.
Siddiqui, M. H., Al-Khaishany, M. Y., Al-Qutami, M. A., Al-Whaibi, M. H., Grover, A., Ali, H. M., Al-Wahibi, M. S., & Bukhari, N. A. (2015). Response of different genotypes of faba bean plant to drought stress. International Journal of Molecular Sciences, 16, 10214–10227.
Singh, B., Singh, B. P., & Cowie, A. L. (2010). Characterisation and evaluation of biochars for their application as a soil amendment. Soil Research, 48, 516–525.
Tahira, T., Riaz, A., Muhammad, F., & Basra, S. M. A. (2018). Improving the drought tolerance in barley by osmopriming and biopriming. International Journal of Agriculture and Biology, 20, 1597–1606.
Vile, D., Garnier, E., Shipley, B., Laurent, G., Navas, M. L., Roumet, C., Lavorel, S., Díaz, S., Hodgson, J. G., & Lloret, F. (2005). Specific leaf area and dry matter content estimate thickness in laminar leaves. Annals of Botany, 96, 1129–1136.
Wang, R., Gao, M., Ji, S., Wang, S., Meng, Y., & Zhou, Z. (2016). Carbon allocation, osmotic adjustment, antioxidant capacity and growth in cotton under long-term soil drought during flowering and boll-forming period. Plant Physiology and Biochemistry, 107, 137–146.
Wang, W., Vignani, R., Scali, M., & Cresti, M. (2006). A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis, 27, 2782–2786.
Warren, C. R. (2011). How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiology, 31, 727–739.
Yang, A., Akhtar, S. S., Fu, Q., Naveed, M., Iqbal, S., Roitsch, T., & Jacobsen, S. E. (2020). Burkholderia phytofirmans PsJN stimulate growth and yield of quinoa under salinity stress. Plants, 9, 672.
Yuan, W., Wenqing, L. I., Binghai, D. U., & Hanhao, L. I. (2021). Effect of biochar applied with plant growth-promoting rhizobacteria (PGPR) on soil microbial community composition and nitrogen utilization in tomato. Pedosphere, 31, 872–881.
Zhang, Z., Chai, X., Tariq, A., Zeng, F., Graciano, C., Li, X., Gao, Y., & Ullah, A. (2022). Coordinated patterns in the allocation, composition, and variability of multiple elements among Organs of two desert shrubs under Nitrogen Addition and Drought. Journal of Soil Science and Plant Nutrition, 22, 47–58.
Zohaib, A., Tabassum, T., Jabbar, A., Anjum, S. A., Abbas, T., Mehmood, A., Irshad, S., Kashif, M., Nawaz, M., & Farooq, N. (2018). Effect of plant density, boron nutrition and growth regulation on seed mass, emergence and offspring growth plasticity in cotton. Scientific Reports, 8, 1–1.
Çığ, F., Sönmez, F., Nadeem, M. A., & Sabagh, A. E. (2021). Effect of biochar and PGPR on the growth and nutrients content of Einkorn Wheat (Triticum monococcum L.) and post-harvest soil properties. Agronomy, 11, 2418.
Acknowledgements
We are also thankful to the Department of Botany, University of Peshawar, for providing all facilities of this work.
Funding
Open access funding provided by ELKH Centre for Agricultural Research. This study received no external funding.
Author information
Authors and Affiliations
Contributions
GL, MAA, and SU conceptualized the study; G, AU, and NI curated the data; GL, AU, MAA, and NI helped in formal analysis; MAA and SU contributed to funding acquisition; GL and AU helped in methodology; SU administrated the project; MAA and SU contributed to resources; GL, NI, AR, AU, and MAA developed the software and wrote the original draft; SU and MAA supervised the study; SU, MAA, and AU validated the study; SU, MAA, AU, NI, and AR helped in writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animal performed by any of the authors.
Consent to Publish
All authors are agreed to publish the work without any conflict.
Consent to participate
All authors declare that they have participated significantly in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gul Lalay and Abd Ullah have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lalay, G., Ullah, A., Iqbal, N. et al. The alleviation of drought-induced damage to growth and physio-biochemical parameters of Brassica napus L. genotypes using an integrated approach of biochar amendment and PGPR application. Environ Dev Sustain 26, 3457–3480 (2024). https://doi.org/10.1007/s10668-022-02841-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-022-02841-2