Abstract
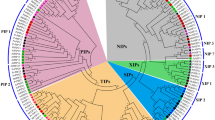
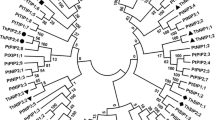
Aquaporins, members of major intrinsic proteins (MIPs), transport water across cellular membranes and play vital roles in all organisms. Adversities such as drought, salinity, or chilling affect water uptake and transport, and numerous plant MIPs are reported to be differentially regulated under such stresses. However, MIP genes have been not yet been characterized in wheat, the largest cereal crop. We have identified 24 PIP and 11 TIP aquaporin genes from wheat by gene isolation and database searches. They vary extensively in lengths, numbers, and sequences of exons and introns, and sequences and cellular locations of predicted proteins, but the intron positions (if present) are characteristic. The putative PIP proteins show a high degree of conservation of signature sequences or residues for membrane integration, water transport, and regulation. The TIPs are more diverse, some with potential for water transport and others with various selectivity filters including a new combination. Most genes appear to be expressed as expressed sequence tags, while two are likely pseudogenes. Many of the genes are highly identical to rice but some are unique, and many correspond to genes that show differential expression under salinity and/or drought. The results provide extensive information for functional studies and developing markers for stress tolerance.





Similar content being viewed by others
References
Barkla BJ, Vera-Estrella R, Pantoja O, Kirch H-H, Bohnert HJ (1999) Aquaporin localization—how valid are the TIP and PIP labels? Trends Plant Sci 4:86–88
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T (2006) Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci USA 103:269–274
Beuron F, Cahérec FL, Guillam M-T, Cavalier A, Garret A, Tassan J-P, Delamarche C, Schultz P, Mallouh V, Rolland J-P, Hubert J-F, Gouranton J, Thomas D (1995) Structural analysis of a MIP family protein from the digestive tract of Cicadella viridis. J Biol Chem 270:17414–17422
Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18:565–570
Brandizzi F, Frangne N, Marc-Martin S, Hawes C, Neuhaus JM, Paris N (2002) The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14:1077–1092
Carvajal M, Cooke DT, Clarkson DT (1996) Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta 199:372–381
Chaumont F, Barrieu F, Jung R, Chrispeels MJ (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol 122:1025–1034
Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125:1206–1215
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97:749–764
Daniels MJ, Mirkov TE, Chrispeels MJ (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol 106:1325–1333
Daniels MJ, Chaumont F, Mirkov TE, Chrispeels MJ (1996) Characterization of a new vacuolar membrane aquaporin sensitive to mercury at a unique site. Plant Cell 8:587–599
de Groot BL, Frigato T, Helms V, Grubmuller H (2003) The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol 333:279–293
Dubcovsky J, Maria GS, Epstein E, Luo MC, Dvorak J (1996) Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theor Appl Genet 92:448–454
Fetter K, Van Wilder V, Moshelion M, Chaumont F (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16:215–228
Forrest KL, Bhave M (2007) Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Funct Integr Genomics 7:263–289
Foster W, Helm A, Turnbull I, Gulati H, Yang B, Verkman AS, Skach WR (2000) Identification of sequence determinants that direct different intracellular folding pathways for aquaporin-1 and aquaporin-4. J Biol Chem 275:34157–34165
Fotiadis D, Jenö P, Mini T, Wirtz S, Müller SA, Fraysse L, Kjellbom P, Engel A (2001) Structural characterization of two aquaporins isolated from native spinach leaf plasma membranes. J Biol Chem 276:1707–1714
Froger A, Tallur B, Thomas D, Delamarche C (1998) Prediction of functional residues in water channels and related proteins. Protein Sci 7:1458–1468
Froger A, Rolland JP, Bron P, Lagree V, Le Caherec F, Deschamps S, Hubert JF, Pellerin I, Thomas D, Delamarche C (2001) Functional characterization of a microbial aquaglyceroporin. Microbiology 147:1129–1135
Fu D, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM (2000) Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290:481–486
Guo L, Wang ZY, Lin H, Cui WE, Chen J, Liu M, Chen ZL, Qu LJ, Gu H (2006) Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res 16:277–286
Gustavsson S, Lebrun A-S, Norden K, Chaumont F, Johanson U (2005) A novel plant major intrinsic protein in Physcomitrella patens most similar to bacterial glycerol channels. Plant Physiol 139:287–295
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Heller KB, Lin ECC, Wilson TH (1980) Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol 144:274–278
Heymann JB, Engel A (1999) Aquaporins: phylogeny, structure, and physiology of water channels. News Physiol Sci 14:187–193
Heymann JB, Engel A (2000) Structural clues in the sequences of the aquaporins. J Mol Biol 295:1039–1053
Houde M, Belcaid M, Ouellet F, Danyluk J, Monroy AF, Dryanova A, Gulick P, Bergeron A, Laroche A, Links MG, MacCarthy L, Crosby WL, Sarhan F (2006) Wheat EST resources for functional genomics of abiotic stress. BMC Genomics 7:149
Ishikawa F, Suga S, Uemura T, Sato MH, Maeshima M (2005) Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett 579:5814–5820
Jauh GY, Phillips TE, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11:1867–1882
Johnson KD, Chrispeels MJ (1992) Tonoplast-bound protein kinase phosphorylates tonoplast intrinsic protein. Plant Physiol 100:1787–1795
Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10:451–459
Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P (2000) The role of aquaporins in cellular and whole plant water balance. Biochim Biophys Acta 1465:324–342
Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126:1358–1369
Jung JS, Preston GM, Smith BL, Guggino WB, Agre P (1994) Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem 269:14648–14654
Kaldenhoff R, Fischer M (2006a) Aquaporins in plants. Acta Physiol 187:169–176
Kaldenhoff R, Fischer M (2006b) Functional aquaporin diversity in plants. Biochim Biophys Acta Biomembr 1758:1134–1141
Kammerloher W, Fischer U, Piechottka GP, Schäffner AR (1994) Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J 6:187–199
Katsuhara M, Akiyama Y, Koshio K, Shibasaka M, Kasamo K (2002) Functional analysis of water channels in barley roots. Plant Cell Physiol 43:885–893
Kawaura K, Mochida K, Yamazaki Y, Ogihara Y (2006) Transcriptome analysis of salinity stress responses in common wheat using a 22k oligo-DNA microarray. Funct Integr Genomics 6:132–142
Kukulski W, Schenk AD, Johanson U, Braun T, de Groot BL, Fotiadis D, Kjellbom P, Engel A (2005) The 5 A structure of heterologously expressed plant aquaporin SoPIP2;1. J Mol Biol 350:611–616
Lagrée V, Froger A, Deschamps S, Hubert J-F, Delamarche C, Bonnec G, Thomas D, Gouranton J, Pellerin I (1999) Switch from an aquaporin to a glycerol channel by two amino acids substitution. J Biol Chem 274:6817–6819
Le Caherec F, Deschamps S, Delamarche C, Pellerin I, Bonnec G, Guillam MT, Thomas D, Gouranton J, Hubert JF (1996) Molecular cloning and characterization of an insect aquaporin functional comparison with aquaporin 1. Eur J Biochem 241:707–715
Lian H-L, Yu X, Ye Q, Ding X, Kitagawa Y, Kwak S-S, Su W-A, Tang Z-C (2004) The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol 45:481–489
Lu Y, Turnbull IR, Bragin A, Carveth K, Verkman AS, Skach WR (2000) Reorientation of aquaporin-1 topology during maturation in the endoplasmic reticulum. Mol Biol Cell 11:2973–2985
Ma S, Quist TM, Ulanov A, Joly R, Bohnert HJ (2004) Loss of TIP1;1 aquaporin in Arabidopsis leads to cell and plant death. Plant J 40:845–859
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Maurel C (1997) Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol 48:399–429
Maurel C, Reizer JU, Schroeder JI, Chrispeels MJ (1993) The vacuolar membrane protein g-TIP creates water specific channels in Xenopus oocytes. EMBO J 12:2241–3347
Maurel C, Kado RT, Guern J, Chrispeels MJ (1995) Phosphorylation regulates the water channel activity of the seed-specific aquaporin a-TIP. EMBO J 14:3028–3035
Maurel C, Javot H, Lauvergeat V, Gerbeau P, Tournaire C, Santoni V, Heyes J (2002) Molecular physiology of aquaporins in plants. Int Rev Cytol 215:105–148
Mizutani M, Watanabe S, Nakagawa T, Maeshima M (2006) Aquaporin NIP2;1 is mainly localized to the ER membrane and shows root-specific accumulation in Arabidopsis thaliana. Plant Cell Physiol 47:1420–1426
Mohammadi M, Kav NNV, Deyholos MK (2007) Transcriptional profiling of hexaploid wheat (Triticum aestivum L.) roots identifies novel, dehydration-responsive genes. Plant Cell Environ 30:630–645
Morillon R, Lassalles J-P (2002) Water deficit during root development: effects on the growth of roots and osmotic water permeability of isolated root protoplasts. Planta 214:392–399
Muramatsu S, Mizuno T (1989) Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res 17:4378
Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (2000) Structural determinants of water permeation through aquaporin-1. Nature 407:599–605
Niemietz CM, Tyerman SD (1997) Characterization of water channels in wheat root membrane vesicles. Plant Physiol 115:561–567
Ott CM, Lingappa VR (2002) Integral membrane protein biosynthesis: why topology is hard to predict. J Cell Sci 115:2003–2009
Page RDM (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88:11110–11114
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
Preston GM, Jung JS, Guggino WB, Agre P (1993) The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem 268:17–20
Quigley F, Rosenberg JM, Shachar-Hill Y, Bohnert HJ (2001) From genome to function: the Arabidopsis aquaporins. Genome Biol 3:research0001.1–0001.17
Roy SW, Gilbert W (2006) The evolution of spliceosomal introns: patterns, puzzles and progress. Nat Rev Genet 7:211–221
Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M (2005) Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol 46:1568–1577
Santoni V, Verdoucq L, Sommerer N, Vinh J, Pflieger D, Maurel C (2006) Methylation of aquaporins in plant plasma membrane. Biochem J 400:189–197
Schunmann PH, Ougham HJ (1996) Identification of three cDNA clones expressed in the leaf extension zone and with altered patterns of expression in the slender mutant of barley: a tonoplast intrinsic protein, a putative structural protein and protochlorophyllide oxidoreductase. Plant Mol Biol 31:529–537
Shi LB, Skach WR, Ma TH, Verkman AS (1995) Distinct biogenesis mechanisms for the water channels MIWC and CHIP28 at the endoplasmic reticulum. Biochemistry 34:8250–8256
Shirasu K, Schulman AH, Lahaye T, Schulze-Lefert P (2000) A contiguous 66-kb barley DNA sequence provides evidence for reversible genome expansion. Genome Res 10:908–915
Skach WR, Shi L, Calayag C, Frigeri A, Lingappa VR, Verkman AS (1994) Biogenesis and transmembrane topology of the CHIP28 water channel at the endoplasmic reticulum. J Cell Biol 125:803–815
Sonnhammer EL, Koonin EV (2002) Orthology, paralogy and proposed classification for paralog subtypes. Trends Genet 18:619–620
Suga S, Komatsu S, Maeshima M (2002) Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiol 43:1229–1237
Sui H, Han B-G, Lee JK, Walian P, Jap BK (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872–878
Tajkhorshid E, Nollert P, Jensen MO, Miercke LJW, O’Connell J, Stroud RM, Schulten K (2002) Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 296:525–530
Thomas D, Bron P, Ranchy G, Duchesne L, Cavalier A, Rolland J-P, Raguenes-Nicol C, Hubert J-F, Haase W, Delamarche C (2002) Aquaglyceroporins, one channel for two molecules. Biochim Biophys Acta Bioenerg 1555:181–186
Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P (2006) Structural mechanism of plant aquaporin gating. Nature 439:688–694
Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425:393–397
Wahlberg JM, Spiess M (1997) Multiple determinants direct the orientation of signal-anchor proteins: the topogenic role of the hydrophobic signal domain. J Cell Biol 137:555–562
Wallace IS, Roberts DM (2004) Homology modeling of representative subfamilies of arabidopsis major intrinsic proteins. Classification based on the aromatic/arginine selectivity filter. Plant Physiol 135:1059–1068
Wallace IS, Wills DM, Guenther JF, Roberts DM (2002) Functional selectivity for glycerol of the nodulin 26 subfamily of plant membrane intrinsic proteins. FEBS Lett 523:109–112
Zardoya R (2005) Phylogeny and evolution of the major intrinsic protein family. Biol Cell 97:397–414
Zardoya R, Villalba S (2001) A phylogenetic framework for the Aquaporin family in eukaryotes. J Mol Evol 52:391–404
Zhang W-H, Tyerman SD (1999) Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiol 120:849–857
Acknowledgements
KF is supported by a Grains Research and Development Corporation (GRDC, Australia) Ph.D. scholarship. We gratefully acknowledge Dr. Greg Grimes (Australian Winter Cereals Collection, Tamworth, Australia) for providing the seed samples, Dr. Paula Moolhuijzen (State Agricultural Biotechnology Centre, Murdoch University, Western Australia) for advice on EST searches, and Prof. Rudi Appels (State Agricultural Biotechnology Centre, Western Australia) for advice on the project. We thank Damian Cockfield for confirming some of the DNA sequences. We are also thankful to the anonymous referees for their critical comments and suggestions, which helped in the revision of the manuscript greatly.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Major differences between PIP1 and PIP2 isoforms of rice (DOC 21.5 kb)
Supplementary Table S2
Summary of PIP and TIP genes obtained through amplification of gDNA (DOC 30.0 kb)
Supplementary Table S3
Sequence identities of PIPs and TIPs of wheat with those of other plants (DOC 50 kb)
Supplementary Table S4
Highest sequence identity scores from ClustalW alignments {score for Cranbrook sequence}: entries indicate poor identity score for TC or EST, therefore, score from cDNA for the gene isolated from cv. Cranbrook was considered also (DOC 31.0 kb)
Supplemental Figure S1
Primer positions on wheat PIP and TIP cDNA sequences from Genbank. Black shading indicates positions of NPA motifs. Gray shading indicates positions of TMHs (DOC 26.0 kb)
Supplemental Figure S2
(DOC 21.0 kb)
Supplemental Figure S3
Sequence alignment of predicted PIP cDNAs (exon contigs) of genes cloned from gDNA of wheat (DOC 64.5 kb)
Supplemental Figure S4
Sequence alignment of predicted TIP cDNAs (exon contigs) of genes cloned from gDNA of wheat (DOC 74.5 kb)
Supplemental Figure S5
Alignment of putative amino acids sequences of wheat PIPs with aquaporins, a glycerol facilitator, and an aquaglyceroporin (DOC 119 kb)
Supplemental Figure S6
Alignment of putative amino acids sequences of wheat TIPs with other aquaporins, a glycerol facilitator, and an aquaglyceroporin (DOC 81.0 kb)
Rights and permissions
About this article
Cite this article
Forrest, K.L., Bhave, M. The PIP and TIP aquaporins in wheat form a large and diverse family with unique gene structures and functionally important features. Funct Integr Genomics 8, 115–133 (2008). https://doi.org/10.1007/s10142-007-0065-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-007-0065-4