Abstract
Persimmon (Diospyros kaki Thunb.) contains numerous uridine diphosphate glucosyltransferases (UGT), and their roles in fruit development and quality formation have not been well studied because of limited genetic information. This study investigated a persimmon DkUGT3 which is highly expressed in young fruits and leaves during development. DkUGT3 can catalyze ABA glycosylation to form ABA-GE, thereby reducing free ABA. Tomato with overexpressed (OE) DkUGT3 significantly induces pale green color phenotypes in both transgenic young plants and fruits. DkUGT3-OE significantly weakens the tomato ABA signaling which affects the expressions of ABA-inducible transcriptional factors (TFs), such as GLK1 and GLK2 and their downstream target genes involved in chlorophyll synthesis, chloroplast development, sugar metabolism and transport, and photosynthesis, thereby impeding leaf and fruit development and quality. Conversely, DkUGT3-RNAi-treatment recovered the OE tomato fruits from yellowing phenotype to green color. This study found that chlorophyll accumulations and ABA level were increased by DkUGT3-RNAi-treatment in young persimmon leaves. These results demonstrate that DkUGT3 plays crucial roles in ABA-mediated leaf and fruit development. This study provides new evidence for the regulation of ABA in early development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
UDP glucosyltransferases (UGTs) are widespread in higher plants and mediate glycosylation reactions through catalyzing the conjugation sugar and aglycone to form glycosides and sugar esters (Schwab and Wüst 2015; Wang and Hou 2009; Xiong et al. 2019a, b). Generally, UGTs catalyze the glycosylation of UDP-sugar to alter their bioactivity, stability, and solubility (Bowles et al. 2005; Song et al. 2018). The glycosylation of abscisic acid (ABA) is catalyzed by ABA UGT, which can adjust the ABA level rapidly by inducing a reversible reaction (Okamoto et al. 2006; Priest et al. 2006) in which the inactive ABA-glucose ester (ABA-GE) can be converted back to free ABA by beta-glucosidases (BGs) (Lim et al. 2005; Xu et al. 2012). In order to meet the needs of growth and adapt to complex environmental changes, plants must continuously adjust their ABA levels. Therefore, ABA UGTs are important regulators to maintain the homeostasis of ABA in tissues and cells during plant development. Several ABA BG/UGTs have been descripted in fruits, for example strawberry FaBG3 (Almeida et al. 2007; Griesser et al. 2008), tomato SlUGT75C1 (Sun et al. 2017), and persimmon DkBG1 (Liang et al. 2020). However, the roles of ABA UGTs in most fleshy fruits remain obscure.
During seedling stage, green tissues become photoautotrophic tissues by transforming plastids into fully functional chloroplasts (Waters and Langdale 2009). The formation of chloroplasts depends on the biosynthesis of chlorophyll and carotenoid activated by light (Bartley and Scolnik 1995; Llorente et al. 2017). Carotenoids are photosynthetic accessory pigments which protect chlorophylls from photooxidation, and carotenoid synthesis in green tissues is tightly coupled with chlorophylls (Bartley and Scolnik 1995). Therefore, inhibiting carotenoid synthesis can lead to albino or pale green seedlings as a result of chloroplast photooxidation (Llorente et al. 2017). Tomato fruits contain a large number of carotenoids which have become a model for the research of carotenoids regulation (Barry 2012). Recent studies suggest that carotenoid accumulation in tomato fruit employs the same biosynthetic pathway as in leaves, albeit with modification (Barry 2012). However, the role of ABA in green fruits during development is still unclear.
Recently, physiological and molecular studies have revealed that fruit ripening is a programmed, biochemical process involving multiple signal integration (Osorio et al. 2013), and ethylene and ABA play important roles in tomato fruit ripening (Klee et al. 2011; Zhang et al. 2009). Tomato and persimmon ripening shares several regulatory pathways in common. The accumulations of starch and nutrients were affected by chlorophyll synthesis and photosynthetic activity in green fruit tissues (Nadakuduti et al. 2014). Persimmon fruit is not only rich in nutrients and well-flavored, but also rich in anti-oxidation, anti-bacterial, and disease resistance properties due to its high amount of soluble tannin. Therefore, persimmon fruits are increasing in consumer preference. In recent years, it has been discovered that the development of persimmon fruits is also regulated by ABA. To date, the contributions of DkUGTs to persimmon fruits have not been well-understood, particularly in young fruit development.
In this study, due to the difficulty to use perennial fruit trees for genetic transformation, and because tomato has the same mature phenomenon as persimmons, tomato plants were used to express persimmon DkUGT3. It was found that the over-expression of DkUGT3 in tomato decreases ABA level/signaling which can down-regulate the expression of ABA-inducible TFs and their target genes involved in chlorophyll synthesis, and thereby influences leaf and young fruit development.
Material and Methods
Persimmon Fruit Sample Collection
To investigate fruit development, ten fruits were sampled from three independent persimmon trees at different development stages. Fruit peel, pulp, and seeds were separated and frozen in liquid nitrogen. The samples were stored at − 80 °C until subsequent analysis.
Enzyme Activity of Recombinant DkUGT3 Protein
Expression, purification, and identification of the recombinant DkUGT3 proteins were performed as described previously (See Sun et al. 2017; Liang et al. 2020 for details) using the primers listed in Table S2.
Generation of DkUGT3-Overexpressed Transgenic Tomato Plants
For the construction of DkUGT3-overexpression vector, the 35S:: full-length DkUGT3 sequence:: Nos-terminator fusion gene was cloned into pCAMBIA1305.1 vector (Takara Bio Inc, Shiga, Japan). The constructor was then introduced into tomato (Solanum lycopersicum L. cv. Ailsa Craig) via Agrobacterium tumefaciens LBA4404-mediated transformation. The T2 homozygous offspring of the transgenic plants were used for analysis, and WT plant was used as control. Primers used are listed in Table S3.
Construction of the Viral Vector and Agroinoculation
pTRV1 and pTRV2 virus-induced gene-silencing vectors (Liu et al. 2002) were used in this study. A 321 bp cDNA fragment of DkUGT3 was amplified using primers 5′-TCTAGACGGGTTTCCCCACCGACTAT-3′ (sense) and 5′-GAGCTC CCCTCACTACCTCCGTGTTCTCC-3′ (antisense), and then it was cloned into EcoRI/SacI-digested pTRV2. Agrobacterium tumefaciens strain GV3101 containing pTRV1, pTRV2, and the pTRV2-derivative pTRV2-DkUGT3 was used for RNAi. 30 leaves from young plants 30 days after planting were divided into two groups for inoculation, and vascular bundle of dorsal leaves were injected with the DkUGT3-RNAi TRV vector (treatment group) and control TRV vectors (no DkUGT3 gene), respectively. The entire leaves were evaluated and sampled 5 days after treatment.
Plant Materials
Tomato plants (Ailsa Craig), including WT and three DkUGT3-OE lines, were grown in a greenhouse (25 ± 5 °C, 70% humidity, 14 h/10 h light/dark regime). Fruits were sampled at different days after full bloom (DAFB): immature green (IM), 25 DAFB; mature green (MG), 30 DAFB; breaker (B), 36 DAFB; orange (Or), 38 DAFB; red (R), 41 DAFB.
Drought Stress and ABA Treatment
Tomato seedlings were grown in 1.5 L pots in the greenhouse with normal watering conditions. Healthy 10-day-old and 7-day-old seedlings were used for the drought and ABA treatment assays, and 20 seedlings of WT and each DkUGT3-OE lines were divided into two groups, respectively. For drought treatment, group I was grown under normal soil moisture as control and group II was subjected to drought stress by halting irrigation for 13 days. For ABA treatment, group I was treated by ABA and group II without ABA treatment as control. The ABA, ABA-GE and chlorophyll contents in leaves were investigated at 10 days (for drought) and 6 days (for ABA treatment) after treatment, respectively.
Effect of Exogenous ABA Treatment on Expression of TF Genes
To evaluate the effect of exogenous ABA treatment, 20 fruits were harvested at mature green stage, divided into two groups (n = 10 per group), and immediately soaked in a 100 μM ABA water solution (group I) or distilled water (group II, control) at − 0.5 MPa vacuum for 10 min. The fruits were then placed in a tissue culture room at 25 ℃ and 95% relative humidity. After 24 h, all fruit were cut into pieces, frozen with liquid nitrogen, and stored at − 80 ℃ before RNA extraction. The leaves of 6-week-old plants were sampled, divided into two groups (n = 10 per group), and soaked in 0 μM or 100 μM ABA solution for 10 h, respectively. After residual liquid on the surface was wiped away, leaves were immediately frozen with liquid nitrogen, and stored at − 80 ℃ before use. All results were expressed as mean ± standard deviation of three biological replicates.
Quantitative Real-Time PCR (qRT–PCR)
Total RNA extraction and cDNA synthesis were performed as previously described. qRT-PCR was conducted through SYBR Premix ExTaq (Perfect Real-Time; TaKaRa Bio) on a Rotor-Gene 3000 system (Corbett Research, Sydney, NSW, Australia) to quantitate gene expression levels. Samples were denatured at 95 °C for 30 s, followed by 40 cycles at 95 °C for 15 s, 60 °C for 20 s, and 72 °C for 15 s. There are three biological replicates and SAND, EXP and CAC were used as reference genes to normalize the data. The relative expression level for each gene was calculated by Rotor-Gene Q software using the 2−ΔΔCT method. Primers of qRT–PCR are listed in Supplementary Table S5.
Measurement of ABA and ABA-GE
The extraction and purification of ABA and ABA-GE from 3 g fruit samples were performed as previously described (Liang et al. 2021). ABA and ABA-GE contents were determined using HPLC (Agilent Technologies 1200) equipped with a 4.8 X 150 mm C18 column (Agilent Technologies). Elution was performed using both solvent A (0.8% v/v glacial acetic acid) and solvent B (100% methanol) with a flow rate of 0.8 ml min−1. ( ±)-abscisic acid and ABA-Glu were bought from Sigma company (USA), and Olomouc company (Czech Republic), respectively, and were used as the standards.
RNA-Seq
Transcriptome was analyzed using the leaves of 28-day-old plants and the fruits at 25, 37 and 41 DAFB during development from three OE lines and WT. Total RNA extraction, mRNA purification and library construction were performed for each sample. Sequencing on an Illumina HiSeq 2000 system generated an average of 90.5 million paired-end reads (2 × 101 nucleotides) per library. A corrected P value of 0.05 and log2 (fold change) of 1 were set as the threshold of statistically significant difference. The details of data analysis are in the Supplementary Information.
Scanning Electron Microscopy
A skin sample of approximately 25 mm2 was rapidly taken from fruits and fixed into 1 ml 2.5% glutaraldehyde buffer under vacuum. To remove the residual fixative on the surface, a phosphate buffer (1.5 ml) with pH of 7.2 was used to wash the system three times (10 min each time). Next, the fixed tissue was dehydrated by a series of aqueous ethyl alcohol with ascending concentrations, and ethanol was washed and replaced by isoamyl acetate in the cells twice (15 min each time). Finally, the solvent in the sample was removed by liquid carbon dioxide through a critical point drying method. The dry fruit skin was mounted on a specimen stub and sputter-coated with gold before examination through the Hitachi Regulus 8100 scanning electron microscope (SEM).
Statistical Analysis
The one-way analysis using SPSS software and Duncan’s test of significance were performed for the statistical analysis of data; *t test P < 0.05; **t test P < 0.01.
Results
Uridine Diphosphate Glucosyltransferases (UGTs) in Persimmon
To date, a publicly available genome sequence of Diospyros plant is absent, therefore the transcriptome of Japanese persimmon (D. kaki) fruit was investigated at 35 and 170 days after full bloom (DAFB). Based on the homology sequence of known Arabidopsis AtUGTs and tomato SlUGTs, 46 putative DkUGT proteins were identified from persimmon transcriptomes (Table S1). Among them were 16 full-length cDNA sequences encoding DkUGT which were designated as DkUGT 1 to DkUGT 16 (Table 1).
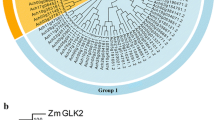
An evolutionary tree was constructed from persimmon DkUGTs, Arabidopsis AtUGTs and tomato SlUGTs, and the DkUGT proteins were clustered into several clades (Fig. 1A). Multiple alignments of the predicted protein sequences showed that most functional residues were well conserved within one gene family (Fig. S1). The amino acid sequence of DkUGT3 was similar to Arabidopsis AtUGT71B6, B7, and B8, all of which have been verified as ABA UGTs, suggesting that DkUGT3 may be related to ABA.
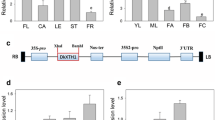
DkUGT3 catalyzes enzymatic reaction of ABA with UDPG. A Phylogenetic tree consisting of persimmon DkUGTs, Arabidopsis AtUGTs, and tomato SlUGTs, constructed using the neighbor-joining method and MEGA6 software with 1000 bootstrap replicates. DkUGTs are marked with a black circle except DkUGT3 which is marked with a red circle. The accession numbers of persimmon are shown in Table 1. B Prokaryotic expression and purification of DkUGT3 protein through SDS-PAGE. M, Protein molecular markers; 1, Without induction; 2, With induction; 3, Purification of DkUGT3. C Chromatogram shows a product (ABA-GE) of enzyme assays containing ABA with UDPG in the presence of DkUGT3 protein. The bottom of the figure shows the control reaction. D Determination of the kinetic parameters from a Lineweaver–Burk plot for the results of kinetic assays of DkUGT3 using ABA as a substrate. S, ABA concentration; V, the reaction rate. E Subcellular localization of DkUGT3
DkUGT3 Catalyzes ABA Glucosylation in Vitro, Forming ABA-GE
To verify the response of these 16 DkUGTs to ABA and abiotic stresses, young fruits were treated with ABA and NaCl, and leaves were dehydrated (Fig. S2). The results showed that expressions of five DkUGTs were altered after ABA treatment and among them, DkUGT3 was down-regulated in both ABA treated fruits and dehydrated leaves (Fig. S2). It was then verified that among ABA-inducible DkUGTs, only DkUGT3 was found to catalyze the glucosylation reaction of ABA using reverse-phase high-performance liquid chromatography (HPLC) in vitro. ABA-GE, a product of ABA glucosylation, was identified with a retention time of 19.15 min when DkUGT3 was added into the reaction mixture containing both UDPG and ABA (Fig. 1B, C). However, ABA-GE could not be detected in the reaction mixture containing denatured DkUGT3. In addition, the enzyme assay in the presence of DkUGT3 saturated with a gradient concentration of substrate ABA was performed in vitro. For DkUGT3, the reaction Vmax was 0.64 µM/min and Km was 1.19 mM (Fig. 1D). In addition, a recombinant DkUGT3 fused with GFP was constructed and expressed in tobacco leaves. The DkUGT3-GFP fusion protein was localized in the cytoplasm of tobacco epidermic cells and observed by fluorescence microscopy (Fig. 1E). These results confirmed that DkUGT3, a novel member of UGT family, can catalyze ABA glucosylation to form ABA-GE, thereby reducing free ABA.
DkUGT3 is Highly Expressed in Young Fruits and Leaves During Development
‘Mopan’ persimmon fruits were 185 days from fruit-set to full-ripe. The expression quantity and patterns of 16 DkUGTs were investigated through quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (Fig. 2A, B). Among them, the transcript level of DkUGT3 decreased during the early stage of fruit development, then it increased rapidly to the maximum value at 80 DAFB and then declined after that. From 95 DAFB, it increased again with a second peaking at 125 DAFB (pre-breaker stage) and then declined, suggesting that DkUGT3 might affect young fruit development and fruit ripening onset. The expression levels of DkUGT3 in leaves were significantly higher than those in stems and flowers (Fig. 2C). These results suggest that the expression level of DkUGT3 may affect the development of young fruits and leaves at their early stages.
Expression levels of DkUGTs in persimmon during development. A and B Expression levels and patterns of 16 DkUGTs in persimmon fruits during development and ripening. C Expression levels of DkUGT3 in stem, leaf, flower, and different fruit tissues in persimmon. qRT–PCR was conducted by three biological replicates and data were normalized against DkSAND and DkACT. *t test P < 0.05; **t test P < 0.01
Overexpression of DkUGT3 Causes Pale Green Color Plants of Transgenic Tomato
To examine the functions of DkUGT3 gene, transgenic tomato was engineered to express DkUGT3, and three independent OE lines were obtained. The transgenic plants were smaller with more internodes (Fig. 3A, Fig. S3B), their flowering time was delayed, and the seed numbers were reduced compared to wild type (WT) plants (Fig. S3C, D, F). In addition, the transgenic DkUGT3-OE lines showed pale green color phenotype, compared to WT plants (Fig. 3A). The transmission electron microscopy (TEM) analysis of the leaves revealed that the chloroplast number in DkUGT3-OE leaves was lower than that in WT plants (Fig. 3B–D). The chlorophyll contents were significantly reduced in the 28-day-old transgenic leaves which may impact photosynthesis (Fig. 3E). To determine whether the pale green color phenotype of transgenic tomato was related to decreased ABA level due to ABA glucosylation, both WT and transgenic plants were grown under normal conditions to compare various parameters. It was confirmed that DkUGT3 was highly expressed in transgenic tomato fruits throughout their development, while it was not present in WT plants (Fig. S3A). The free ABA content and chlorophyll accumulation in transgenic tomato were reduced while the ABA-GE level was increased compared to WT plants (Fig. 3E, F, G), suggesting that DkUGT3-mediated ABA glucosylation might be associated with decreased chlorophyll level in the transgenic tomato. There was no difference in the expressions of SlNCED1 and SlCYP707A1 between WT and transgenic tomato (Fig. S4) which is consistent with a previous report that DkBG1-OE did not influence the expressions of SlNCED1 and SlCYP707A1 (Liang et al., 2020). These results indicate that DkUGT3 does not influence the de novo synthesis and degradation of ABA during tomato plant development. Therefore, the decreased ABA levels found in transgenic tomatoes were due to the overexpression of DkUGT3.
Overexpression of DkUGT3 leads to pale green color plants and reduced ABA in transgenic tomato. A Phenotype of plants. Left panel, 28-day-old plants; Right panel, 90-day-old plants. B, C Mesophyll cell and ultrastructure of chloroplast. Upper panel, WT leaves. Below panel, OE-1 leaves. ST, starch granule; TH, thylakoid. D Chloroplast numbers in a mesophyll cell. E–G Chlorophyll, ABA, and ABA-GE contents in 28-day-old leaves. Error bars on each column indicate the standard deviation (SD) from three biological replicates for (D, E, F and G). *t test P < 0.05; **t test P < 0.01. H–K Expressions of genes involved in ABA signaling, chlorophyll, chloroplast, sugar metabolism and transport in WT and transgenic tomato leaves. Data are from RNA-seq analysis of young leaves. The significant differential expression was set as log2-fold change > 1 and < 1 with false detection rate < 0.005
To verify whether the pale green color phenotype of DkUGT3-OE plants was caused by the reduction of free ABA, the DkUGT3-OE plants were further evaluated under both drought stress and ABA treatment (Fig. S5–S7). Under ABA and drought treatments, the ABA, chlorophyll and sugar content increased with the treatment time, and the yellowish leaves gradually turned to green but not as green as WT, suggesting that the pale green color phenotype in the transgenic lines were partly recovered by the application of exogenous ABA and drought stress (Fig. S5–S7). The results indicate that the decreased ABA level in transgenic lines is related to pale green color phenotype.
DkUGT3 Affects Regulation of Seed Germination and Stress Responses by Modulating ABA level in Tomato
A reduced degree of seed dormancy was observed in DkUGT3-OE seeds, while under ABA treatment, the germination of seeds and growth of primary roots were rapid (Fig. 4A–C). Consistently, DkUGT3-OE plants displayed a decreased ABA sensitivity, for example, DkUGT3-OE plants were more prone to wilt, their detached leaves exhibited increased water loss compared to those of the WT plants (Fig. 4F). Drought sensitivity was also compared, and DkUGT3-OE fruits wilted faster after harvest compared to WT fruits (Fig. 4D, E). Collectively, these results demonstrate that DkUGT3 overexpression may affect the regulation of seed germination and stress responses by modulating ABA level in tomato, which in turn enhances the stomatal aperture size and transpiration.
DkUGT3 affects regulation of seed germination and stress responses by modulating ABA level in tomato. A, B Seed germination on 1/2 MS medium with or without 3 µM ABA. C Primary root growth on 1/2 MS medium with or without 10 µM ABA. D, E Water loss rate in the harvested fruits under 24 °C. F Water loss rate in the detached leaves under 24 °C. Data are represented as means ± SE from three biological replicates. *t test < 0.05; **t test < 0.01
To assess whether tomato ABA signaling was altered by DkUGT3-OE, an RNA sequencing (RNA-seq) analysis of 28-day-old OE leaves was performed. A total of 540 (OE1), 491 (OE14) and 728 (OE36) differentially expressed genes (DEGs) were detected (Fig. S8). The data showed that the expressions of most ABA signaling genes (PYLs, PP2Cs, SnRK2.5, and AREB2) in the OE leaves were down-regulated (Fig. 3I), which affected the expression of ABA-inducible downstream genes. Several ABA-inducible transcription factors (TFs) (Fig. 5) and their target genes involved in chlorophyll synthesis, chloroplast development, sugar metabolism and transport, and photosynthesis were down-regulated in transgenic leaves (Fig. 3H, J, K; Fig. S9).
Response of transcription factors (TFs) to exogenous ABA. Effect of ABA treatment on the expression of TFs which are related to chlorophyll accumulation and chloroplast development in (A) leaves and (B) fruits. qRT–PCR was conducted by three biological replicates and data were normalized against SlSAND, SlEXP and SlCAC. *t test P < 0.05; **t test P < 0.01
The expression profiles of some TFs in tomato were validated using qRT-PCR which showed consistent results with the transcriptome data, and the expressions of these TF genes were ABA-inducible in fruits and leaves (Fig. 5). Among them, GLK1 (GOLDEN2-LIKE), APRR2 (ARABIDOPSIS PSEUDO RESPONSE REGULATOR 2-LIKE) and TKN2 are master TFs in chloroplast development (Powell et al. 2012; Pan et al. 2013; Nguyen et al. 2014; Liu et al. 2015a, b). BBX20, a B-box (BBX) zinc-finger TF, is a positive regulator of carotenoid accumulation in tomato (Xiong et al. 2019a, b). MBP11, a MADS-box gene, plays an important role in regulating plant architecture and reproductive development in tomato (Guo et al. 2017). NAP1/NAP2 (NAC-like TF) has a complex role in establishing ABA homeostasis during leaf senescence (Ma et al. 2018). SlWRKY81 can induce photoinhibition and reduce the net photosynthetic rate under drought stress in tomato leaves (Ahammed et al. 2021). The transcript levels of SlGLK1, TKN2 and APRR2 were significantly decreased in transgenic leaves which could explain, at least partially, why the expressions of chlorophyll synthetic genes were repressed in DkUGT3-OE plants (Figs. 3H, 5A).
The expression profiles of these TF genes between leaves and fruits were different. For example, the expression of GLK1 and NAP1 is mainly in the leaves while the expression of GLK2 and NAP2 is chiefly in the fruits. In addition, ABA-inducible TF numbers in fruits are more than those in the leaves (Fig. 5B). These data suggest that TFs for tissue-specific control of certain types exhibit differential expression patterns in downstream networks.
Tomato Fruits Expressing DkUGT3 Showed Yellowish Green Color
Compared to green WT fruits, the pericarp color of DkUGT3-OE fruit was yellow-green in young fruits (Fig. 6Abcd). The transmission electron microscopy (TEM) analysis of young fruits revealed that the chloroplast number decreased in DkUGT3-OE fruits compared to WT fruits (Fig. 6B, C, D). The chlorophyll contents in the whole fruit at 10 and 20 DAF, and in the pericarp at 25 and 30 DAF of the DkUGT3-OE lines were lower than those of WT fruits (Fig. 6E, F). The ABA accumulations were significantly down-regulated, while the levels of ABA-GE were up-regulated in the transgenic lines compared to WT fruits (Fig. 6G, H).
Phenotype analyses of WT and transgenic tomato fruits with overexpressed DkUGT3. A Phenotype of young fruits. (a) WT fruits; (b–d) DkUGT3-OE fruits; (e) DkUGT3-RNAi-treated WT fruits at 5 DAT; (f–h) DkUGT3-RNAi-treated OE fruits at 5 DAT. B Flesh cell in the WT (upper) and OE pericarp (below). C Ultrastructure of chloroplast in WT (upper) and OE pericarp (below). D Chloroplast numbers in pericarp at 20 DAF. E, F Total chlorophyll content of the entire fruit and pericarp. G, H ABA and ABA-GE contents in fruits. The standard deviations (SD) are from three biological replicates for D-H. *t test P < 0.05; **t test P < 0.01
VIGS-induced DkUGT3-RNAi was used to suppress the expressions of DkUGT3 in DkUGT3-OE fruits (Fig. 6 Af–h). Ten fruits attached to plants grown in a greenhouse were injected with DkUGT3-RNAi TRV vector. The fruits were evaluated 5 days after RNAi-treatment (DAT). Changes in fruit color after DkUGT3-RNAi-treatment were examined at 20 and 25 DAF of WT and OE fruits, respectively. When WT and OE fruits were treated by DkUGT3-RNAi, the OE fruit color started to change to green at 5 DAT (Fig. 6Af–h), while WT fruits had no changes in color (Fig. 6Ae). The transcript levels of DkUGT3 gene decreased after RNAi inoculation, concomitant with the increase of GLK2 expression (Fig. S10), indicating that DkUGT3 expression affects fruit chlorophyll synthesis via GLK2. The ABA accumulation and chlorophyll contents in transgenic OE fruits at 20 DAF was lower than those in WT fruits, but both contents in the OE fruits treated with DkUGT3-RNAi were higher than those of untreated OE fruits (Fig. S10C, D). Thus, DkUGT3 may play an important role in the ABA-mediated regulation of young fruit development.
Furthermore, RNA-seq analysis showed that the expressions of the target genes of TFs participating in chlorophyll synthesis, chloroplast development and photosynthesis in DkUGT3-OE fruits, were lower than those of the WT fruits (Fig. 7A–F). The expression levels of some TFs in tomato were validated using qRT-PCR which showed consistent results with the transcriptome data (Fig. 7, right A-R). In addition, the RNA-seq analysis showed significant down-regulation of DEGs in OE fruits which were responsible for chlorophyll metabolism, sugar biosynthesis and signal transduction, and photosynthesis (Fig. S8–10). Most of these genes encode chlorophyll-binding proteins, structural photosystem proteins and chlorophyll biosynthetic enzymes. This may at least partly explain the 50% reduction of total chlorophyll level observed in DkUGT3-OE immature green fruits (Fig. 3E). The analysis of chlorophyll biosynthetic POR and CABs genes showed reduced mRNA levels in DkUGT3-OE lines (Fig. 7). These results suggest that the transcription of genes involved in chlorophyll metabolism and photosynthesis in tomato fruits are affected by the DkUGT3-mediated ABA and ABA signaling in transgenic tomato. Thus, DkUGT3 negatively impacts fruit development in transgenic tomato plants.
Expressions of transcription factors and target genes in young fruits of WT and OE lines. (Right:A-R). Relative expression levels of genes related to chlorophyll accumulation, chloroplast development and photosynthesis in young fruits. qRT–PCR was conducted with three biological replicates for A-S, and data were normalized against SlSAND, SlEXP and SlCAC. *t test P < 0.05; **t test P < 0.01. (Left: A-F) Expressions of development-related TFs and target genes. Data are from RNA-seq analysis of pericarp at 25 DAFB. The significant differential expression is set as log2-fold change > 1 and < 1 with false detection rate < 0.005. The heat map is made using MEV4.9.0 software
DkUGT3-Induced Alterations in Fruit Compositions can Adversely Impact Fruit Quality
The effect of DkUGT3-OE on fruit ripening and quality were observed during fruit ripening stage, and several ripening-related physiological parameters were measured in fruits. The ripening time from fruit-set stage to breaker stage in the WT fruits was 36 days, which was 2–3 days earlier than that of transgenic fruits (Fig. S3, S11, S12). The OE fruits were orange instead of red at the harvest stage (Fig. 8A); in agreement with fruit phenotype, ripe DkUGT3-OE fruits showed a half reduction of total carotenoids, lycopene and beta-carotene contents compared to those of WT fruits (Fig. 8B). In addition, the ABA content, starch, sucrose and fructose were down-regulated while the ABA-GE content were up-regulated in transgenic tomato fruits (Fig. 8C–G). These results show that DkUGT3-OE affects fruit ripening and quality in transgenic tomato.
DkUGT3-OE decreases fruit quality in transgenic tomato fruits. A Fruit ripening. B Total carotenoids, lycopene, and beta-carotene contents at B + 3 stage. C, D ABA and ABA-GE contents. E-G Starch and sugar contents. Data are from three biological replicates. *t test P < 0.05; **t test P < 0.01. Relative expression of genes related to ripening-related TFs (H), carotenoids (I), ethylene synthesis (J), and ethylene signaling (K). Data are based on the RNA-seq analysis of pericarp. The significant differential expression is set as log2-fold change > 1 and < 1 with false detection rate < 0.005. The heat map is made using MEV4.9.0 software.
RNA-seq analysis showed that the overexpression of DkUGT3 affected multiple metabolic pathways, including carotenoid biosynthesis, flavonoid biosynthesis, fatty acid biosynthesis, cell wall, lipids, terpenes, and flavonoids (Datasets). The gene expressions related to fruit ripening TFs, carotenoids metabolism, ethylene synthesis and signaling were down-regulated at 37 DAFB (Fig. 8H–K). These results indicate that DkUGT3-OE leads to decreased ABA accumulation and ABA signaling, which impacts the expressions of ripening-related TFs and target genes, thereby influencing the ripening and quality of tomato fruits.
DkUGT3-RNAi-Treated Persimmon Leaves Enhanced Chlorophyll and ABA Contents
VIGS-induced DkUGT3-RNAi was used to suppress DkUGT3 in young persimmon leaves. 20 leaves from young plants were injected with the DkUGT3-RNAi TRV vector and control TRV vectors, respectively. In the DkUGT3-RNAi-treated leaves, the color of leaves after 5 days changed to green (Fig. 9A). The transcript levels of DkUGT3 gene were decreased after RNAi inoculation, concomitant with the increased expression of GLK1 (Fig. 9B, C), indicating that DkUGT3-mediated ABA affects the development of young leaves via GLK1. The ABA accumulation and chlorophyll contents were higher in DkUGT3-RNAi-treated young leaves than those of the control leaves at 5 DAT (Fig. 9D, E). Thus, DkUGT3 plays an important role in the ABA-mediated regulation of the development of young leaves.
Phenotype of DkUGT3-RNAi-treated leaves in young persimmon plants. 30 leaves from young plants were divided into two groups (control and treatment groups) for virus-induced gene-silencing experiments. DkUGT3-RNAi TRV and TRV vectors (control) were injected into the vascular bundle of dorsal leaves, respectively. The entire leaves were evaluated and sampled 5 days after inoculation. A Phenotype of leaves treated by DkUGT3-RNAi-vectors (red arrows) and control (blue arrows) 5 days after treatments. The expression of DkUGT3 (B) and DkGLK1 (C) in the DkUGT3-RNAi-treated and control leaves. ABA (D) and Total chlorophyll contents (E) in the DkUGT3-RNAi-treated and control leaves. Error bars on each column indicate the standard deviation (SD) from three biological replicates. *t test P < 0.05; **t test P < 0.01
Discussion
Lower Level of ABA Content Mediated by DkUGT3 Leads to Pale Green Color Plants and Fruits
ABA UGT can affect the development and stress response of plants through ABA glycosylation. However, the role of ABA UGT in fruit and leaf development has not been fully understood. In this study, persimmon DkUGT3, which mediated constitutively decreased ABA in transgenic tomato, was analyzed.
Tomato plants expressing DkUGT3 showed obvious defects in chlorophyll synthesis with reduced chloroplast numbers, indicating that ABA and the concomitantly triggered ABA responses are likely to mediate chlorophyll metabolism and photosynthesis with a low efficiency (Fig. 3A–E). To verify this point, drought stress and ABA treatment on transgenic plants showed increased both ABA levels and chlorophyll contents along with the leaves color turn green under both tests (Figs. S5, S6). Thus, increased ABA during drought stress and ABA treatment can recover the green color of leaves, although it could not fully recover them as WT plants, suggesting that decreased chlorophyll content in the pale green color leaves of transgenic plants may be caused by the decreased ABA level mediated by DkUGT3 -OE (Fig. S5-S7).
In addition, it was found that ABA signaling is influenced in the transgenic tomato plant (Fig. 4). ABA acts mainly through ABA signaling which is composed of PYL-PP2C-SnRK2. RNA-seq analysis showed that the expressions of ABA signaling genes in both young fruits and leaves were down-regulated, meaning that ABA signaling cascades were reduced in DkUGT3-OE lines (Figs. 3I, 7A). AREBs are the primary factors to activate downstream ABA-dependent genes through binding to ABREs (ABA-responsive elements) in the promoter region of the target genes (Yanez et al. 2009; Fujita et al. 2013). As we expected, the expressions of SlAREB1 and SlAREB2 were down-regulated in leaves and fruits, respectively. Several ABA-inducible TF genes were significantly down-regulated in transgenic fruits and leaves (Figs. 3, 5, and 7). These TF genes simultaneously regulate the expression of genes related to chlorophyll accumulation, chloroplast development, and photosynthesis (Figs. 3 and 7). For example, SlGLK1 and SlGLK2, which belong to the GARP subfamily of the MYB TF superfamily in plants, are master TFs for chloroplast development (Powell et al. 2012; Nguyen et al. 2014). APRR2 can promote pigment accumulation and chloroplast development in tomato fruits (Pan et al. 2013; Liu et al. 2015a, b). TKN2/4, which belongs to the Class I KNOTTED1-LIKE HOMEOBOX (KNOX) TF family, can positively modulate SlGLK2 and SlAPRR2-LIKE to promote fruit chloroplast development (Nadakuduti et al. 2014). Protochlorophyllide oxidoreductase (POR) catalyzes the conversion from chlorophyllide a to chlorophyll a and chlorophyllide b (Meng 2018). Chlorophyll a/b binding genes (CABs) encode the chlorophyll biosynthetic enzymes in the chlorophyll biosynthetic pathway. It is known that the photosynthetic efficiency of green tissue is regulated by the expression of photosynthesis-related genes (Llorente et al. 2020), which interact with downstream factors to determine the physiological outcome. Photosynthesis converts light to chemical energy through two light-driven reactions, photosystem I (PSI) and photosystem II (PSII), which are connected by the electron transport chain and are arranged on the thylakoid membrane in a highly ordered manner, followed by the Calvin-Benson cycle. Light harvesting complex II (LHC II) from PS II is excited by light to produce electrons, which are then transported through cytochrome b6f complex, PSI complex, ferredoxin (Fd), and ferredoxin-NADP+ reductase (FNR). FNR reduces NADP+ to NADPH, which can assimilate CO2 through the Calvin–Benson cycle (Fromme et al. 2001). In this process, ATP and NADPH generated during the light reactions are used to fix and change atmospheric CO2 to carbohydrates, such as sucrose and starch, in the chloroplast and cytosol (Cocaliadis et al. 2014). Under the catalysis of phosphoenolpyruvate carboxylase (PEPC), HCO3− is used to produce oxaloacetate (OAA). In this study, the expressions of most genes mentioned above were down-regulated in transgenic leaves and young fruits. Furthermore, VIGS-induced DkUGT3-RNAi was used to suppress the expression of DkUGT3 in transgenic young fruits, and, as a result, the green color in OE fruits was recovered, suggesting that the pale green color phenotype of transgenic tomatoes was caused by DkUGT3-OE (Fig. 6 Af–h). These results suggest that the overexpression of DkUGT3 in tomato can decrease ABA level and weaken ABA signaling which down-regulates ABA-inducible TFs and target genes involved in chlorophyll synthesis, chloroplast development, and photosynthesis, and thereby induces pale green color plants and fruits in transgenic tomato. It is noted that GLK1 and GLK2 expressions were down-regulated by exogenous ABA (Fig. 5), indicating that endogenous- and exogenous ABA regulations of GLK1 and GLK2 are different.
The above results show the effect of DkUGT3 heterologous expression in tomato. DkUGT3 was then silenced in the leaves of persimmon seedlings through VIGS technology (Fig. 9). As expected, in DkUGT3-RNAi-treated leaves, GLK1 expression level, ABA accumulation and chlorophyll content were increased with the decreased DkUGT3 level, leading to greener leaves because of the more accumulated chlorophyll (Fig. 9). The results indicate that the effect of DkUGT3 on persimmon development is consistent with its effect on tomato through heterologous expression, suggesting that the functions of well-known ABA UGTs identified in model organisms are probably well conserved in D. kaki, and the transcriptional regulation of DkUGT3 possibly causes pale green color phenotype in green tissues. Taken together, these data demonstrate that decreased ABA level/signaling caused by DkUGT3-OE is responsible for this pale green color phenotype.
In conclusion, persimmon DkUGT3 can catalyze ABA glycosylation to reduce free ABA. DkUGT3 is highly expressed in leaves, young fruits and pre-breaker fruits in both persimmon and transgenic tomato. DkUGT3 expressed in tomato can dramatically induce the pale green color phenotype of plants and fruits due to the decreased ABA level/signaling which affects ABA-inducible TFs, such as GLK1 and GLK2, and target genes involved in chlorophyll production and photosynthesis. DkUGT3-induced alterations in fruit composition adversely impact fruit quality. DkUGT3-RNAi-treatment can change yellow-green fruits of transgenic tomato to a dark green color and can enhance chlorophyll and ABA contents of young persimmon leaves. Taken together, these results demonstrate that DkUGT3 plays crucial roles in ABA-mediated plant and fruit development and quality, and they provide new evidence of the regulatory function of ABA.
Data Availability
All relevant data can be found within the paper and in the supporting materials.
References
Ahammed GJ, Li X, Mao Q, Wan H, Zhou G, Cheng Y (2021) The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Physiol Plant 172:885–895
Almeida JR, D’Amico E, Preuss A et al (2007) Characterization of major enzymes and genes involved in flavonoid and proanthocyanidin biosynthesis during fruit development in strawberry (Fragaria xananassa). Arch Biochem Biophys 465:61–71
Barry CS (2012) Altered chloroplast development and delayed fruit ripening caused by mutations in a zinc metalloprotease at the lutescent2 locus oftomato. Plant Physiol 159:1086–1098
Bartley GE, Scolnik PA (1995) Plant carotenoids: pigments for photo- protection, visual attraction, and human health. Plant Cell 7:1027–1038
Bowles D, Isayenkova J, Lim EK, Poppenberger B (2005) Glycosyltransferases: managers of small molecules. Curr Opin Plant Biol 8:254–263
Cocaliadis FM, Fernández-Muñoz R, PonsOrzaez CD, Granell A (2014) Increasing tomato fruit quality by enhancing fruit chloroplast function. A double-edged sword? J Exp Bot 65:4589–4598
Fromme P, Jordan P, Krauss N (2001) Structure of photosystem I. Biochim Biophys Acta 1507:5–31
Fujita S et al (2013) An atypical tubulin kinase mediates stress-induced microtubule depolymerization in Arabidopsis. Curr Biol 23:1969–1978
Griesser M et al (2008) Multi-substrate flavonol O-glucosyltransferases from strawberry (Fragaria x ananassa) achene and receptacle. J Exp Bot 59:2611–2625
Guo XH, Chen GP, Naeem M et al (2017) The MADS-box gene SlMBP11 regulates plant architecture and affects reproductive development in tomato plants. Plant Sci 258:90–101
Klee HJ, Giovannoni JJ et al (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59
Liang B et al (2020) Overexpression of the persimmon abscisic acid β-glucosidase gene (DkBG1) alters fruit ripening in transgenic tomato. Plant J 102:1220–1233
Liang B et al (2021) Tomato protein phosphatase 2C (SlPP2C3). influences fruit ripening onset and fruit glossiness. J Exp Bot 72:2403–2418
Lim EK, Doucet CJ, Hou B et al (2005) Resolution of (+)-abscisic acid using an Arabidopsis glycosyl- transferase. Tetrahedron Asymmetry 16:143–147
Liu YL, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31:777–786
Liu Z, Yan JP, Li DK et al (2015a) UDP-glucosyltransferase71C5, a major glucosyltransferase, mediates abscisic acid homeostasis in Arabidopsis. Plant Physiol 167:1659–1670
Liu L, Shao Z, Zhang M, Wang Q (2015b) Regulation of carotenoid metabolism in tomato. Mol Plant 8:28–39
Llorente B, Martinez-Garcia JF, Stange C, Rodriguez-Concepcion M (2017) Illuminating colors: regulation of carotenoid biosynthesis and accumulation by light. Curr Opin Plant Biol 37:49–55
Llorente B et al (2020) Synthetic conversion of leaf chloroplasts into carotenoid-rich plastids reveals mechanistic basis of natural chromoplast development. Proc. Natl Acad. Sci. USA 117:21796–21803
Ma XM, Zhang YJ, Turečková V et al (2018) The NAC transcription factor SlNAP2 regulates leaf senescence and fruit yield in tomato. Plant Physiol 177:1286–1302
Meng LH (2018) BEL1-LIKE HOMEODOMAIN 11 regulates chloroplast development and chlorophyll synthesis in tomato fruit. Plant J 94:1126–1140
Nadakuduti SS, Holdsworth WL, Klein CL, Barry CS (2014) KNOX genes influence a gradient of fruit chloroplast development through regulation of GOLDEN2-LIKE expression in tomato. Plant J 78:1022–1033
Nguyen CV, Vrebalov JT, Gapper NE et al (2014) Tomato GOLDEN2-LIKE transcription factors reveal molecular gradients that function during fruit development and ripening. Plant Cell 26:585–601
Okamoto M, Kuwahara A, Seo M, Kushiro T et al (2006) CYP707A1 and CYP707A2, which encode abscisic acid 8’-hydroxylases, are indispens- able for proper control of seed dormancy and germination in Arabidop- sis. Plant Physiol 141:97–107
Osorio S, Scossa F, Fernie A (2013) Molecular regulation of fruit ripening. Front Plant Sci 4:198
Pan Y, Bradley G, Pyke K et al (2013) Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol 161(3):1476–1485
Powell AL et al (2012) Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 336:1711–1715
Priest DM, Ambrose SJ, Vaistij FE et al (2006) Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J 46:492–502
Schwab W, Wüst M (2015) Understanding the constitutive and induced biosynthesis of mono- and sesquiterpenes in grapes (Vitis vinifera): A key to unlocking the biochemical secrets of unique grape aroma profiles. J Agric Food Chem 63:10591–10603
Song C, Härtl K, McGraphery K et al (2018) Attractive but toxic: emerging roles of glycosidically bound volatiles and glycosyltransferases involved in their formation. Mol Plant 11:1225–1236
Sun YF et al (2017) Suppressing ABA uridine diphosphate glucosyltransferase (SlUGT75C1) alters fruit ripening and the stress response in tomato. Plant J 91:574–589
Wang J, Hou B (2009) Glycosyltransferases: key players involved in the modification of plant secondary metabolites. Front Biol China 4:39–46
Waters MT, Langdale JA (2009) The making of a chloroplast. The EMBO J. 28:2861–2873
Xiong C et al (2019a) A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. N Phytol 221:279–294
Xiong F, Ren J, Yu Q et al (2019b) AtU2AF65b functions in abscisic acid mediated flowering via regulating the precursor messenger RNA splicing of ABI5 and FLC in Arabidopsis. New Phytol 223:277–292
Xu ZY, Lee KH, Dong T et al (2012) A vacuolar b-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 24:2184–2199
Yanez A, Murciano C, Llopis S et al (2009) In vivo and in vitro studies on virulence and host responses to Saccharomyces cerevisiae clinical and non-clinical isolates. Open Mycol J 3:37–47
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60:1579–1588
Funding
This work was financially supported by the NSFC (Grant Nos. 31772270 and 31572095) and the Israel Science Foundation (ISF)–National Natural Science Foundation of China (NSFC) Joint Scientific Research Program (Grant No. 31661143046). It was also supported by the 2115 Talent Development Program of China Agricultural University, and the Construction of Beijing Science and Technology Innovation and Service Capacity in Top Subjects (CEFF-PXM2019_014207_000032).
Author information
Authors and Affiliations
Contributions
PL and QL made the experimental plan and wrote the manuscript; JW undertook the experiments and analyzed the data; BL was responsible for protein activity assay; YX assisted in experimental operations. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Francesca Cardinale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Xu, Y., Yin, Z. et al. Overexpression of the Persimmon Abscisic Acid DkUGT3 Gene Alters Plant/Fruit Development in Transgenic Tomato. J Plant Growth Regul 42, 4324–4338 (2023). https://doi.org/10.1007/s00344-022-10895-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10895-9