Abstract
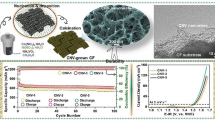
In this paper, with the aim of improving hydrogen storage efficiency, cerium vanadate/reduced graphene oxide (CeVO4/rGO) nanocomposite was synthesized through a one-step hydrothermal method due to its unique characteristics, including suitable electrochemical behavior, high specific surface area, and high porosity. In addition to controlling the growth of CeVO4 nanoparticles, hydrazine reduces graphene oxide (GO) to reduced graphene oxide (rGO) and enables one-step synthesis. FESEM images showed that the rod-like CeVO4 nanoparticles were dispersed on the graphene plates. According to the obtained storage results, with the addition of graphene, the synergistic effect between the layers has increased and, as a result, the hydrogen storage capacity has increased. The highest amount of electrochemical storage of hydrogen in the synthesized nanocomposite was about 5430 mAh/g, which is a significant result compared to other nanostructures used.










Similar content being viewed by others
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
A. Zonarsaghar, M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, Hydrothermal synthesis of CeVO4 nanostructures with different morphologies for electrochemical hydrogen storage. Ceram. Int. 47, 35248 (2021)
K. Spyrou, D. Gournis, P. Rudolf, Hydrogen storage in graphene-based materials: efforts towards enhanced hydrogen absorption. ECS J. Solid State Sci. Technol. 2, M3160 (2013)
U. Eberle, M. Felderhoff, F. Schueth, Chemical and physical solutions for hydrogen storage. Angew. Chem. Int. Ed. 48, 6608 (2009)
P.E. de Jongh, P. Adelhelm, Nanosizing and nanoconfinement: new strategies towards meeting hydrogen storage goals. Chem. Sus. Chem. 3, 1332 (2010)
B. Vinayan, R. Nagar, S. Ramaprabhu, Solar light assisted green synthesis of palladium nanoparticle decorated nitrogen doped graphene for hydrogen storage application. J. Mater. Chem. A 1, 11192 (2013)
T. Gholami, M. Pirsaheb, Review on effective parameters in electrochemical hydrogen storage. Int. J. Hydrogen Energy 46, 783 (2020)
A. Zonarsaghar, M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, Sonochemical synthesis of CeVO4 nanoparticles for electrochemical hydrogen storage. Int. J. Hydrogen Energy 47, 5403 (2022)
E. Boateng, A. Chen, Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 6, 100022 (2020)
S. Seenithurai, R.K. Pandyan, S.V. Kumar, C. Saranya, M. Mahendran, Li-decorated double vacancy graphene for hydrogen storage application: a first principles study. Int. J. Hydrogen Energy 39, 11016 (2014)
H.G. Shiraz, M.G. Shiraz, Palladium nanoparticle and decorated carbon nanotube for electrochemical hydrogen storage. Int. J. Hydrogen Energy 42, 11528 (2017)
K. Manoharan, V.K. Palaniswamy, K. Raman, R. Sundaram, Investigation of solid state hydrogen storage performances of novel NaBH4/Ah-BN nanocomposite as hydrogen storage medium for fuel cell applications. J. Alloys Compd. 860, 158444 (2021)
N. Ekthammathat, T. Thongtem, A. Phuruangrat, S. Thongtem, Synthesis and characterization of CeVO4 by microwave radiation method and its photocatalytic activity. J. Nanomater. 2013, 434197 (2013)
Q. Liu, C. Fan, H. Tang, T. Ma, J. Shen, One-step synthesis of recycled 3D CeVO4/rGO composite aerogels for efficient degradation of organic dyes. RSC Adv. 6, 85779 (2016)
S. Abdolhosseinzadeh, H. Asgharzadeh, H.S. Kim, Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 5, 1 (2015)
E. Jaafar, M. Kashif, S.K. Sahari, Z. Ngaini, Study on morphological, optical and electrical properties of graphene oxide (GO) and reduced graphene oxide (rGO). Mater. Sci. Forum. 917, 112 (2018)
M. Fathy, A. Gomaa, F.A. Taher, M.M. El-Fass, A.E.-H.B. Kashyout, Optimizing the preparation parameters of GO and rGO for large-scale production. J. Mater. Sci. 51, 5664 (2016)
M.P. Lavin-Lopez, A. Paton-Carrero, L. Sanchez-Silva, J. Valverde, A. Romero, Influence of the reduction strategy in the synthesis of reduced graphene oxide. Adv. Powder Technol. 28, 3195 (2017)
M. Mousavi-Kamazani, R. Rahmatolahzadeh, S.A. Shobeiri, F. Beshkar, Sonochemical synthesis, formation mechanism, and solar cell application of tellurium nanoparticles. Ultrason. Sonochem. 39, 233 (2017)
N. Cao, Y. Zhang, Study of reduced graphene oxide preparation by Hummers’ method and related characterization. J. Nanomater. 2015, 168125 (2015)
P. Intaphong, S. Jonjana, A. Phuruangrat, P. Pookmanee, S. Artkla, S. Thongtem, T. Thongtem, Synthesis and photocatalytic properties of CeVO4 nanoparticles under UV irradiation. Dig. J. Nanomater. Bios. 10, 1281 (2015)
I. Othman, J.H. Zain, M.A. Haija, F. Banat, Catalytic activation of peroxymonosulfate using CeVO4 for phenol degradation: an insight into the reaction pathway. Appl. Catal. B 266, 118601 (2020)
V. Ţucureanu, A. Matei, A.M. Avram, FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 46, 502 (2016)
G. Surekha, K.V. Krishnaiah, N. Ravi, R.P. Suvarna, FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method. J. Phys. Conf. Ser. 1495, 012012 (2020)
T.F. Emiru, D.W. Ayele, Controlled synthesis, characterization and reduction of graphene oxide: a convenient method for large scale production, Egypt. J. Basic Appl. Sci. 4, 74 (2017)
D. He, Z. Peng, W. Gong, Y. Luo, P. Zhao, L. Kong, Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv. 5, 11966 (2015)
M.H. Choi, Y.J. Min, G.H. Gwak, S.M. Paek, J.M. Oh, A nanostructured Ni/graphene hybrid for enhanced electrochemical hydrogen storage. J. Alloys Compd. 610, 231 (2014)
M. Kaur, K. Pal, An investigation for hydrogen storage capability of zirconia-reduced graphene oxide nanocomposite. Int. J. Hydrogen Energy 41, 21861 (2016)
F. Ambroz, T.J. Macdonald, V. Martis, I.P. Parkin, Evaluation of the BET theory for the characterization of meso and microporous MOFs. Small Methods 2, 1800173 (2018)
M. Kaur, K. Pal, Potential electrochemical hydrogen storage in nickel and cobalt nanoparticles-induced zirconia-graphene nanocomposite. J. Mater. Sci. Mater. Electron. 31, 10903 (2020)
Z.A. ALOthman, A review: fundamental aspects of silicate mesoporous materials. Materials 5, 2874 (2012)
Z. Hu, L. Zhang, J. Huang, Z. Feng, Q. Xiong, Z. Ye et al., Self-supported nickel-doped molybdenum carbide nanoflower clusters on carbon fiber paper for an efficient hydrogen evolution reaction. Nanoscale 13, 8264 (2021)
M. Ghodrati, M. Mousavi-Kamazania, S. Zinatloo-Ajabshir, Zn3V3O8 nanostructures: facile hydrothermal/solvothermal synthesis, characterization, and electrochemical hydrogen storage. Ceram. Int. 46, 28894 (2020)
S. Ashrafi, M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, A. Asghari, Novel sonochemical synthesis of Zn2V2O7 nanostructures for electrochemical hydrogen storage. Int. J. Hydrogen Energy 45, 21611 (2020)
Acknowledgements
The authors would like to thank Semnan University Research Council for the financial support of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MR, MM-K, and SZ-A. The first draft of the manuscript was written by MR, MM-K, and SZ-A, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rezayeenik, M., Mousavi-Kamazani, M. & Zinatloo-Ajabshir, S. CeVO4/rGO nanocomposite: facile hydrothermal synthesis, characterization, and electrochemical hydrogen storage. Appl. Phys. A 129, 47 (2023). https://doi.org/10.1007/s00339-022-06325-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06325-y