Abstract
In a controlled environment experiment, we studied how physiological changes in leaves during the vegetative phase regulate final grain yield of wheat crops in salt-affected soils. We also hypothesized that amendments such as biochar (SB) and selenium-chitosan nanoparticles (Se-NPs) can protect wheat plants from salt injury. 20-day-old wheat plants were submitted to 4-week salt stress (3000 ppm NaCl). Soybean straw biochar was mixed with soil media at planting and Se-NPs (30 ppm) was sprayed 5 days after the first salt stress treatment. At the end of 4-week Se-NPs treatment, one set of plants was harvested for studying leaf level physiological changes. The salt-stressed plants accumulated significantly high leaf Na+ (~ 13-fold increase), which trigged oxidative and osmotic damage. This salt-induced cellular injury was evident from significantly high levels of lipid membrane peroxidation and inhibited photosynthesis. Our study suggested that leaf physiological impairment in wheat plants was translated into poor biomass production and grain yield loss at crop maturity. Compared with control, salt-stressed plants produced 43% lesser biomass during vegetative phase, and 62% lesser grain yield at maturity. Amendments such as SB and Se-NPs protected the plants from salt-induced cellular injury by restricting Na+ transport toward leaf tissues. Plants treated with NaCl + SB + Se-NPs accumulated 50% less Na+ concentrations in leaves compared with NaCl-treated plants. Our study also suggested that SB and Se-NPs can restore ionic homeostasis and carbon assimilation in salt-stressed wheat by upregulating key transporter genes in leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Feeding the ever-growing world population in an unceasingly changing climate is one of the most significant challenges for crop producers worldwide. To meet human food consumption, an estimated 119% increase in the productivity of major food crops is needed by 2050 (Berners-Lee et al 2018). Crop productivity in many parts of the world is already challenged by the depleting natural resources. This issue has further been complicated by extreme environmental factors such as salinity and drought, which are the key growth and yield limiting factors for many food crops (Munns and Tester 2008). For instance, approximately 60 million hectares of global land area are affected by soil salinity, accounting for 20% of the total irrigated area (Machado and Serralheiro 2017). Salt levels in irrigated lands predominantly increase due to inappropriate irrigation practices, leading to sodium chloride (NaCl) build-up (Munns et al. 2019). Plants growing on the salt-affected soils take up excessive Na+ ions, which impair their normal metabolic functioning. For instance, oxidative stress caused by cellular Na+ ion build-up alters water and nutrient uptake, carbon assimilation, and biomass production (Gurmani et al. 2013; Soliman et al. 2020a). Some plant species have developed sophisticated mechanisms of mitigating salinity stress, i.e., through anatomical modifications and physiological and metabolic adaptations (Munns and Tester 2008). Nevertheless, an effective defense against oxidative stress is being played by the modulation of antioxidant enzymes in plants (Soliman et al. 2020b). In addition, several methods have been proposed to increase salt tolerance in plants; however, lesser attention has been paid to developing environment-friendly techniques (Ghoname et al. 2010).
One such environment-friendly approach for improving crop performance under stressed environments is addition of biochar to the affected soils. Biochar (SB), a solid and rich form of carbon, is produced by heating organic waste from large biomass through pyrolysis. In agricultural systems, SB is often used as a soil refinement for improving fertility, e.g., soil pH, electrical conductivity (EC), and water holding capacity (Sun et al. 2020). Addition of organic carbon and enhanced mineral composition contribute to superior crop performance under stressed environments (Hanna et al. 2021). Positive effects of SB supplementation leaf physiological processes such as photosynthesis and carbon assimilation have also been reported on salt-stressed crops (Iqbal et al. 2021). High adsorption potential of biochar has been linked to mitigating adverse salt effects by minimizing Na+ uptake, hence reducing electrolyte leakages even under high salt concentrations (Akhtar et al. 2015).
Selenium (Se) is an essential trace element necessary for normal plant functioning (Feng and Wei 2012). Interestingly, Se has been found effective in counteracting the harmful effects of environmental stresses such as heavy metals, salinity, and pathogens on plants (Schiavon et al. 2017). At low concentrations, Se acts as an anti-oxidative agent and promotes plant growth but it may suppress growth when applied in large quantities (Feng and Wei 2012; Cittrarasu et al. 2021). Appropriate Se concentration can help plants maintaining membrane structure and fluidity and protect cellular organs from damage (Diao et al. 2014). Salts such as sodium selenite (Na2Se) and potassium selenate (KSe) have been used for promoting crop growth and stress tolerance; however, high Se concentrations could accelerate ROS generation and oxidative damage (Hugouvieux et al. 2009). It has been found that elevated levels of Se can reduce leaf area, biomass production by disrupting cellular structure in plants (Molnár et al. 2018). Nanoparticles offer an attractive solution for managing agricultural production systems due to their tiny size and surface characteristics (Pramanik et al. 2020). Slow-release of nutrients from nanoparticles could promote plant growth by optimizing nutrient supplies and supporting antioxidant defense system (Hamouda et al. 2019). More recently, there has been an increased interest in nanoparticles such as selenium nanoparticles (Se-NPs), which can improve plant performance under stressed environments by stimulating their growth recovery (Mosallam et al. 2018; Farooq et al. 2022).
The negative impact of salinity on plant growth is simultaneously characterized by ionic toxicity, osmotic, and oxidative stress (Tanveer and Shabala 2018). Maintaining cellular osmotic potential is a significant challenge for plants to sustain growth under salt-stressed environments. In living organisms, including plants, aquaporins (AQPs) are the most common transmembrane transporters of water and small ionic substrates such as glycerol, urea, CO2, NH3, metalloids, and reactive oxygen species (ROS) (Groszmann et al. 2017). Based on amino acid sequences, four different members of plant AQPs family have been defined (Johanson and Gustavsson 2002). These include plasma membrane intrinsic proteins (PIPs); tonoplast intrinsic proteins (TIPs) (Johansson et al. 2000); nodulin 26-like intrinsic proteins (NIPs) (Roberts and Routray 2017); and small essential intrinsic protein (SIPs) (Ahmed et al. 2020). Twenty-four PIP and 11 TIP genes were recently identified in wheat (Forrest and Bhave 2008; Maurel et al. 2015); however, no NIP genes have been reported in wheat to date.
Wheat (Triticum aestivum L.) is an important food crop cultivated in arid and semiarid regions. Despite undeniable status in the human food supply chain, wheat production has been facing a stiff hindrance due to environmental stresses. For instance, soil salinity alone can cause up to 60% loss in wheat grain yield in many parts of the world (Wani et al. 2018). Thus, improving wheat performance under saline environments is crucial for sustaining wheat grain supplies. In this study, we explored the impact of long-term salinity on growth and biomass production in wheat, intending to unravel the biochemical and genetic pathways affected by salt stress. We also investigated the potential of using amendments such as soil SB and Se-NPs to regulate salt tolerance in wheat plants. Relationship between post-stress leaf physiology and grain yield at maturity is also explored to understand how these amendments protect wheat plants from salt-induced cellular injury.
Materials and Methods
Experimental Setup
One week before the start of experiment, plastic pots (40 × 40 cm) were filled with 2 kg sterilized sandy, loamy soil. For biochar amendment (SB), half of these pots contained 5% (W/V) soybean straw. Healthy and uniform grains of a salt-tolerant wheat (Triticum aestivum L.) cultivar Sakha 93 (Mahmoud et al. 2019) were obtained from Agriculture Research Centre, Giza, Egypt. The grains were surface sterilized with 0.01% HgCl2 solution and then thoroughly washed with distilled water. Four grains per pot were established, and the pots were organized to achieve a target density of 100 plants m−2. All pots were kept in a greenhouse under natural day/night conditions [65 ± 2% relative humidity, 23 °C/17 °C (± 3 °C) average day/night temperature and 680 μmol m−2 s−1 photosynthetically active radiation (PAR). The pots were watered using 100 mL Hoagland nutrient solution on alternate days. Eight treatments with three independent replicates were arranged in a complete randomized block design.
Characterization of Soil and Biochar
The soil and soybean straw SB used in this experiment were oven-dried at 50 °C for 24 h, then homogenized in a grinder and sieved through 2-mm openings and mixed. The soil characteristics such as soil pH and texture were presented in Table 1. Electrical conductivity (EC) and pH were measured in deionized water at 1:5 soil/water suspensions. The basic properties of biochar used in this study are shown in Table 2. Soil organic matter was measured according to (Sikora and Moore 2014). Soil organic carbons and SB samples were measured as described by Nelson protocol (Nelson and Sommers 1996). Total nitrogen in soil and SB were measured according to the sulfuric-salicylic acid mixture method by (Brush et al. 1982). The extractable potassium was measured in soil and SB by the flame atomic absorption spectrophotometer (Thermo Fisher). Total P in soil and SB were performed as described in Olsen and Sommers (1982) method (Table 3).
The Selenium Nanoparticles and SS Biochar Treatments
The selenium nanoparticles (Se-NPs) were obtained from (Sigma-Aldrich, Germany) in physical dispersion form with average particle size ranging from 80 to 100 nm and concentration 0.15 wt% of selenium in water. Soybean straw biochar (SB) was obtained from the experimental farm at the Faculty of Agriculture, Alexandria University. Briefly, the biochar was prepared from soybean straw material. The plant material was thoroughly dried under the sun and converted into biochar under pyrolysis temperature of 400 °C in an oven for 2 h (Joseph 2015). Then, the biochar was ground, sieved, and stored for further use.
Salinity Stress and Se-NPs Treatment
Twenty days after germination, one set of plants was submitted to salinity treatment by irrigating the pots with 3000 ppm saline water (NaCl) for 4 weeks. Five days after the start of salinity treatment, the plants (25-day-old) were sprayed with 30 ppm Se-NPs once a week. Se-NPs were dissolved in 2 mL of ethanol and then desired concentration (30 ppm) was prepared by adding double-distilled water. Twenty mL of Se-NPs solution per pot was sprayed on leaves each time, at the same time control plants were also sprayed with water (20 mL pot−1). Experimental treatments included: (a) control, (b) salinity stressed, (c) Se-NPs, (d) soil amended with biochar (SB), (e) salinity + Se-NPs, (f) salinity + SB, (g) SB + Se-NPs and (h) salinity + SB + Se-NPs. After the fourth Se-NPs spray, one set of 55-day-old plants was harvested for further analysis, while the remaining plants were allowed to growth under optimum conditions till maturity.
Plant Growth Parameters and Yield Parameters
Plant height was measured with a scale at harvest, and then the plants were separated into stem and leaves. Green leaf area was measured following Quarrie (Quarrie and Jones 1977).
Shoot and root dry weight was measured after oven-drying the samples at 70 °C for 24 h. Mature plants of each treatment were harvested for grain yield components such as number of tillers, spike length, number of spikelet per spike plants.
Measurement of Photosynthesis, PSII Activity, Water Use Efficiency and Leaf Water Potential
Leaf chlorophyll contents were determined by Arnon method (Arnon 1949) and the absorbance of reagent solution containing leaf sample was recorded at 663 and 645 nm with UV/VIS spectrophotometer (Genway, Japan). Chlorophyll fluorescence (Fv/Fm) and photosynthetic rate (Pn) were measured using a portable gas-exchange system equipped with fluorimeter and infrared gas analyzer (IRGA) system (TPS-2, USA). The measurements were taken under 22 °C temperature and 50–70% relative humidity. Mature leaves having total light exposure (maximum leaf area) were selected to measure leaf water potential using a psychrometer. Each value was an average of at least10 independent measurements. Water use efficiency (WUE) was calculated from the ratio of Pn and Tr following Zhang (2016) protocol. Measurements were made on fully expanded leaves with a fluorometer after plant adaptation in the dark for 30 min.
Estimation of Stress-Induced Biomarkers
Malondialdehyde Contents
Leaf MDA contents were measured using the thiobarbituric acid (TBA) method according to Heath and Packer (1968) and Erofeeva (2014) with slight modifications. For calculation, a molar extension coefficient of 155 mmol L−1 cm−1 was used. Absorbance of the mixture was taken at 532 nm and MDA contents were expressed as nmol g−1 FW.
Hydrogen Peroxide Contents
Reactive oxygen species such as hydrogen peroxide level was determined from the stressed and non-stressed samples according to Velikova et al (2000). In brief, leaf samples were homogenized in 2 mL 0.1% trichloroacetic acid (TCA) solution. After centrifugation of homogenates at 12,000×g for 15 min, 0.5 mL of the supernatant was added into the reaction mixture containing 0.5 mL of 10 mM K phosphate buffer (pH 7.0) and 1 mL of 1 M KI. The absorbance of the mixture was determined at 390 nm.
Electrolytes Leakage
Electrolytes leakage (EL) was measured in 0.2 g leaf segments. Samples were immersed in a test tube containing 4 mL of demineralized water for 30 min, rinsed 3 times to eliminate surface electrolytes (Blum and Ebercon 1981), and then placed on a dry filter paper under dark (Fan and Blake 1994). After 2 h incubation, the segments were placed into another set of tubes containing 15 mL of demineralized water. The conductivity of the solution was determined using a conductivity meter.
Determination of Osmolytes
Glycine betaine contents were measured by homogenizing (0.5 g) leaf samples with 10 mL milli-Q water and incubated for 24 h at 25 °C (Grieve and Grattan 1983). The homogenate was filtered and mixed with 2 N sulfuric acid in a 1:1 (v: v) ratio. 0.5 mL of the mixture was incubated on ice for 1 h and then 0.2 mL cold KI reagent was added into the solution 4 °C. The next day, mixture was centrifuged at 14,000×g for 15 min. C. Absorbance of the supernatant was read at 365 nm. A standard curve was prepared and glycine betaine contents were expressed as µmol g−1 FW.
Total water soluble carbohydrates (WSC) were measured by Anthrone method (Yemm and Willis 1954). The absorption of samples was taken at 625 nm using a PD-303 model spectrophotometer. WSC contents were determined from a standard glucose curve and expressed as mg g−1 DW.
Proline concentration from fresh leaf samples was measured as described by Bates et al (1973). Phenolic contents (mg 100 g−1 DW) were determined with Folin–Ciocalteu reagent (Cumplido-Nájera et al. 2019). Total flavonoids in the extracts were determined by a colorimetric method described by Chang et al (2002).
Total Antioxidant and Oxidant Capacity
The percent ratio of total antioxidant capacity (TAC) and total oxidant capacity (TOC) was assessed following Erel (2004) method.
Antioxidant Enzymes Assay
Fresh (0.1 g) wheat leaf samples were used for enzyme assays from all treatments. Superoxide dismutase (SOD) was assayed according to Misra and Fridovich (1972). Glutathione reductase (GR) activity was determined by Carlberg and Mannervik (1975) method. Monodehydroascorbate reductase (MDAR) activity was determined by Hossain et al (1984) protocol. Catalase (CAT) activity was determined by recording consumption of H2O2 for 30–90 s of the reaction, according to Aebi (1984). Ascorbate peroxidase (APX) activity was measured by Asada (1992) method.
Estimation of Element Contents
Dried leaf samples were used for measuring elemental concentrations in wheat tissues. P were measured using vanadomolybdate indication method, and total N was measured by McGill and Figueiredo (1993). Na and K ion concentrations were estimated by a flame photometer (Fisher scientific, USA). Estimation of Ca and Mg were performed according to Padhye (1957).
Ion transporter Genes and AQPs Expression Analysis
Gene expression analysis was performed following the MIQE guidelines (Bustin et al, 2009). Total mRNA was isolated from leaf samples (0.25 g) of all the treatments including control plants, using a Spectrum™ Plant Total RNA Kit (Sigma-Aldrich, USA). The extracted RNA was treated with DNase I (Sigma-Aldrich, USA) for the removal of any gDNA contamination and purified RNA concentration was determined using a SPECTROstar Nano (BMG LABTECH, Ortenberg, Germany) spectrophotometer at 260/280 nm with 1% agarose gel. From total RNA samples, cDNA was constructed using a GoScript™ Reverse Transcriptase assay kit (Promega, Germany). A reaction mixture (20 mL) containing 10.8 µL RNase free water, 2.5 µL 10 × buffer with MgCl2, (10 mM), 3 µL RNA (30 ng), 2.5 µL deoxynucleotide triphosphates (dNTPs), 1 µL oligo (dT) primer (10 pmol µL−1), and 0.2 µL reverse transcriptase enzyme was used (Promega, Walldorf, Germany). PCR was performed using a SureCycler 8800 (Agilent, Santa Clara, CA, USA) programmed at 42 °C for 2 h, and then at 70 °C for 5 min, and the product was stored at − 80 °C. qPCR reactions were performed to quantify mRNA level using a Rotor-Gene-6000-system (Qiagen, Valencia, CA, United States), real-time PCR through Sybr Green GoTaq® qPCR Master Mix (2X) (Promega, Germany). A reaction mixture was prepared, comprising 2 µL of template, 10 µL of GoTaq® qPCR Master Mix (2X) (Promega, Germany), 2 µL of reverse primer, 2 µL of forward primer, and DD water was added for a total volume of 20 µL. PCR assays were performed under the following conditions: 95 °C for 2 min, 95 °C for 15 s and 60 °C for 60 s followed by 40 cycles. The wheat β-actin gene (housekeeping gene) was used as an internal control for all qRT-PCR expression analysis. The reaction was performed in three biological replicates. The expression level of gene of interest (targeted AQPs gene) was normalized to that of β-Actin (internal control) by subtracting β-Actin gene CT from target gene CT. The relative gene expression was determined using 2−ΔΔCt method (Togawa et al. 2008). For each sample, triplicate biological and technical replications were done. Primer sequences used in this analysis are given in Table 2.
Data Analysis
Data were presented as mean ± CI (95% confidence interval) of three independent replicates. Data were analyzed in R (R Core Team (2018). One-way analysis of variance (ANOVA) was performed, followed by Duncan’s multiple range test to determine significant differences (at P ≤ 0.05) among treatment means. Association between leaf physiological traits and grain yield components of wheat was calculated using a heat map.
Results
Plant Growth and Biomass Production
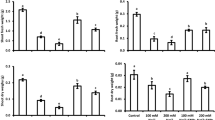
Long-term (4 weeks) salinity significantly inhibited growth and biomass production of wheat plants without any immediate recovery. For instance, salt-stressed plants (harvested 11 days after the end of salt treatment) experienced 56%, 53%, 45%, and 40% reduction in leaf area, plant height, root, and shoot dry biomass, respectively, compared with control (Fig. 1). In the absence of salt stress, Se-NPs and SB treatments had no significant effect on plant growth except root biomass, which was significantly increased. In contrast, SB and/or Se-NPs significantly improved the growth of salt-stressed plants. On average, Se-NPs more effectively promoted growth recovery of salt-stressed wheat plants than SB in this study, with maximum a biomass production was achieved under their combined application. For example, salt-stressed plants treated with SB, Se-NPs, and biochar + Se-NPs produced 25%, 33%, and 44% more root dry biomass, respectively, compared with salt treatments alone (Fig. 1).
Changes in a height, b leaf area, c shoot biomass and d root biomass in wheat plants treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected 55 days after sowing and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Grain Yield Components
Excessive salt concentration in the growth media during vegetative phase significantly inhibited grain yield formation at maturity, causing 61%, 47%, 25% reduction in total grain yield per pot, the number of fertile tillers, and the number of spikelet per spike, respectively (Fig. 2). However, spike length remained significantly unaffected (Fig. 2b). A relatively more significant reduction in grain yield than the number of fertile tillers and spikelet suggested that salt stress negatively affected both grain number and weight in wheat. Individual or combined application of amendments such as Se-NPs and SB significantly improved grain yield components, i.e., number of tillers and spikes both under salt-stressed or control conditions. Individual application of Se-NPs and SB had no significant effect on wheat grain yield but BC + Se-NPs-treated plants produced 32% and 41% higher grain yield than their respective untreated control (non-stressed) and salt-stressed plants, respectively (Fig. 2b).
Changes in a grain yield, b spike length, c number of fertile tillers and d number of spikelets per spike in wheat plants treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected at crop maturity and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Leaf Water Relations
Leaf greenness and other physiological traits of wheat plants were significantly affected by the 4 weeks of growth under salt-affected environments. For instance, compared with control, the salt-stressed plants experienced 39%, 48%, 20% and 31% reduction in chlorophyll, Pn, WUE and PSII efficiency, respectively (Fig. 3). In contrast, LWP of was significantly increased under salt stress. Amendments such as Se-NPs, biochar and Se-NPs + biochar variably affected these traits under different treatments with relatively more significant under salt-stressed than unstressed environments. Individual or combined SB and Se-NPs treatments significantly reduced LWP of salt-stressed plants but this reduction in control (non-stressed) plants was significant under Se-NPs + SB treatment only (Fig. 3a). In contrast, Se-NPs and/or SB significantly increased WUE of wheat plants under non-stressed environments only (Fig. 3b). Se-NPs + SB treatments significantly increased leaf Pn (25% and 17%) chlorophyll (38% and 28%) and PSII efficiency (18% and 12%) in salt-stressed and non-stressed plants (Fig. 3c, d, e).
Changes in a leaf water potential, b water use efficiency, c chlorophyll, d net photosynthesis and e PSII efficiency in wheat plants treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected 55 days after sowing and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Osmolytes in Leaf Tissues
Salt stress and amendments significantly affected concentrations of various osmolytes in wheat leaves (Fig. 4). For instance, compared with control, the salt-stressed plants accumulated significantly lower proline (44%) and glycine betaine (34%) contents in leaves, but there was a significant increase in WSC, phenolic, and flavonoids contents. On average, salt-stressed plants accumulated 73%, 103%, and 136% more phenolic, flavonoids, and WSC contents in leaf tissues, respectively, compared with control (Fig. 4b d, e). Se-NPs + SB, restored osmolytes production in plants with a significant impact under salt-stressed environments. Proline contents in wheat leaves were significantly increased under Se-NPs applied as individually or in combination with SB both under salt-stressed and non-stressed environments (Fig. 4a). A significant increase in WSC contents was recorded in wheat leaves in response to Se-NPs + SB treatment, with treated leaves contained 47% and 224% greater WSC contents that their respective salt-stressed and non-stressed leaves, respectively (Fig. 4b). No significant change in glycine betaine was observed in response to any amendment treatments under non-stressed environments but Se-NPs + SB significantly increased glycine betaine contents in salt-stressed plants (Fig. 4c). Leaf phenolic and flavonoid contents were significantly reduced in response to Se-NPs + SB treatments both under salt-stressed and non-stressed environments (Fig. 4d, e).
Changes in a proline, b water soluble carbohydrates, c glycine betaine, d phenolics and e flavonoids in wheat plants treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected 55 days after sowing and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Reactive Oxygen Species and Malondialdehyde
Elevated salt levels in the growth media significantly induced oxidative damage to wheat leaves. For instance, the plants exposed to 4-week salinity had 44%, 37%, and 50% higher leaf MDA, H2O2 contents, and EL (%), respectively, compared with control (Fig. 5). Amendments such as Se-NPs and SB significantly reduced oxidative stress, particularly in salt-stressed plants. For example, leaf MDA contents of salt-stressed plants were significantly reduced by SB as alone or with Se-NPs, although Se-NPs alone had no significant effect on leaf MDA under any environments (Fig. 5a). Leaf H2O2 and EL in the salt-stressed plants were significantly reduced by the individual or combined applications of Se-NPs and SB (Fig. 5b, c).
Changes in a lipid peroxidation, b hydrogen peroxide and c electrolyte leakage in wheat plants treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected 55 days after sowing and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Antioxidant Enzymes
Antioxidant enzyme activities of wheat leaves responded variably to different treatments used in this study. For instance, salt stress significantly upregulated most of the tested enzymes, i.e., SOD, APX, GR, and TOC but it significantly deceased CAT and TAC activity (Fig. 6). Amendments also variably affected the antioxidant enzymes under different environments. SB and Se-NPs treatments significantly increased SOD activity of salt-stressed and non-stressed plants, with a maximum increased achieved under Se-NPs applications alone (Fig. 6a). Similarly, Se-NPs, was relatively more effective in upregulating CAT and TAC enzymes in leaves than SB under saline or non-stressed environment (Fig. 6b, f). GR and APX responded significantly more strongly to SB + Se-NPs than their individual applications (Fig. 6c, d). TOC activity was significantly reduced by individual and combined SB and Se-NPs treatments both under salt-stressed and non-stressed environments (Fig. 6e).
Changes in activity of a SOD, b CAT, c GR, d APX, e total oxidant capacity and f total antioxidant capacity in wheat plants treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected 55 days after sowing and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Nutrient Concentrations in Wheat Leaves
Salt stress significantly inhibited the accumulation of different macro- and micro-nutrients in wheat leaves, except Na, which showed a significantly greater accumulation in salt-stressed plants. On average, salt-stressed plants contained 35%, 61%, 59%, 12%, and 11% lower N, P, K, Ca, and Mg in leaf tissues than control plants (Fig. 7). The combined application of Se-NPs and biochar significantly promoted the uptake of these ions both under salt-stressed and unstressed environments. Further, individual effect of these amendments was also significant on macronutrients uptake particularly under non-stressed environments. Individual or combined application of Se-NPs and biochar significantly reduced Na accumulation in salt-stressed wheat leaves.
Changes in content of a nitrogen, b phosphorous, c potassium, d magnesium, e calcium and f sodium in flag leaves of wheat treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected 55 days after sowing and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Changes in Expression of Key Genes
Soil salinity significantly induced the expression of various ion transporter genes, i.e., plasma membrane H+-ATPase (ATPase) Ca++ protein exchanger (CAX1), sodium/proton exchanger (NHX1), and salt overly sensitive (SOS1) in wheat leaves (Fig. 8). In the absence of salt stress, amendments had no significant effect on expression of these genes when applied alone, but there was a significant increase in the expression of ion transporter genes when Se-NPs were applied to SB-treated plants. All the tested ion transporter genes in salt-stressed leaves were further induced by SB + Se-NPs treatments, although the effect was variable when SB or Se-NPs were applied individually. For example, Se-NPs upregulated ATPase and SOS1, while SB induced expression of CAX1 in salt-stressed leaves. In contrast, NHX1 expression in salt-stressed leaves was significantly downregulated by individual application of SB or Se-NPs (Fig. 8c).
Changes in expression of a sodium/proton exchanger (NHX1); b salt overly sensitive 1 (SOS1); c Ca++ protein exchanger (CAX1); d plasma membrane H+-ATPase (ATPase) in wheat treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected 55 days after sowing and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Salt stress also significantly induced water transporter proteins, i.e., nodulin intrinsic protein (NIP), plasma membrane intrinsic protein (PIP1), and tonoplast intrinsic protein (TIP1) in leaves. Amendments, i.e., SB and/or Se-NPs significantly induced expression of these genes in control leaves, but their effect on salt-stressed plants was significant when applied in combination (Fig. 9). Induction of ionic and water transporter genes in response to SB + Se-NPs application played a crucial role in ion homeostasis and ROS regulation in salt-stressed wheat plants (Fig. 10).
Changes in expression of a nodulin intrinsic protein (NIP), b plasma membrane intrinsic protein (PIP1) and c tonoplast intrinsic protein (TIP1.1) in wheat treated with salinity, biochar (SB) and selenium nanoparticles (Se-NPs). Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. Starting from 20 days after sowing, every alternate day the plants were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, plants were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. Data were collected 55 days after sowing and were presented as mean ± CI (95% confidence interval) of three independent replicates. Variable means sharing similar letters are not significantly (P < 0.05) different from each other
Correlation Between Key Leaf Physiological Traits and Expression of Transporter Genes
Leaf levels changes in wheat physiological traits under salt and amendment treatments can be categorized into three distinct groups (1) traits regulating biomass accumulation and grain yield formation, (2) changes associated with cellular damage and (3) key transporter genes (Fig. 11). The expression of key ion and water transporter genes in wheat leaves were strongly positively correlated with each other (r2 values of 0.94 to 0.37) as well as with growth related traits (except NHX1, which had a relatively poor association). Similarly, stress related traits, i.e., leaf proline, ROS and LWP and Na concentration were strongly positively correlated with each other (r2 values of 0.92 to 0.57) but negatively associated with growth related traits (r2 values of 0.94 to 0.34).
Heatmap of correlations of grain yield and biomass production in wheat plants treated with salinity, biochar SB amendment, and foliar application of selenium nanoparticles with key leaf physiological traits. Leaf physiology data were collected at 55 days after sowing and grain yield components at crop maturity. Soybean straw biochar (SB) was mixed with soil (5%, W/V) before planting. The plants (20-day-old) were irrigated with saline water (3000 ppm NaCl) for 4 weeks. Five days after the start of salinity treatment, the plants (25-day-old) were sprayed with Se-NPs (30 ppm) or water (control) on a weekly basis for 4 weeks. These changes can be categorized into three distinct groups (1) traits regulating biomass accumulation and grain yield formation, (2) changes associated with cellular damage and (3) key transporter genes. WUE water use efficiency, LWP leaf water potential, MDA leaf malondialdehyde contents, PIP1 plasma membrane intrinsic protein, NIP nodulin intrinsic protein, NHX1 sodium/proton exchanger, TIP1.1 tonoplast intrinsic protein, SOS1 salt overly sensitive gene, NHX1 sodium/proton exchanger
Discussion
Soil Salinity Inhibits Biomass Assimilation Processes
Our study suggested that increased salt levels in soils can arrest plant growth and biomass assimilation, leading to a significant reduction in grain yield at maturity. Detrimental effects of salt stress on growth and yield of crops, including wheat, have already been reported (Gurmani et al. 2013; Ahanger et al. 2019). Increased salt levels in rhizosphere can inhibit root development, water and mineral nutrient uptake (Abbas et al. 2017), inducing osmotic stress. Under inhibited water supply, leaves tend to close their stomata, thereby restricting leaf water potential and carbon assimilation, as has been evident in this study (Fig. 3a, d). Under salt-stressed environments, there was a significant increase in Na concentrations in leaf tissues (Fig. 7f), suggesting that wheat plants translocated excessive Na+ into the aboveground tissues. This induces ionic stress and imbalances cytosolic Na+/K+ ratio (Assaha et al. 2017). Further, excessive salt in leaves accelerated ROS generation, damaging membranous cellular organelles as evident from an increased level of MDA and electrolyte leakage in salt-stressed leaves (Fig. 5). This was further supported by the strong positive correlation (r2 = 0.58–0.99) of Na+ concentration with the key stress indicators such as H2O2, MDA and phenolic compounds in leaf tissues (Fig. 11).
Our study suggest that soil salinity induces osmotic, ionic and oxidative stresses in wheat leaves, thereby impairing chloroplast structure (as evidenced by chlorophyll loss) and carbon assimilation process. Although plant species may overcome oxidative stress by activating their antioxidant enzyme system (Sofy 2016), in this study, the antioxidant enzyme capacity of wheat plants appeared to be limited due to an excessive cellular damage (Figs. 4 and 5). This was also confirmed by a limited post-stress recovery in leaf physiological traits (data collected 1 week after the last salt treatment) and reduced grain yield formation at maturity (Fig. 2).
Biochar and Se-NPs Protect Leaf Physiological Traits from Salt Injury by Restricting Na+ Uptake
Extensive research is underway on the introduction of novel management techniques to improve plant performance on salt-affected soils. In the present study, soil amendment through SB application and foliar supplementation significantly improved the growth and yield of wheat plants both under stressed and unstressed environments. Positive effects of SB (Cui et al. 2021a) and Se (Shekari et al. 2017; Shalaby et al. 2021) on the growth of stressed plants have already been reported. Cellular injury and impaired physiological functioning in salt stress plants are associated with excessive tissue Na+ concentrations (Theerawitaya et al. 2020). Further, excessive salt in the root zone can inhibit uptake of essential minerals (Fig. 7). In this study, both SB and Se-NPs significantly restricted Na+ uptake and restored uptake of essential nutrients in the salt-stressed plants (Fig. 7) and improving their physiological functioning. Increased mineral uptake and assimilation are associated with the growth recovery as these elements regulate plant function, e.g., enzyme activity, chlorophyll synthesis, photosynthesis, and stress tolerance (Fig. 11).
Biochar and Se-NPs Regulate ROS Oxidative Damage in Salt Stress Wheat Leaves
Excessive ROS accumulation in plant cells impairs the structural integrity of key macromolecules, including proteins and lipids, thereby influencing their normal functioning (Mittler 2002). In addition to increasing ion homeostasis in salt-stressed wheat, SB and Se-NPs protected cellular organelles from injury by capturing these excessive ROS. This was achieved through the modification of antioxidant enzyme activities. In salinity stressed plants, an increased lipid peroxidation and subsequent electrolyte leakage have been observed as a result of unregulated ROS, which is directly related to lipoxygenase activity (Ahanger et al. 2019) and can be reversed by amendments such as SB and Se. Hence, in the present study, reduced ROS accumulation and oxidative damage in SB and Se-treated wheat plants is likely associated with increased ROS-scavenging enzymes (Fig. 11).
Biochar-induced recovery of carbon assimilation has been linked with upregulated antioxidant functioning, and PSII stability in drought-stressed Phragmites karka (Abideen et al. 2020) and salt-stressed soybean (Mehmood et al. 2020). Similar positive effects of Se have been recorded on photosynthesis and membrane stability of maize (Jiang et al. 2017) and strawberry plants (Refaat et al. 2021). These studies suggested that the anti-oxidative defense mechanism plays a crucial role in protecting major cellular organelles from salinity. For example, critical antioxidant enzymes such SOD mediates dismutation of toxic superoxide. At the same time, H2O2 is neutralized by either CAT or through an intriguing pathway called ascorbate–glutathione (AsA-GSH) of which APX, GR, and MDAR are the key components (Alhaithloul et al. 2020). Increased functioning of AsA-GSH cycle prevents H2O2 mediated oxidative effects and protects the major functioning of cells by maintaining redox homeostasis, thereby protecting major metabolic pathways, including photosynthesis, enzyme functioning (Alam et al. 2020). In this study, SB and Se-NP modulated various antioxidant enzymes to protect growth and physiological functioning of wheat plants under saline conditions.
Secondary metabolites such as phenols and flavonoids also mediate osmoregulation and ROS scavenging, thereby strengthening the enzymatic antioxidant system to withstand oxidative stress (Austen et al. 2019). The synthesis of secondary metabolites is regulated by various environmental factors, including biotic and abiotic stresses (Ramakrishna and Ravishankar 2013). In this study, SB and Se-NP significantly amplified phenols and flavonoids levels in the salt-stressed wheat with a concomitant increase in osmolytes, including glycine betaine, proline, and carbohydrates. Increased accumulation of phenols and flavonoids strengthened the antioxidant system, thus preventing damaging effects of ROS on cellular organelles. In earlier studies by Hussein et al. (2019) and Zahedi et al. (2019) have also suggested an increased synthesis of secondary metabolites in plants in response to Se-NP application. Similarly, Ciccolini et al. (2017) demonstrated that SB-induced phenols and flavonoids accumulation in lettuce promoted antioxidant efficiency and stress adaptation. Osmolytes also scavenge ROS, maintain cellular osmolarity and eventually maintain water contents, and mediate stress signaling for quick elicitation of stress response to prevent oxidative effects. Recently, Qin et al. (2021) demonstrated that application of acetylcholine, a glycine betaine precursor, can activate signaling pathways and induce salinity stress tolerance in Nicotiana tobaccumAQ. In the present study, SB or Se-NP application also resulted in a significant enhancement in the accumulation of compatible osmolytes, contributing to strengthening tolerance mechanisms against salinity (Fig. 10).
Activation of Water Transporter Genes Increases Salt Stress Tolerance in Wheat
Increased carbon assimilation and biomass production in response to SB and Se-NPs in wheat observed in this study could be linked with upregulation of water transporter genes. Aquaporins play an important role in water transport; particularly PIPs not only promote uptake and transport of water to roots and leaves (Saibi 2021; Cui et al. 2021b), but boost CO2 diffusion, ensuring abundant substrate supply for photosynthesis and carbohydrate assimilation (Groszmann et al. 2017). The findings of this study confirm previous observations, where upregulation of AQP genes was linked with an enhanced water uptake for maintaining cell homeostasis as in Brassica rapa (Kayum et al. 2017). Similarly, upregulated AQPs genes can promote CO2 concentration in mesophyll cells by enhancing stomatal conductance (Groszmann et al. 2017). A strong positive correlation of water transporter genes with plant growth attributes and leaf physiological traits as observed in this study also supported our hypothesis (Fig. 11).
AQPs mediate and regulate rapid transmembrane water flow during growth and developmental processes from the physiological point of view. This has been confirmed through upregulation of wheat ion transporter genes and aquaporin, which are regulate ion homeostasis under salinity conditions (Santander et al. 2021). Moreover, increased Ca2++ content in the SB + Se-NPs-treated plants might have induced salt tolerance in wheat plants by modifying cellular signaling pathways (Qin et al. 2019). Consequently, these treatments increased K+ /Na+ ratio and Ca2+ concentrations primarily via Ca2+ -dependent SOS pathway. Ion transport proteins such as NHX1, HKT1 and SOS1 play a key role in regulating salinity tolerance and elicitation of signaling events (Assaha et al. 2017; Li et al. 2021). For instance, NHX1, HKT1 mediate cellular Na sequestration (Liu et al. 2018), while SOS1 forms the salt's key component over a sensitive pathway for mediating ion homeostasis (Ji et al. 2013). In this study, NHX1, CAX1, SOS1, and H + -ATPase were switched off under non-stressed environments but upregulated under salt and amendment treatments, with a significantly higher expression under their combined (SB + Se-NPs) than individual (SB or Se-NPs) application (Fig. 8). This suggests a synergy between SB and Se-NPs for stabilizing membrane potential for optimal functioning of H+ATPase, favoring K uptake and protecting wheat plants from salt-induced injury. In terms of aquaporin (AQP) proteins expression, which belongs to a major intrinsic protein superfamily, it has a crucial role in transporting water and some other molecules in a plant cell by phosphorylation. In this experiment, three wheat AQPs, i.e., P1P1, NIP, and N1P1 were upregulated in response salinity and were further significantly induced when SB + Se-NPs were applied (Fig. 9). This upregulation of aquaporin (AQP) proteins promoted water status equilibrium in plant cells and adjusted their position concerning ROS accumulation and membrane damage.
Conclusion
This study suggests that elevated salt concentrations in plant tissues impairs leaf functioning and biomass assimilation through oxidative and osmotic stresses. However, SB and Se-NPs applied individually or combined, can protect wheat plants from this salt-induced injury by restricting Na+ transport and restoring leaf functions. Our findings suggest that upregulation antioxidant system plays a key role in alleviation of negative effects of salinity on wheat physiological functioning. SB and/or Se-NPs can activate this plant defense mechanism by inducing AQPs and ion transporter genes and promoting synthesis of protective metabolites such as phenols and flavonoids and compatible osmolytes. Further, increased mineral uptake and restricted Na accumulation in SB and Se-treated plants also promoted photosynthesis and WUE, thereby contributing to growth and yield enhancement. Further studies are required to unravel the molecular mechanistic and signaling pathways associated with salt stress tolerance in wheat.
Abbreviations
- SB:
-
Biochar
- Se-NPs:
-
Selenium-chitosan nanoparticles
- EL:
-
Electrolyte leakage
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
References
Abbas G, Saqib M, Akhtar J, Murtaza G (2017) Physiological and biochemical characterization of Acacia stenophylla and Acacia albida exposed to salinity under hydroponic conditions. Can J for Res 47(9):1293–1301. https://doi.org/10.1139/cjfr-2016-0499
Abideen Z, Koyro HW, Huchzermeyer B, Ansari R, Zulfiqar F, Gul B (2020) Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol 22(2):259–266. https://doi.org/10.1111/plb.13054
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Ahanger MA, Qin C, Begum N, Maodong Q, Dong XX, El-Esawi M, El-Sheikh MA, Alatar AA, Zhang L (2019) Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol 19(1):1–12. https://doi.org/10.1186/s12870-019-2085-3
Ahmed J, Mercx S, Boutry M, Chaumont F (2020) Evolutionary and predictive functional insights into the aquaporin gene family in the allotetraploid plant Nicotiana tabacum. Int J Mol Sci 21(13):4743. https://doi.org/10.3390/ijms21134743
Akhtar SS, Andersen MN, Liu F (2015) Biochar mitigates salinity stress in potato. J Agron Crop Sci 201(5):368–378. https://doi.org/10.1111/jac.12132
Alam MJ, Ahmed KS, Nahar MK, Akter S, Uddin MA (2020) Effect of different sowing dates on the performance of maize. J Krishi Vigyan 8(2):75–81. https://doi.org/10.1111/jac.12132
Alhaithloul HA, Soliman MH, Ameta KL, El-Esawi MA, Elkelish A (2020) Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 10(1):43. https://doi.org/10.3390/biom10010043
Arnon DI (1949) Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1. https://doi.org/10.1104/pp.24.1.1
Asada K (1992) Ascorbate peroxidase–a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85(2):235–241. https://doi.org/10.1111/j.1399-3054.1992.tb04728.x
Assaha DV, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509. https://doi.org/10.3389/fphys.2017.00509
Austen N, Walker HJ, Lake JA, Phoenix GK, Cameron DD (2019) The regulation of plant secondary metabolism in response to abiotic stress: interactions between heat shock and elevated CO2. Front Plant Sci 10:1463. https://doi.org/10.3389/fpls.2019.01463
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207. https://doi.org/10.1007/bf00018060
Berners-Lee M, Kennelly C, Watson R, Hewitt CN, Kapuscinski AR, Locke KA, Peters CJ (2018) Current global food production is sufficient to meet human nutritional needs in 2050 provided there is radical societal adaptation. Elementa. https://doi.org/10.1525/elementa.310
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21(1):43–47
Brush GS, Martin EA, DeFries RS, Rice CA (1982) Comparisons of 210Pb and pollen methods for determining rates of estuarine sediment accumulation. Quatern Res 18(2):196–217. https://doi.org/10.1016/0033-5894(82)90070-9
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250(14):5475–5480. https://doi.org/10.1016/s0021-9258(19)41206-4
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal https://doi.org/10.38212/2224-6614.2748
Ciccolini V, Pellegrino E, Coccina A, Fiaschi AI, Cerretani D, Sgherri C, Quartacci MF, Ercoli L (2017) Biofortification with iron and zinc improves nutritional and nutraceutical properties of common wheat flour and bread. J Agric Food Chem 65(27):5443–5452. https://doi.org/10.1021/acs.jafc.7b01176
Cittrarasu V, Kaliannan D, Dharman K, Maluventhen V, Easwaran M, Liu WC, Balasubramanian B, Arumugam M (2021) Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci Rep 11(1):1–15. https://doi.org/10.1038/s41598-020-80327-9
Cui Y, Mao F, Zhang J, He Y, Tong YW, Peng Y (2021a) Biochar enhanced high-solid mesophilic anaerobic digestion of food waste: Cell viability and methanogenic pathways. Chemosphere 272:129863. https://doi.org/10.1016/j.chemosphere.2021.129863
Cui Y, Zhao Y, Lu Y, Su X, Chen Y, Shen Y, Lin J, Li X (2021b) In vivo single-particle tracking of the aquaporin AtPIP2; 1 in stomata reveals cell type-specific dynamics. Plant Physiol 185(4):1666–1681. https://doi.org/10.1093/plphys/kiab007
Cumplido-Nájera CF, González-Morales S, Ortega-Ortíz H, Cadenas-Pliego G, Benavides-Mendoza A, Juárez-Maldonado A (2019) The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci Hortic 245:82–89. https://doi.org/10.1016/j.scienta.2018.10.007
Diao M, Ma L, Wang J, Cui J, Fu A, Liu H-y (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33(3):671–682. https://doi.org/10.1007/s00344-014-9416-2
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37(4):277–285. https://doi.org/10.1016/j.clinbiochem.2003.11.015
Erofeeva EA (2014) Hormesis and paradoxical effects of wheat seedling (Triticum aestivum L.) parameters upon exposure to different pollutants in a wide range of doses. Dose-Response 12(1):13–017. https://doi.org/10.2203/dose-response.13-017.erofeeva
Fan S, Blake TJ (1994) Abscisic acid induced electrolyte leakage in woody species with contrasting ecological requirements. Physiol Plant 90(2):414–419. https://doi.org/10.1111/j.1399-3054.1994.tb00407.x
Farooq MA, Islam F, Ayyaz A, Chen W, Noor Y, Hu W, Hannan F, Zhou W (2022) Mitigation effects of exogenous melatonin-selenium nanoparticles on arsenic-induced stress in Brassica napus. Environ Pollut 1(292):118473. https://doi.org/10.1016/j.envpol.2021.118473
Feng R, Wei C (2012) Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant. Plant Soil Environ 58(3):105–110
Forrest KL, Bhave M (2008) The PIP and TIP aquaporins in wheat form a large and diverse family with unique gene structures and functionally important features. Funct Integrat Genom 8(2):115–133
Ghoname A, El-Nemr M, Abdel-Mawgoud A, El-Tohamy W (2010) Enhancement of sweet pepper crop growth and production by application of biological, organic and nutritional solutions. Res J Agric Biol Sci 6(3):349–355. https://doi.org/10.1007/s10343-012-0272-3
Grieve C, Grattan S (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70(2):303–307. https://doi.org/10.1007/bf02374789
Groszmann M, Osborn HL, Evans JR (2017) Carbon dioxide and water transport through plant aquaporins. Plant Cell Environ 40(6):938–961. https://doi.org/10.1111/pce.12844
Gurmani AR, Bano A, Najeeb U, Zhang J, Khan SU, Flowers TJ (2013) Exogenously applied silicate and abscisic acid ameliorates the growth of salinity stressed wheat (Triticum aestivum L.) seedlings through Na+ exclusion. Aust J Crop Sci 7:1123–1130. https://doi.org/10.2135/cropsci2000.4061702x
Hannan F, Islam F, Huang Q, Farooq MA, Ayyaz A, Fang R, Ali B, Xie X, Zhou W (2021) Interactive effects of biochar and mussel shell activated concoctions on immobilization of nickel and their amelioration on the growth of rapeseed in contaminated aged soil. Chemosphere 282:130897. https://doi.org/10.1016/j.chemosphere.2021.130897
Hamouda RA, Hussein MH, Abo-Elmagd RA, Bawazir SS (2019) Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci Rep 9(1):1–17. https://doi.org/10.1038/s41598-019-49444-y
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25(3):385–395. https://doi.org/10.1093/oxfordjournals.pcp.a076726
Hugouvieux V, Dutilleul C, Jourdain A, Reynaud F, Lopez V, Bourguignon J (2009) Arabidopsis putative selenium-binding protein1 expression is tightly linked to cellular sulfur demand and can reduce sensitivity to stresses requiring glutathione for tolerance. Plant Physiol 151(2):768–781. https://doi.org/10.1104/pp.109.144808
Hussein H-AA, Darwesh OM, Mekki BB (2019) Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal Agric Biotechnol 18:101080
Iqbal J, Shah NS, Sayed M, Niazi NK, Imran M, Khan JA, Khan ZUH, Hussien AGS, Polychronopoulou K, Howari F (2021) Nano-zerovalent manganese/biochar composite for the adsorptive and oxidative removal of Congo-red dye from aqueous solutions. J Hazard Mater 403:123854. https://doi.org/10.1016/j.jhazmat.2020.123854
Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X (2013) The salt overly sensitive (SOS) pathway: established and emerging roles. Mol Plant 6(2):275–286. https://doi.org/10.1093/mp/sst017
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7(1):1–14. https://doi.org/10.1038/srep42039
Johanson U, Gustavsson S (2002) A new subfamily of major intrinsic proteins in plants. Mol Biol Evol 19(4):456–461. https://doi.org/10.1093/oxfordjournals.molbev.a004101
Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P (2000) The role of aquaporins in cellular and whole plant water balance. Biochimica Et Biophysica Acta (BBA) Biomembranes 1465(12):324–342
Joseph S (2015) Biochar for environmental management: science, technology and implementation. Routledge. https://doi.org/10.4324/9780203762264
Kayum M, Park J-I, Nath UK, Biswas MK, Kim H-T, Nou I-S (2017) Genome-wide expression profiling of aquaporin genes confer responses to abiotic and biotic stresses in Brassica rapa. BMC Plant Biol 17(1):1–18. https://doi.org/10.1186/s12870-017-0979-5
Li S-J, Wu G-Q, Lin L-Y (2021) AKT1, HAK5, SKOR, HKT1;5, SOS1 and NHX1 synergistically control Na+ and K+ homeostasis in sugar beet (Beta vulgaris L.) seedlings under saline conditions. J Plant Biochem Biotechnol. https://doi.org/10.1007/s13562-021-00656-2
Liu M, Song X, Jiang Y (2018) Growth, ionic response, and gene expression of shoots and roots of perennial ryegrass under salinity stress. Acta Physiol Plant 40(6):1–8. https://doi.org/10.1007/s11738-018-2687-7
Machado RMA, Serralheiro RP (2017) Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 3(2):30. https://doi.org/10.3390/horticulturae3020030
Mahmoud M, Salim MA, El-Monem A, Amany A, El-Mahdy AA (2019) Effect of magnetic brackish-water treatments on morphology, anatomy and yield productivity of wheat (Triticum aestivum. Alexandria Sci Exchange J 40:604–617. https://doi.org/10.21608/ASEJAIQJSAE.2019.63578
Maurel C, Boursiac Y, Luu D-T, Santoni V, Shahzad Z, Verdoucq L (2015) Aquaporins in plants. Physiol Rev 95(4):1321–1358. https://doi.org/10.1152/physrev.00008.2015
McGill W, Figueiredo C (1993) Total nitrogen. Soil sampling and methods of analysis https://doi.org/10.1201/9781420005271.ch22
Mehmood S, Ahmed W, Ikram M, Imtiaz M, Mahmood S, Tu S, Chen D (2020) Chitosan modified biochar increases soybean (Glycine max L.) resistance to salt-stress by augmenting root morphology, antioxidant defense mechanisms and the expression of stress-responsive genes. Plants 9(9):1173. https://doi.org/10.3390/plants9091173
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175. https://doi.org/10.1016/s0021-9258(19)45228-9
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Molnár Á, Kolbert Z, Kéri K, Feigl G, Ördög A, Szőllősi R, Erdei L (2018) Selenite-induced nitro-oxidative stress processes in Arabidopsis thaliana and Brassica juncea. Ecotoxicol Environ Saf 148:664–674. https://doi.org/10.1016/j.ecoenv.2017.11.035
Mosallam FM, El-Sayyad GS, Fathy RM, El-Batal AI (2018) Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microb Pathog 122:108–116. https://doi.org/10.1016/j.micpath.2018.06.013
Munns R, Day DA, Fricke W, Watt M, Arsova B, Barkla B, Bose J, Byrt C, Chen Z, Foster K, Gilliham M, Henderson S, Jenkins C, Kronzucker H, Miklavcic S, Plett D, Roy S, Shabala S, Shelden M, Soole K, Taylor N, Tester M, Wege S, Wegner L, Tyerman S (2019) Energy costs of salt tolerance in crop plants. New Phytol 225(3):1072–1090. https://doi.org/10.1111/nph.15864
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. Methods of soil analysis: Part 3 Chemical methods 5:961–1010 https://doi.org/10.2134/agronmonogr9.2.2ed.c29
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (ed) Methods of soil snalysis. Part 2. Chemical and microbiological properties. Agronomy mongraphs, vol 9
Padhye V (1957) A rapid method for the determination of calcium and magnesium in plant material by titration with disodium ethylenediaminetetra-acetate. Analyst 82(978):634–638. https://doi.org/10.1039/an9578200634
Pramanik P, Krishnan P, Maity A, Mridha N, Mukherjee A, Rai V (2020) Application of nanotechnology in agriculture. In: Environmental Nanotechnology. vol 4. Springer, pp 317–348. https://doi.org/10.1007/978-3-030-26668-4_9
Qin C, Ahanger MA, Lin B, Huang Z, Zhou J, Ahmed N, Ai S, Mustafa NSA, Ashraf M, Zhang L (2021) Comparative transcriptome analysis reveals the regulatory effects of acetylcholine on salt tolerance of Nicotiana benthamiana. Phytochemistry 181:112582. https://doi.org/10.1016/j.phytochem.2020.112582
Qin H, Wang J, Chen X, Wang F, Peng P, Zhou Y, Miao Y, Zhang Y, Gao Y, Qi Y (2019) Rice Os DOF 15 contributes to ethylene-inhibited primary root elongation under salt stress. New Phytol 223(2):798–813. https://doi.org/10.1111/nph.15824
Quarrie S, Jones HG (1977) Effects of abscisic acid and water stress on development and morphology of wheat. J Exp Bot 28(1):192–203. https://doi.org/10.1093/jxb/28.1.192
R Core Team (2018) R a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2014
Ramakrishna A, Ravishankar G (2013) Role of plant metabolites in abiotic stress tolerance under changing climatic conditions with special reference to secondary compounds. Climate change and plant abiotic stress tolerance. https://doi.org/10.1002/9783527675265.ch26
Refaat AM, El-Sheikh E, Ragheb D, Shalaby A (2021) The comparative efficacy of spinosad, lufenuron and hexythiazox on some strawberry pests under laboratory and field conditions. Zagazig J Agric Res 48(3):679–688. https://doi.org/10.21608/ZJAR.2021.191288
Roberts DM, Routray P (2017) The nodulin 26 intrinsic protein subfamily. In: Plant Aquaporins. Springer, pp 267–296. https://doi.org/10.1007/978-3-319-49395-4_13
Saibi W (2021) Brini faiçal. Oxidative stress and antioxidant defense in Brassicaceae plants under abiotic stresses, pp 232–244. https://doi.org/10.25177/jps.5.1.ra.10694
Santander C, Aroca R, Cartes P, Vidal G, Cornejo P (2021) Aquaporins and cation transporters are differentially regulated by two arbuscular mycorrhizal fungi strains in lettuce cultivars growing under salinity conditions. Plant Physiol Biochem 158:396–409. https://doi.org/10.1016/j.plaphy.2020.11.025
Schiavon M, Lima LW, Jiang Y, Hawkesford MJ (2017) Effects of selenium on plant metabolism and implications for crops and consumers. In: Selenium in plants. Springer, pp 257–275. https://doi.org/10.1007/978-3-319-56249-0_15
Shalaby SE, Al-Balakocy NG, Beliakova MK, Othman AM (2021) Effect of wet processing operations on the functional properties imparted to polyester fabrics loaded with different metal oxides NPs part II: effect of the different sequences of dyeing. J Eng Fibers Fabr 16:15589250211005760. https://doi.org/10.1177/15589250211005760
Shekari Z, Zare HR, Falahati A (2017) Developing an impedimetric aptasensor for selective label–free detection of CEA as a cancer biomarker based on gold nanoparticles loaded in functionalized mesoporous silica films. J Electrochem Soc 164(13):B739
Sikora F, Moore K (2014) Soil test methods from the Southeastern United States. Southern Cooperat Series Bullet 419:54–58
Sofy MR (2016) Effect of gibberellic acid, paclobutrazol and zinc on growth, physiological attributes and the antioxidant defense system of soybean (Glycine max) under salinity stress. Int J Plant Res 6(3):64–87
Soliman M, Elkelish A, Souad T, Alhaithloul H, Farooq M (2020a) Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol Mol Biol Plants 26(3):501–511. https://doi.org/10.1007/s12298-020-00765-7
Soliman MH, Alnusaire TS, Abdelbaky NF, Alayafi AAM, Hasanuzzaman M, Rowezak MM, El-Esawi M, Elkelish A (2020b) Trichoderma-Induced Improvement in Growth, Photosynthetic Pigments, Proline, and Glutathione Levels in Cucurbita pepo Seedlings under Salt Stress. Phyton 89(3):473–486. https://doi.org/10.32604/phyton.2020.08795
Sun Y, Yang J, Yao R, Chen X, Wang X (2020) Biochar and fulvic acid amendments mitigate negative effects of coastal saline soil and improve crop yields in a three year field trial. Sci Rep 10(1):8946. https://doi.org/10.1038/s41598-020-65730-6
Tanveer M, Shabala S (2018) Targeting redox regulatory mechanisms for salinity stress tolerance in crops. In: Salinity Responses and Tolerance in Plants, Volume 1. Springer, pp 213–234
Theerawitaya C, Tisarum R, Samphumphuang T, Takabe T, Cha-Um S (2020) Expression levels of the Na+/K+ transporter OsHKT2; 1 and vacuolar Na+/H+ exchanger OsNHX1, Na enrichment, maintaining the photosynthetic abilities and growth performances of indica rice seedlings under salt stress. Physiol Mol Biol Plants 26(3):513–523. https://doi.org/10.1007/978-3-319-75671-4_8
Togawa T, Dunn WA, Emmons AC, Nagao J, Willis JH (2008) Developmental expression patterns of cuticular protein genes with the R&R consensus from Anopheles gambiae. Insect Biochem Mol Biol 38(5):508–519. https://doi.org/10.1016/j.ibmb.2007.12.008
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wani SH, Tripathi P, Zaid A, Challa GS, Kumar A, Kumar V, Upadhyay J, Joshi R, Bhatt M (2018) Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.). Plant Mol Biol 97(6):469–487. https://doi.org/10.1007/s11103-018-0761-6
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508
Zahedi SM, Abdelrahman M, Hosseini MS, Hoveizeh NF, Tran L-SP (2019) Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ Pollut 253:246–258
Zhang P, Senge M, Dai Y (2016) Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev Agric Sci 4:46–55. https://doi.org/10.1139/cjfr-2016-0499
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
MHS, GSHA and UN contributed to the conception and design of the experiments. MHS, TSA, AMA and HSA conducted experiments, collected and analysed plant samples. Data analysis and write up were performed by MHS, AAK, MAF and MY. The first draft of the manuscript was written by MHS and UN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Handling Editor: Heather Nonhebel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soliman, M.H., Alnusairi, G.S.H., Khan, A.A. et al. Biochar and Selenium Nanoparticles Induce Water Transporter Genes for Sustaining Carbon Assimilation and Grain Production in Salt-Stressed Wheat. J Plant Growth Regul 42, 1522–1543 (2023). https://doi.org/10.1007/s00344-022-10636-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10636-y