Abstract
Organismal symbioses are fundamental to biodiversity, evolution, and ecosystem functioning. On coral reefs, many decapod species have formed distinct epibiotic symbioses through decoration tendencies that enhance diet, camouflage, and defence. The red decorator crab, Schizophrys aspera (Majidae: Decapoda), has a broad Indo-Pacific distribution and is a successful predator of juvenile crown-of-thorns seastars (CoTS; Acanthaster sp.). However, little is known of the biology and decorating symbioses of S. aspera on the Great Barrier Reef (GBR), where CoTS pose ongoing management challenges. We characterised S. aspera and its epibiont community collected in coral rubble patches on the southern GBR. S. aspera predominantly used sponges (94 ± 1%; mean ± SE) in its decoration, with greater proportions of the carapace covered for juveniles (58 ± 5%) and females (46 ± 4%) compared to males (24 ± 4%). In short-term (8-d) experiments, S. aspera substantially reduced sponge (31%) and algal (47%) cover on rubble pieces, demonstrating its potential to alter sessile communities. The close association of S. aspera with sponges and algae likely reflects its diet and enhances camouflage and chemical defence in its coral rubble niche on the GBR. As sessile taxa are often noxious, we postulate that these symbioses may confer resilience of S. aspera to plancitoxins in its consumption of CoTS. Evaluating how epibiont diversity and biochemistry shape the habitat associations, distribution, and role of S. aspera as predator and prey may be important to understanding its ability to mediate CoTS densities on the GBR and elsewhere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many organisms depend on symbioses for survival and reproduction (Thompson 1999). Indeed, positive mutualisms are ubiquitous and may lie at the root of evolutionary phenomena such as the origin of eukaryotic cells and radiation of flowering plants (Stachowicz 2001). On coral reefs, mutualistic, parasitic, and commensal relationships are common and can be key drivers of physiology, biodiversity, fisheries productivity, climate resilience, and ecosystem functioning (Baskett et al. 2009; Birkeland 1997; Kinzie III, 1999; Wolfe et al. 2020). There are innumerous organismal symbioses on coral reefs beyond the coral-zooxanthellae archetype, including many novel relationships that involve crustaceans (Stachowicz 2001; Stella et al. 2011). For example, boxer crabs (Xanthidae) are considered kleptoparasitic, holding other animals (usually sea anemones) in their chelae to aid in protection from predators and food capture (Schnytzer et al. 2013, 2022), while coral crabs (e.g. Tetralidae and Trapeziidae) are obligate symbionts of corals and other colonial cnidarians where they play crucial roles in maintaining coral health, including defending their host from predators (Castro and Titelius 2007; Stella et al. 2011).
Many crustaceans are known for their decorative symbiotic relationships (Wicksten 1993). In decapods, decoration involves placement of small inorganic and organic materials on the exoskeleton in a process known as epibiosis (Fernandez-Leborans 2010; Guinot and Wicksten 2015). This process is an association between the epibiont, which is attached to a living surface, and the basibiont, which hosts and supports the epibiont. Epibiosis can be beneficial for the epibiont, as it enhances food acquisition, increases epibiont mobility and affords a degree of protection from predators (Fernandez-Leborans 2010; McGaw 2006; Wahl 1989). In turn, the basibiont benefits from enhanced camouflage against predators (Dudgeon 1980; Hazlett and Estabrook 1974) and/or storage of edible materials for later consumption (Kilar and Lou 1986; Wicksten 1980; Wirtz and Diesel 1983; Woods and McLay 1994). On the contrary, there can be negative effects for the basibiont, where epibionts can increase the host’s weight, reducing mobility and increasing energetic demands (Ibrahim 2014). Given these tradeoffs, the characteristics and extent of decoration vary among species of decapod.
Spider crabs (Majidae) are a diverse group of decapod (Poore and Ahyong 2023) that can decorate heavily, often covering the entire dorsal surface of their carapace and walking legs with biological (and other) material (Guinot and Wicksten 2015; Wicksten 1993). Majid species use their chelae to harvest segments of benthic organisms, including sponges, algae, and hydroids, which are affixed to hooked setae on the carapace (Wicksten 1976, 1980). Epibiont communities therefore consist of organisms that are actively selected and attached by the host but may also include organisms that passively settle directly to and encrust over the basibiont (Guinot and Wicksten 2015; Hartnoll 1993; Sanka et al. 2016). Acquisition and composition of epibionts vary among Majids, often reflecting species-specific selectivity and localised resource availability (Hultgren and Stachowicz 2008; Maldonado and Uriz 1992; Woods and Page 1999).
Decorative behaviour is analogous to optimum foraging theory, whereby basibionts select for preferred organisms when resources are abundant but broaden organism selection when resources are scarce (Kilar and Lou 1984). The relative availability of sessile taxa in the immediate environment can alter resource use and preference toward camouflage or diet (Kilar and Lou 1986; Stachowicz and Hay 2000). When resources are scarce, dietary needs take preference with stored epibionts removed from the carapace for consumption (Kilar and Lou 1986; Wicksten 1980; Wirtz and Diesel 1983; Woods and McLay 1994). When resources are abundant, epibiont selectivity can favour camouflage to ensure the basibiont best matches its environment (Ibrahim 2012; Sánchez-Vargas and Hendrickx 1987; Stachowicz and Hay 2000; Woods and Page 1999). In addition, epibiont species can be selected for based on biochemistry, with some majids favouring toxin-containing taxa such as algae and sponges, to enhance their own chemical defences (Dick et al. 1998; Stachowicz and Hay 2000), which can reduce predation (Stachowicz and Hay 1999). Decoration with non-preferred species can increase predation risk and mortality (Stachowicz 2001), with predation pressure a major driver of specialisation among decorator crabs (Stachowicz and Hay 1999). Indeed, characterisation of decoration tendencies in majids is the first step to understanding how their epibiont symbioses may shape the abundance, distribution, and ecology of the basibiont.
The red decorator crab, Schizophrys aspera (Milne-Edwards 1831), has a broad distribution from the Red Sea to the Great Barrier Reef (GBR) and throughout the Indo-Pacific (Wolfe et al. 2023a). In the Red Sea, S. aspera shows an affinity for polychaetes and barnacles as epibionts to match the artificial structures, such as buoys and jetties, it inhabits (Ibrahim 2012). However, comparatively little is known of the biology or decorating tendencies of S. aspera on the GBR, where it seems to occupy a different ecological niche in coral rubble (Desbiens et al. 2023). As coral rubble habitats are dominated primarily by sponges, algae, and ascidians (Wolfe et al. 2023b, 2021), whether S. aspera on the GBR has distinct decorating tendencies to that of the Red Sea population is of interest. Moreover, on the GBR, S. aspera has recently been documented as a successful predator of the crown-of-thorns seastar (CoTS) during its juvenile life stage (Desbiens et al. 2023). Given the negative impacts CoTS can have on the GBR and coral reefs globally (Pratchett et al. 2014), characterisation of S. aspera is crucial to determining its potential impact as a natural top-down control mechanism on CoTS in the region. In this light, it seems pertinent to understand the epibiotic symbioses of S. aspera to begin to identify potential drivers of its habitat associations, distribution, and trophic ecology.
In this study, we aimed to characterise the biology and decorating tendencies of S. aspera on Heron Reef, in the southern GBR. Through collection of 116 individuals, we provide general population descriptions for S. aspera and document epibiont community composition in this locality. Using short-term (8-day) experiments in aquaria, we also describe patterns in epibiont uptake from rubble pieces. Our results help to clarify the novel ecological niche of S. aspera on the GBR and we discuss the implications of our findings in the context of its broader trophic ecology.
Methods
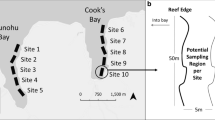
Schizophrys aspera were collected from October 2022 to March 2023 at shallow (< 15 m depth) sites along the south-western slope of Heron Reef (− 23° 26′ S, 151° 54′ E) in the southern GBR, Australia (Fig. 1a). Individuals (n = 116) were collected by hand by overturning large rubble pieces on SCUBA (as described in Desbiens et al. 2023 and Wolfe et al. 2023a, b), under the Great Barrier Reef Marine Park Authority permit (G22/47448.1) and The University of Queensland Animal Ethics permit (2019/AE000388).
Biology and epibiont community
Once collected, S. aspera were returned to the laboratory in buckets of seawater where it was housed in aquaria with natural rubble shelter and flowing seawater (> 1 L min−1) at ambient temperature (~ 27 °C). Size (mm) was determined for each individual by measuring the widest span of the carapace. Individuals were then weighed (g) to establish a length–weight relationship, and sexed based on abdomen shape, with those < 22 mm termed juveniles here based on the consistent lack of adult features (e.g. large male chelae) (Davie et al. 2015). We note that this blanket categorisation of juveniles may have included small sexually mature individuals as males, for example, can be reproductively mature before the development of bulkier male chelipeds (Davie et al. 2015).
To characterise the epibiont community of S. aspera, the carapace of each specimen (n = 116) was photographed from above (Fig. 1c) using an Olympus TG6 camera immediately after collection. All photos were taken submerged to ensure image quality for later identification of the epibionts aided by a series of close-up photos of each epibiont. Carapace photos were analysed in Fiji (Schindelin et al. 2012) using the known carapace width of each individual to set the scale. In all cases, the carapace and each epibiont were individually traced (Fig. 1c) so proportional cover could be quantified. Taxonomically, species identification of sessile taxa is challenging with considerable disagreement in approaches and requirement of molecular approaches (e.g. CCA: Dean et al. 2015; sponges: Vicente et al. 2022), so this was not attempted. Instead, epibiont cover was categorised using major functional groups of sessile taxa, including sponges, serpulids, bryozoans, ascidians, macroalgae, vermetids, and hydrozoans. No other macroorganisms were present. As hooked setae are found on all regions of the Schizophrys carapace (Osman et al. 2021), we did not attempt to determine spatial distribution of epibiont coverage.
Patterns in decoration tendencies
Short-term (8-day) experiments were conducted on a subset of individuals (n = 15) to observe changes in epibiont uptake by S. aspera in a controlled aquarium setting. Specimens (n = 3 juveniles; n = 6 females; n = 6 males) were collected through February and March 2023, as above, and placed in individual 6 L tanks (19 × 19 × 16.3 cm) supplied with flowing seawater (~ 0.8 L min−1) at ambient temperature (27.5 ± 0.1 °C; n = 42). Individuals were provided with rubble pieces (mean size ± SE: 6048 ± 1388 mm2) collected from the same habitat, ensuring sufficient shelter and a selection of sessile taxa typical of their immediate environment for use as epibionts and/or food. Individuals were not stripped of their original epibionts before the experiments commenced. Four additional tanks of rubble without S. aspera were used as procedural controls.
All S. aspera and rubble pieces were measured and photographed on days 0 (initial), 4 (middle), and 8 (final). Epibiont cover on the carapace of S. aspera was quantified in Fiji as for wild caught individuals, described above. Rubble pieces were photographed top and bottom at each time point to estimate whole-of-piece cover of each benthic functional group. Photos were taken from the same perspective at each time point with pieces submerged in seawater on a gridded scale. Closeup images were taken of all epibionts to aid in identification, as above, though two additional epibiont groups (anemones and crustose coralline algae) were present on rubble pieces. Proportional changes in epibiont cover on both S. aspera carapaces and rubble pieces were determined, with differences assumed to result from use as decoration or consumption. All aquaria were checked daily to record whether epibionts were severed from rubble but not used (i.e. stray in tanks), which was never found. S. aspera were fed small portions of bait prawns 2–3 times over the experiment to ensure it was sustained, with waste products siphoned out regularly to avoid fouling.
Additional observations
Given the paucity of information on S. aspera on the GBR, and elsewhere, we considered it important to document interesting observations during animal husbandry, including occurrences of spawning, moulting, and copulation. One juvenile that moulted in captivity was housed separately to observe post-moult changes in its epibiont community. Photos of this individual were taken every three days for ~ 1 month (25/01/2023–27/02/2023) and changes in its proportional cover of epibionts determined, as above. As in experiments above, the individual was given rubble from its natural habitat as shelter with live epibionts for decoration and food, supplemented with chopped bait prawns.
Statistical analyses
Linear models (analysis of variance) in the base stats package (R Core Team 2022) were used to determine the influence of sex (male, female, and juvenile) on the size (carapace width) and total carapace cover of S. aspera. Permutational Analysis of Variance (PERMANOVA) was then performed to determine differences in the proportional cover of different epibiont groups among sexes (juveniles, females, males). A Gower distribution was used to account for proportional data (Anderson et al. 2008; Gower 1966), and pairwise comparison used to determine differences among sexes using the vegan package (Oksanen et al. 2022). A similarity of percentages (SIMPER) test was then performed to determine which epibionts displayed the largest differences among sexes. All size and proportional cover data were log-transformed before analysis.
Relative changes in total carapace cover on S. aspera in aquaria over time (days 0, 4, and 8) were analysed using a linear mixed-effects model with the lmer function of the lme4 package (Bates 2010). Specimen sex (juveniles, females, males) and time (day) were used as fixed factors with rubble piece area (mm2) used as a random term to account for rubble size, along with individual to account for the repeated measures component of this dataset. All proportional and size data were log-transformed, as above. Then, proportional changes in the cover of epibiont functional groups on both the carapace of S. aspera and rubble pieces were calculated between initial (day 0) and final (day 8) data. To analyse changes in epibiont cover on the carapace of S. aspera, sex and epibiont group were used as fixed effects, with rubble piece size as a random term. For epibiont cover on rubble pieces, fixed factors were treatment (i.e. tanks with or without S. aspera) and epibiont group, with rubble piece size as a random term. Since values ranged from positive to negative change—representing increases and decreases in epibiont cover, respectively—a log-transformation accounting for negative values was used: \({\text{sin}}\left(x\right)\times {\text{log}}(1+\left|x\right|)\). For all lmer tests, model selection was assessed using Akaike Information Criterion values with the AICc function in the MuMIn package (Bartoń, 2018). In each case, rubble piece size was retained as a random factor though variance terms indicated that this had little influence on results compared to the main significant effects.
Lastly, we assessed epibiont selection by S. aspera using data on the epibiont cover on rubble pieces and the carapace of S. aspera in a modified versions of Ivlev’s electivity index (Jacobs 1974). Due to a lack of selectivity owing to high epibiont availability on rubble, this analysis is reserved for Supplementary Material (see: Supplementary Text 1 and Fig. S1).
All statistical analyses were conducted in R (R Core Team 2022). Assumptions of normality and homogeneity of variance were checked and confirmed where relevant using the DHARMa package (Hartig 2022), and Tukey’s HSD tests performed post-hoc to explore significant results with the agricolae (Mendiburu, 2019) or emmeans (Lenth 2023) packages.
Results
Biology and epibiont community
Schizophrys aspera had a total mean size (carapace width) of 23.5 mm (SD ± 5 mm, range = 5–35 mm, n = 116), which differed depending on sex (Table S1a; Figs. 2a, 3). The adult sex ratio of S. aspera collected was skewed ~ 2:1 towards females, which comprised 51% (n = 59) of collected individuals, followed by juveniles (27%; n = 31) and males (22%; n = 26). On average, males were larger than females while juveniles were the smallest (Fig. 2a), as would be expected. The length–weight relationship of S. aspera was well-fit (R2 = 0.88) and showed an exponential pattern of growth (Fig. 3). Adult males tended to weigh more than adult females of a given size (Fig. 3), likely owing to the dimorphic male chelae (see Fig. 1b), which are evident (and indicate maturation) from ~ 22 mm carapace width.
Box and whisker plots showing a carapace size (mm) and b proportion of carapace covered by epibionts on juvenile, female and male Schizophrys aspera. Boxes represent the interquartile range (25th and 75th percentile), horizontal line is the median, and whiskers represent the data range (i.e. minimum and maximum). Groups that significantly differed (p < 0.05) are labelled with different letters (Tukey’s HSD)
The mean (± SE) proportion of the S. aspera carapace covered by epibionts differed by sex (p < 0.001, Table S1b). Carapace cover was greatest on juveniles (58 ± 5%) and females (46 ± 4%), while males had the lowest cover of epibionts on their carapace (24 ± 4%) (Fig. 2b). In four females and two juveniles, total carapace cover exceeded 100% (Fig. 2b) as epibiont growth extended beyond the carapace. The relative contribution of different epibiont groups varied also (Table S2), with sponges comprising the majority (94 ± 1%) of the epibiont community on the carapace of S. aspera, followed by algae (2.8 ± 0.6%) and ascidians (1.7 ± 0.6%). Bryozoan, hydrozoan, serpulid, and vermetid epibionts were proportionally rare on the carapace of S. aspera (Fig. 4).
Epibiont community composition varied among sexes of S. aspera (Fig. 5; Table S3), wherein females (p = 0.05) and juveniles (p < 0.001) differed to males (Table S4. Females hosted proportionally more sponges (p = 0.002) and fewer serpulids (p = 0.003) on their carapace than males (Table S5), while males hosted fewer sponges than juveniles (p = 0.005) (Fig. 5; Table S5c). Juveniles and females did not differ in the relative composition of epibionts on their carapace (Table S4), though juveniles had no serpulids or vermetids within their epibiont community (Fig. 5; Table S5b, c).
Patterns in decoration tendencies
There were no significant changes in total carapace cover of S. aspera over 8 days in controlled aquaria, though total cover differed among sexes (Table S6a). The carapace of females was higher in total cover of epibionts compared to males, but this did not change over the 8-days (Fig. 6a). However, the type of epibiont taxa present on S. aspera varied over time (Fig. 6b; Table S6b). From day 0 to 8, the proportion of algae increased on S. aspera while the proportion of sponge cover decreased (Fig. 6b). These results were skewed by one juvenile that experienced a relative increase in algae cover > 900%, though sex did not determine differences in the mean change of epibiont taxa on S. aspera (Table S6b). In aquaria with S. aspera, the proportion of algae on rubble pieces decreased, but algal cover increased marginally on rubble pieces in control tanks with no crabs (Fig. 6c; Table S6c). In no case was positive selection of epibionts evident (Fig. S1), suggesting low use compared to the high availability of preferred taxa (i.e. algae and sponges) on rubble pieces. However, Schizophrys aspera appeared to utilise algae and sponges over other epibionts (Fig. 6c). Since no stray epibionts were found inside the tanks, it was assumed that organisms were either placed on the carapace or consumed. The increase in algae on the carapace of S. aspera (Fig. 6b) suggests algae taken from rubble was adopted as an epibiont, while the decrease in sponges on the carapace of S. aspera (Fig. 6b) and removal of sponges from rubble pieces (Fig. 6c) reflected consumption, though changes in biomass were not quantified.
Results from the 8-day aquarium experiment, including mean (± SE) a total carapace cover of Schizophrys aspera through time, and total change (day 0 to day 8) in the cover of epibiont taxa on b the carapace of S. aspera (juveniles: n = 3, females: n = 6, males: n = 6) and c rubble pieces in aquaria with (n = 15) and without (n = 4) S. aspera. Groups that significantly differed (p < 0.05) are labelled with different letters (Tukey’s HSD)
Additional observations
There were five recorded cases of S. aspera females spawning in captivity (Table S7) with many more gravid individuals collected. We observed copulation in holding tanks on three occasions (Table S7). Males initiated each encounter, arranging themselves and the female to meet ventrally. The couples remained in this position for 10–15 min. There was no clear trend in spawning, moulting nor copulation relative to the lunar phase (Table S7). Three individuals moulted in aquaria, with 3 to 4 mm increases in carapace size observed in each instance (Table S7).
One juvenile male moulted twice over an approximate 1-month period (Table S7, Fig. 7), and was monitored closely. This juvenile initially had high cover of sponges (98%), typical of wild caught individuals (Figs. 3, 4), with a sharp decrease in epibiont cover post-moult (Fig. 7). Total epibiont cover decreased over its 36 days in captivity with an increased ratio of algae used over sponges (Fig. 7), though we did not quantify epibiont availability on rubble pieces to determine selectivity. Immediately after moulting, the juvenile was observed to harvest epibionts from its shed exoskeleton, as well as from the rubble provided in the aquarium, to consume and re-adhere to its fresh carapace (Fig. 7a–c). This resulted in an increase in carapace cover between moults (Fig. 7). After the first moult, conspecifics attacked and ingested two appendages from the juvenile before it was isolated. No other instances of cannibalism were observed throughout this experiment and the juvenile regrew its two missing appendages after the second moult.
Change in epibiont contribution to carapace cover on a juvenile of Schizophrys aspera that moulted twice (black arrows) in captivity over 36 days. Carapace photos indicate the juvenile (a) at the time of collection, and < 1 d after the b first moult and c second moult. White arrows show the same purple sponge harvested and re-adhered between moults
Discussion
Decoration tendencies in majids are important to characterise as they are highly variable among species (Guinot and Wicksten 2015; Stachowicz 2001). This may be especially important to understand in species with key ecological roles, such as the red decorator crab, Schizophrys aspera, a newfound predator of CoTS juveniles on the GBR (Desbiens et al. 2023). We found that epibiont coverage was greatest in juveniles and females of S. aspera. The primary resources for epibiosis were sponges, which constituted 94% of the epibiont community. Algae and ascidians were also utilised, including in aquaria, which reflects the sessile community typical of the coral rubble habitat occupied by S. aspera in the region (Wolfe et al. 2023b). Here, we discuss our findings on the biology and decoration of S. aspera in its novel ecological niche on the GBR, and its symbiotic relationships that could confer resilience in its consumption of CoTS in the region.
Few studies have documented and described S. aspera beyond occasional sightings (Wolfe et al. 2023a), with most research derived on populations in the Red Sea (El-Serehy et al., 2015; Ibrahim 2012, 2014). Schizophrys aspera were generally smaller in our surveys on the GBR (range: 5–35 mm) than documented in the Red Sea (range: 25–75 mm). This variation in size may reflect localised adaptations, as S. aspera in the Red Sea were collected exposed on buoys and jetties (Ibrahim 2012) compared to the cryptic undersides of coral rubble pieces on the GBR (Desbiens et al. 2023; Wolfe et al. 2023a), where many small individuals were found. The adult population of S. aspera on the GBR was skewed 2:1 towards females, a common occurrence among crustaceans that may indicate females live longer than males (Wenner 1972). The Red Sea population was skewed 3:2 towards males (Ibrahim 2012), which may reflect their exposed habitat of collection that would favour large mechanically defended males. Decoration also differed between the two populations, with epibiont communities that reflect their immediate habitat, which we discuss in greater detail below. Overall, the notable differences between the Red Sea and GBR populations of S. aspera highlight the importance of understanding the biology and ecology of species across its range, and support the suggestion that taxonomic revision of the Schizophrys genus is required (Lee et al. 2018).
On the GBR, males of S. aspera were larger and heavier than females and juveniles, which is not surprising owing to their large chelae. This dimorphism is a common feature of decapods (Homola et al. 1991; Poore and Ahyong 2023; Sagi et al. 1994) and can result in niche differentiation of resource use between sexes and across their ontogeny (Cobo, 2005). Regardless, the small size of S. aspera compared to other majoids likely contributes to their life-long crypsis (Berke and Woodin 2008; Hultgren and Stachowicz 2009). We found males of S. aspera had a lower cover of epibionts on their carapace (24%) than females (46%) and juveniles (58%), which differs to the Red Sea population where epibiont coverage between males and females were similar (Ibrahim 2012). Perhaps juveniles, which tend to have a faster intermoult period (Wainwright and Armstrong 1993), are more active at gathering epibionts to reduce vulnerability to predation. Males may be better able to defend themselves than juveniles and females with less requirement for camouflage and chemical defence. Large male chelae could also mechanically inhibit epibiont selection owing to limitations in dexterity and articulation in the harvest of small objects from coral rubble, which may result in further dietary specialisation and energy intake (Guinot and Wicksten 2015), but the implications of this have not been investigated (Davie et al. 2015). Not all majoids decorate throughout their life histories, so our finding here for S. aspera promotes the hypothesis that ecological and evolutionary processes, rather than phylogeny, contribute to this behaviour (Berke and Woodin 2008). Understanding how body and chelae size influence the diet of S. aspera across its ontogeny warrants further investigation, especially in context of its ability to consume juvenile CoTS (Desbiens et al. 2023).
Sponges comprised the majority of epibionts utilised by S. aspera in its decoration, which suggests selection over macroalgae and crustose coralline algae that are more prevalent in its rubble habitat in the region (Wolfe et al. 2023b). Sponges typically encrusted closely over the carapace of S. aspera, which supports the previous suggestion that this species does not ‘decorate’ (Guinot and Wicksten 2015), at least as heavily as other species. Still, preferential use of sponges likely enhances camouflage and protection from predators, as suggested for the heavily decorated crabs, Inachus phalangium (Martinelli et al. 2006) and Camposcia retusa (Brooker et al. 2018). Decoration with encrusting sponges would provide S. aspera a variably coloured and multipatterned surface that would be very effective camouflage, while minimising movement constraints in the coral rubble substrate it inhabits on the GBR. Additionally, sponges and other marine species, including ascidians, algae, and even CoTS, employ hepatotoxicity as a chemical defence (Abd El Moneam et al. 2018; Braekman and Daloze 1986; Furey et al. 2003; Pawlik 1993; Tianero et al. 2015; Watanabe et al. 2009), which would add to the biochemical defences of S. aspera when adopted as epibionts. Some marine organisms, such as nudibranchs, selectively prey on toxic species to accumulate and acquire deterrent metabolites in bodily tissue (da Cruz et al. 2012). Whether S. aspera decorate with and consume sponges and other noxious taxa (including CoTS) to avail of biochemical compounds requires attention.
Algae was the second most prevalent epibiont found on wild-caught S. aspera, which provided vertical complexity to the epibiont community and demonstrates that S. aspera are active in their decoration choices not solely colonised by settling and encrusting organisms (Guinot and Wicksten 2015). Our 8-day aquarium experiments revealed a disproportionate use of algae and sponges by S. aspera, implying these resources are favoured for decoration and/or diet in the GBR population. In other species of majid, algae and sponges are a primary epibiont choice (Hultgren and Stachowicz 2008; Sánchez-Vargas and Hendrickx 1987) as they reduce predation risk (Stachowicz and Hay 1999) and can contribute significantly to their diet; up to ~ 97% in some species (Woods 1993). In aquaria, S. aspera increased the cover of algae on its carapace but decreased its cover of sponge. This shift implies that algae became the preferred epibiont while sponges not retained on the carapace were repurposed as food. It was expected that S. aspera would discard and select epibionts to match its new environment, as observed in other decorating crabs (Stachowicz 2001; Woods and Page 1999). The apparent switch to include more algae in their decoration than wild specimens may reflect environment matching in aquaria along with possible dietary and energetic requirements (e.g. epibiont nutrition, palatability, and chemistry) not determined here. Though, while we observed S. aspera to consume algae, sponges, and other sessile taxa (e.g. sea anemones) during the experiment, we did not aim to characterise the diet of S. aspera here, which could be explored through quantification of epibiont biomass and basibiont gut content analysis.
The role of S. aspera in coral reef food webs is important to characterise, including whether sessile taxa are indeed its preferred food choice. While S. aspera altered its relative use of sponges and algae in aquaria, we found no change in total carapace cover over the eight days nor positive selection for any sessile taxa relative to its availability, suggesting individuals were satiated by epibiont choice. Considering the ability of S. aspera to prey heavily on echinoderms (Desbiens et al. 2023), perhaps motile animals are a dietary preference when available. However, the capacity of S. aspera to rapidly reduce algal and sponge cover on rubble pieces by 47% and 31%, respectively, demonstrates that its combined feeding and decoration behaviours can have top-down effects on sessile communities. Whether S. aspera select for and shape the structure of sessile communities in situ is of interest given its localised residence under large rubble pieces (Desbiens et al. 2023; Wolfe et al. 2023a), including the potential preference for epibiont and motile food options not examined here.
Tradeoffs between epibiont selection for camouflage or diet are crucial to determine for majoids as decoration with non-preferred species can increase predation risk and mortality (Stachowicz 2001). Decapods in rubble are indeed a primary food source of invertivorous fishes on coral reefs (Wolfe et al. 2021) but the extent to which higher-order fishes prey upon S. aspera is currently unknown, as is the comparable effectiveness of different epibiont species in reducing its risk of predation. The abundance and habitat associations of S. aspera seem to correlate with rubble piece size and patch morphology (Wolfe et al. 2023a), but whether this relates more specifically to epibiont availability within the rubble is yet to be determined. It is possible that S. aspera selects for rubble high in sponge and/or algal cover to ensure greater resource availability (diet) and protection from predators (camouflage). Indeed, only three small juveniles of S. aspera have been found under rubble overlying sand (Wolfe et al. 2023a) where sediment accumulation impairs the growth of sessile taxa (Kenyon et al. 2023). The lack of adults in this bare rubble type indicates that success of S. aspera across its ontogeny is associated with a more complex and biodiverse rubble biome.
Based on our observations, it appears that S. aspera cache sponges, algae and other epibionts as a later food source. The lower proportion of epibionts on males suggests that they either require food more frequently, leaving little time for epibiont accumulation on their carapace, or that they source food externally through grazing and/or predation. Previous research has found that male and female crabs vary in dietary composition (Cerda and Wolff 1993). Perhaps females rely more heavily on epibiont stores than males to ensure consistent maternal provisions, and juveniles to ensure food availability for rapid early development (Cerda and Wolff 1993). This was made evident by the juvenile maintained in aquaria over 36 days, which fluctuated in its cover of sponge and algae and consumed both epibionts from rubble pieces and its moulted exoskeleton (see: Fig. 7a–c). This juvenile rapidly adopted a new epibiont community post-ecdysis compared to other individuals in the aquarium experiment that did not change total carapace cover over the eight days. It seems the ecdysis process forces rapid decoration that is perhaps more likely to reflect the immediate habitat, as observed in the majid, Thacanophrys filholi (Woods and Page 1999). We predict the juvenile consumed most of its original epibionts as they were no longer present on the shed exoskeleton nor in the aquarium post-ecdysis, though one distinct purple sponge was re-adhered to the exoskeleton after both moults (see: Fig. 7a–c). It seems of interest to evaluate epibiont selection at greater taxonomic resolution to determine why some species may be consecutively preferred in decoration over diet.
The single juvenile monitored closely demonstrated that epibiont uptake happens immediately post-ecdysis, consistent with other reports of spider crabs (Wicksten 1975). The intermoult is a metabolically active period when newly-moulted crabs are soft-shelled and lack mobility, making shed epibionts an invaluable resource for energy and to avoid predation (McLay 2015), the latter of which occurred in our holding tank prior to its isolation. Limb regrowth after ecdysis is a common occurrence in decapods (Hartnoll 1993), as demonstrated by the juvenile here. Though many decapods cease ecdysis at reproductive maturity whereby limbs cannot be replaced after their terminal moult (Hartnoll 1993). Considering that large adults were regularly found with missing appendages, we predict that S. aspera undergo this pattern of determinate growth (McLay 2015). On this note, juveniles of S. aspera hosted low (to no) cover of settling epibionts (serpulids and vermetids) compared to males and females. Higher ecdysis frequency of juveniles would limit the longevity of settling organisms, as well as their smaller carapace area available as a settlement target. Conversely, large individuals likely to have reached their terminal moult (especially males) had a higher proportion of serpulids and vermetids despite lower total epibiont cover. This may also explain why the larger-bodied S. aspera in the Red Sea primarily hosted settling cirriped (barnacle) and serpulid (polychaete) epibionts (Ibrahim 2012), common taxa of the region (Elkhawass 2006). Indeed, settling epibionts that calcify increase the weight and drag of the basibiont (Ibrahim 2014), which would be of great detriment to juvenile growth, feeding and survival, along with interstitial movement through the rubble habitat of S. aspera on the GBR.
Schizophrys aspera plays a potentially important role through its consumption of juvenile CoTS (Desbiens et al. 2023), though whether this scales in situ and whether CoTS or other motile fauna are preferred over sessile species are unknown. Varied use of sponges and algae here, including in aquarium experiments, indicates S. aspera is flexible in its diet and decoration, which seems of interest to explore in context of its habitat associations and reef scale distribution, especially as its novel ecological niche in coral rubble on the GBR overlaps with the early-life history stages of CoTS. Use of epibionts that contain hepatotoxins may indeed aid in the tolerance of S. aspera to the toxicity of CoTS, though the biochemical properties of this relationship are yet to be explored. The diet, biochemistry, and habitat preferences of S. aspera on the GBR are key to determining the relationship this majid has with CoTS and its potential to add to the natural control of CoTS populations on coral reefs.
References
Abd El Moneam NM, Shreadah MA, El-Assar SA, De Voogd NJ, Nabil-Adam A (2018) Hepatoprotective effect of red sea sponge extract against the toxicity of a real-life mixture of persistent organic pollutants. Biotechnol Biotechnol Equip 32(3):734–743. https://doi.org/10.1080/13102818.2018.1441747
Anderson M, Gorley RN, Clarke K (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. Primer-E Limited
Bartoń K (2018) MuMIn: Multi-model inference
Baskett ML, Gaines SD, Nisbet RM (2009) Symbiont diversity may help coral reefs survive moderate climate change. Ecol Appl 19(1):3–17
Bates DM (2010) lme4: Mixed-effects modeling with R. Springer, New York
Berke SK, Woodin SA (2008) Energetic costs, ontogenetic shifts and sexual dimorphism in spider crab decoration. Funct Ecol 22:1125–1133
Birkeland C (1997) Symbiosis, fisheries and economic development on coral reefs. Trends Ecol Evol 12(9):364–367
Braekman JC, Daloze D (1986) Chemical defence in sponges. Pure Appl Chem 58(3):357–364
Brooker RM, Muñoz Ruiz EC, Sih TL, Dixson DL (2018) Shelter availability mediates decorating in the majoid crab. Camposcia Retusa Behav Ecol 29(1):179–185
Castro P, Titelius MM (2007) Trapeziidae Miers, 1886 and Tetraliidae Castro, Ng and Ahyong, 2004 (Crustacea, Brachyura): coral crabs of Western Australia, with notes on their biogeography. Crustac Collect West Aust Mus/woodside Energy Ltd Partnersh Explor Mar Biodivers Dampier Archipelago West Aust 2002:303–314
Cerda G, Wolff M (1993) Feeding ecology of the crab Cancer polyodon in La Herradura Bay, Northern Chile. II. Food spectrum and prey consumption. Mar Ecol 100(1–2):119–125. https://doi.org/10.3354/meps100119
Cobo V (2005) Population biology of the spider crab, Mithraculus forceps (A. Milne-Edwards, 1875) (Majidae, Mithracinae) on the Southeastern Brazilian coast. Crustaceana 78(9):1079–1087. https://doi.org/10.1163/156854005775361016
da Cruz JF, Gaspar H, Calado G (2012) Turning the game around: toxicity in a nudibranch-sponge predator–prey association. Chemoecology 22(1):47–53. https://doi.org/10.1007/s00049-011-0097-z
Davie PJF, Guinot D, Ng PKL (2015) Anatomy and functional morphology of Brachyura. In: Castro P, Davie PJF, Guinot D, Schram F, Von Vaupel KC (eds) Treatise on Zoology—Anatomy, Taxonomy, Biology. The Crustacea, vol 9. Brill, p 638
Dean AJ, Steneck RS, Tager D, Pandolfi JM (2015) Distribution, abundance and diversity of crustose coralline algae on the Great Barrier Reef. Coral Reefs 34(2):581–594. https://doi.org/10.1007/s00338-015-1263-5
Desbiens AA, Mumby PJ, Dworjanyn S, Plagányi ÉE, Uthicke S, Wolfe K (2023) Novel rubble-dwelling predators of herbivorous juvenile crown-of-thorns starfish (Acanthaster sp.). Coral Reefs 42(2):579–591. https://doi.org/10.1007/s00338-023-02364-w
Dick MH, Donaldson WE, Vining IW (1998) Epibionts of the tanner crab Chionoecetes bairdi in the region of Kodiak Island. Alsk J Crustac Biol 18(3):519–527
Dudgeon D (1980) Some inter-and intraspecific differences in the decorating patterns of majid crabs (Crustacea: Decapoda) from the coastal waters of Hong Kong. In: Proceedings of the First International Marine Biological Workshop: The Marine Flora and Fauna of Hong Kong and Southern China, Hong Kong
Elkhawass EA (2006) Acorn barnacles and effects of environmental factors on their growth and distribution in Lake Timsah. M.Sc., Faculty of science, Suez Canal University
El-Serehy HA, Al-Rasheid KA, Ibrahim NK, Al-Misned FA (2015) Reproductive biology of the Suez Canal spider crab Schizophrys aspera (H. Milne Edwards, 1834 Crustacea: Brachyura: Majidae). Saudi J Biol Sci 22(6):789–794
Fernandez-Leborans G (2010) Epibiosis in Crustacea: an overview. Crustaceana 83:549–640
Furey A, Crowley J, Shuilleabhain AN, Skulberg OM, James KJ (2003) The first identification of the rare cyanobacterial toxin, homoanatoxin-a. Ireland Toxicon 41(3):297–303
Gower JC (1966) Gower JC. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53:325–338
Guinot D, Wicksten MK (2015) Camouflage: carrying behaviour, decoration behaviour, and other modalities of concealment in Brachyura, vol 9. Brill, Leiden. https://doi.org/10.1163/9789004190832
Hartig F (2022) DHARMa: residual diagnostics for hierarchical (Multi-Level/Mixed) Regression Models. https://CRAN.R-project.org/package=DHARMa
Hartnoll R (1993) The epibiota of spider crabs. Sci Ann Sch Biol 1(1):163–176
Hazlett BA, Estabrook GF (1974) Examination of agonistic behavior by character analysis I Spider Grab Microphrys bicornutus. Behaviour 48(1/2):131–144
Homola E, Sagi A, Laufer H (1991) Relationship of claw form and exoskeleton condition to reproductive system size and methyl farnesoate in the male spider crab. Libinia Emarginata Invertebr Reprod Dev 20(3):219–225. https://doi.org/10.1080/07924259.1991.9672202
Hultgren KM, Stachowicz JJ (2008) Alternative camouflage strategies mediate predation risk among closely related co-occurring kelp crabs. Oecologia 155(3):519–528
Hultgren K, Stachowicz J (2009) Evolution of decoration in Majoid crabs: a comparative phylogenetic analysis of the role of body size and alternative defensive strategies. Am Nat 173(5):566–578. https://doi.org/10.1086/597797
Ibrahim N (2012) Epibiont communities of the two spider crabs Schizophrys aspera (H. Milne Edwards, 1834) and Hyastenus hilgendorfi (De Man, 1887) in Great Bitter Lakes, Suez Canal Egypt. Egypt J Aquat Biol Fish 16:133–144. https://doi.org/10.21608/ejabf.2012.2118
Ibrahim N (2014) Biochemical composition of the edible parts of the spider crab Schizophrys aspera in the Great Bitter Lake of the Suez Canal. Egypt J Aquat Biol Fish 18:47–54. https://doi.org/10.21608/ejabf.2014.2228
Jacobs J (1974) Quantitative measurement of food selection. Oecologia 14(4):413–417. https://doi.org/10.1007/BF00384581
Kenyon TM, Doropoulos C, Wolfe K, Webb GE, Dove S, Harris D, Mumby PJ (2023) Coral rubble dynamics in the Anthropocene and implications for reef recovery. Limnol Oceanogr 68(1):110–147
Kilar JA, Lou RM (1984) Ecological and behavioral studies of the decorator crab, Microphrys bicornutus Latreille (Decapoda: Brachyura): a test of optimum foraging theory. J Exp Mar Biol Ecol 74(2):157–167
Kilar JA, Lou RM (1986) The subtleties of camouflage and dietary preference of the decorator crab, Microphrys bicornutus Latreille (Decapoda: Brachyura). J Exp Mar Biol Ecol 101(1–2):143–160
Kinzie RA III (1999) Sex, symbiosis and coral reef communities. Am Zool 39(1):80–91
Lee BY, Low ME, Ng PK (2018) A nomenclatural review of the genus Schizophrys White, 1848 (Decapoda: Brachyura: Majoidea: Majidae). Raffles Bull Zool 66:12–22
Lenth RV (2023) Emmeans: estimated marginal means, aka least-squares means. https://CRAN.R-project.org/package=emmeans
Maldonado M, Uriz M (1992) Relationship between sponges and crabs: patterns of epibiosis on. Inachus
Martinelli M, Calcinai B, Bavestrello G (2006) Use of sponges in the decoration of Inachus phalangium (Decapoda, Majidae) from the Adriatic Sea. Ital J Zool 73(4):347–353
McGaw IJ (2006) Epibionts of sympatric species of Cancer crabs in Barkley sound, British Columbia. J Crustac Biol 26(1):85–93
McLay CL (2015) Moulting and growth in Brachyura. In: Castro P, Davie PJF, Guinot D, Schram F, Von Vaupel KC (eds) Treatise on Zoology—Anatomy Taxonomy, Biology. The Crustacea, vol 9. Brill, Leiden, pp 245–316
Mendiburu, F. d. (2023) Agricolae: statistical procedures for agricultural research. https://CRAN.R-project.org/package=agricolae
Milne-Edwards H (1831) Observations sur les Crustacés du genre Mithrax. Magasin De Zool 2:1–16
Oksanen J, Simpson G, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Weedon J (2022) Vegan: Community Ecology Package. https://CRAN.R-project.org/package=vegan
Osman MM, Sallam WS, Madkour FF, Wicksten MK (2021) Morphology of camouflage by encrustation in the spider crabs Schizophrys dahlak and Hyastenus hilgendorfi (Decapoda: Brachyura: Majoidea: Epialtidae) from the Suez Canal. Egypt J Nat Hist 55(33–34):2047–2064. https://doi.org/10.1080/00222933.2021.1973132
Pawlik JR (1993) Marine invertebrate chemical defenses. Chem Rev 93(5):1911–1922
Poore GC, Ahyong ST (2023) Marine Decapod Crustacea: a guide to families and genera of the world. CSIRO Publishing
Pratchett MS, Caballes CF, Rivera-Posada JA, Sweatman H (2014) Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr Mar Biol Annu Rev 52:133–200
R Core Team (2022) R: a language and environment for statistical computing. In: R Foundation for Statistical Computing. https://www.R-project.org/
Sagi A, Ahl JSB, Danaee H, Laufer H (1994) Methyl farnesoate levels in male spider crabs exhibiting active reproductive behavior. Horm Behav 28(3):261–272. https://doi.org/10.1006/hbeh.1994.1022
Sánchez-Vargas D, Hendrickx M (1987) Utilization of algae and sponges by tropical decorating crabs (Majidae) in the Southeastern Gulf of California. Rev Biol Trop 35(1):161–164
Sanka I, Suyono EA, Rivero-Müller A, Alam P (2016) Carapace surface architecture facilitates camouflage of the decorator crab Tiarinia cornigera. Acta Biomater 41:52–59. https://doi.org/10.1016/j.actbio.2016.05.021
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682
Schnytzer Y, Giman Y, Karplus I, Achituv Y (2013) Bonsai anemones: growth suppression of sea anemones by their associated kleptoparasitic boxer crab. J Exp Mar Biol Ecol 448:265–270
Schnytzer Y, Achituv Y, Fiedler GC, Karplus I (2022) The intimate relationship between boxer crabs and sea anemones: what is known and what is not. Oceanogr Mar Biol 60:495–531
Stachowicz JJ (2001) Positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend mutualism, facilitation, and the structure of ecological communities. Bioscience 51(3):235–246. https://doi.org/10.1641/0006-3568(2001)051[0235:mfatso]2.0.co;2
Stachowicz JJ, Hay ME (1999) Mutualism and coral persistence: the role of herbivore resistance to algal chemical defense. Ecology 80(6):2085–2101. https://doi.org/10.2307/176680
Stachowicz JJ, Hay ME (2000) Geographic variation in camouflage specialization by a decorator crab. Am Nat 156(1):59–71
Stella J, Pratchett MS, Hutchings PA, Jones GP (2011) Coral-associated invertebrates. Oceanogr Mar Biol Annu Rev 49:43–104
Thompson JN (1999) The evolution of species interactions. Science 284(5423):2116–2118
Tianero MDB, Kwan JC, Wyche TP, Presson AP, Koch M, Barrows LR, Bugni TS, Schmidt EW (2015) Species specificity of symbiosis and secondary metabolism in ascidians. ISME J 9(3):615–628. https://doi.org/10.1038/ismej.2014.152
Vicente J, Webb MK, Paulay G, Rakchai W, Timmers MA, Jury CP, Bahr K, Toonen RJ (2022) Unveiling hidden sponge biodiversity within the Hawaiian reef cryptofauna. Coral Reefs 41:727–742
Wahl M (1989) Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar Ecol Prog Ser 58:175–189
Wainwright TC, Armstrong DA (1993) Growth patterns in the Dungeness Crab (Cancer Magister Dana): synthesis of data and comparison of models. J Crustac Biol 13(1):36–50. https://doi.org/10.1163/193724093X00435
Watanabe A, Nagai H, Nagashima Y, Shiomi K (2009) Structural characterization of plancitoxin I, a deoxyribonuclease II-like lethal factor from the crown-of-thorns starfish Acanthaster planci, by expression in Chinese hamster ovary cells. Fish Sci 75(1):225–231. https://doi.org/10.1007/s12562-008-0004-x
Wenner AM (1972) Sex ratio as a function of size in marine Crustacea. Am Nat 106(949):321–350
Wicksten MK (1975) Observations on decorating behavior following molting in Loxorhynchus crispatus Stimpson (Decapoda, Majidae). Crustaceana 29(3):315–316
Wicksten M (1976) Studies on the hooked setae of Hyas lyratus. Syesis 9:367–368
Wicksten MK (1980) Decorator crabs. Sci Am 242(2):146–157
Wicksten MK (1993) A review and a model of decorating behavior in spider crabs (Decapoda, Brachyura, Majidae). Crustaceana 64(3):314–325
Wirtz P, Diesel R (1983) The social structure of Inachus phalangium, a spider crab associated with the sea anemone: Anemonia sulcata. Z Tierpsychol 62(3):209–234
Wolfe K, Anthony K, Babcock RC, Bay L, Bourne DG, Burrows D, Byrne M, Deaker DJ, Diaz-Pulido G, Frade PR (2020) Priority species to support the functional integrity of coral reefs. Oceanography and Marine Biology. Taylor & Francis, Routledge
Wolfe K, Kenyon TM, Mumby PJ (2021) The biology and ecology of coral rubble and implications for the future of coral reefs. Coral Reefs 40:1769–1806
Wolfe K, Desbiens AA, Pietsch E, Mumby PJ (2023a) Habitat and distribution of the red decorator crab, Schizophrys aspera, a cryptic crown-of-thorns seastar predator. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsad136
Wolfe K, Kenyon TM, Desbiens A, de la Motte K, Mumby PJ (2023b) Hierarchical drivers of cryptic biodiversity on coral reefs. Ecol Monogr. https://doi.org/10.1002/ecm.1586
Woods CMC (1993) Natural diet of the crab Notomithrax ursus (Brachyura: Majidae) at Oaro, South Island, New Zealand. NZ J Mar Freshwat Res 27(3):309–315. https://doi.org/10.1080/00288330.1993.9516571
Woods CM, McLay CL (1994) Use of camouflage materials as a food store by the spider crab Notomithrax ursus (Brachyura: Majidae). NZ J Mar Freshwat Res 28(1):97–104
Woods CM, Page MJ (1999) Sponge masking and related preferences in the spider crab Thacanophrys filholi (Brachyura: Majidae). Mar Freshw Res 50(2):135–143
Acknowledgements
We acknowledge Gidarjil and the Traditional Owners of the Sea Country of which this research was undertaken. We thank Peter Davie for taxonomic advice, and appreciate Tania Kenyon, Karen Eigeland, and the staff of Heron Island Research Station for field assistance. This project funded by the Winifred Violet Scott Trust (KW) and the CoTS Control Innovation Program, which is supported by the partnership between the Australian Government’s Reef Trust and the Great Barrier Reef Foundation (KW, AD). We thank both anonymous reviewers for their comments on this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goodhill, C., Desbiens, A.A. & Wolfe, K. Biology and epibiont community of the red decorator crab, Schizophrys aspera, on the southern Great Barrier Reef. Coral Reefs 43, 455–466 (2024). https://doi.org/10.1007/s00338-024-02479-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02479-8