Abstract
Crown-of-thorns starfish (CoTS) are a pervasive coral predator prone to population outbreaks that have damaged coral reefs across Australia and the wider Indo-Pacific. CoTS population control through predation has been suggested as a primary mechanism that suppresses their outbreaks. However, the nature and rates of predation on CoTS are poorly resolved, especially for early life-history stages where they are expected to be most vulnerable. Here, we provide results from the first investigation of predators of CoTS during their rubble-dwelling, herbivorous, juvenile phase. We assessed the capacity of 104 common species of the rubble cryptofauna found across Heron Reef, Great Barrier Reef, Australia, to consume early-stage juvenile CoTS (0.8–3.8 mm) using controlled feeding experiments with laboratory-raised juveniles. We identified 26 novel CoTS predators, but only 10 species that regularly consumed juvenile CoTS in their entirety. Most cases of predation resulted in severed bodies and missing arms (i.e. sublethal predation) but not total consumption. We highlight one crustacean predator, Schizophrys aspera, the red decorator crab, which consumed whole juvenile CoTS in 89% of feeding trials and in excess of 5 CoTS d−1 in natural rubble mesocosms with alternative prey. This work emphasises the importance of predators at the critical juvenile stage that may control the build-up of CoTS populations prior to being detectable as an outbreak population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crown-of-thorns starfish, Acanthaster spp. (hereafter CoTS), are corallivorous asteroids common across coral reefs of the Indo-Pacific. At low densities, CoTS play an important role in coral reef functioning and can enhance local coral diversity (Birkeland 1989a; Keesing 1990; Uthicke et al. 2009; Pratchett et al. 2014). However, CoTS populations are prone to population fluctuations resulting in periodic outbreaks. Localised adult CoTS densities can increase by several orders of magnitude within 1–2 yrs (Birkeland and Lucas 1990). Rampant CoTS population proliferation is often followed by rapid and extensive coral loss (Pratchett et al. 2014). As a result, CoTS outbreaks are considered a major contributor to persistent declines in coral cover across the Indo-Pacific (Osborne et al. 2011; Trapon et al. 2011; De'ath et al. 2012; Vercelloni et al. 2017; Bozec et al. 2021).
Several hypotheses have been proposed to explain the proximal causes of CoTS outbreaks, but they are widely debated and remain largely unresolved (Moran 1986; Birkeland and Lucas 1990; Pratchett et al. 2017). Outbreaks have been suggested as a natural consequence of exceptional but variable reproductive output (the ‘natural causes’ hypothesis) (Dana et al. 1972; Vine 1973; Babcock et al. 2016; Caballes et al. 2021). Concurrently, regional outbreaks may be facilitated by location-specific patterns in dispersal (connectivity) caused by inherent hydrodynamic and geographical properties (Hock et al. 2014). Alternatively, CoTS outbreaks may be caused (or exacerbated) by anthropogenic degradation of coral reef ecosystems, due to eutrophication and/or overfishing and modification of trophic networks (Pratchett et al. 2017, 2021).
Many hypothesised causes of CoTS outbreaks focus on factors affecting the persistence of early life-history stages, as processes that dictate larval and recruit success can drive adult abundances (Keesing and Halford 1992b; Gosselin and Qian 1997; Wilmes et al. 2018). Significant research effort has focused on the potential for variations in larval nutrition (the ‘nutrient enrichment’ hypothesis) to contribute to periodic CoTS population booms (Pearson and Endean 1969; Lucas 1973). Increased phytoplankton density as a result of high nutrient loads from agricultural land use may release CoTS larvae from food limitation in otherwise oligotrophic tropical waters (Brodie et al. 2005; Fabricius et al. 2010). As possible coping mechanisms to thrive in water with low particulate food, CoTS larvae have also demonstrated resilience to a range of nutrient-poor conditions (Wolfe et al. 2015a, 2017), through developmental phenotypic plasticity (Wolfe et al. 2015b) and associations with microbial phototrophs (Carrier et al. 2018). Similarly, persistence and growth plasticity of CoTS when food-limited have also been demonstrated in the juvenile stage (Deaker et al. 2020), suggesting that early life-history stages of CoTS may be inherently robust to fluctuations in environmental conditions.
The ‘predator-removal’ hypothesis postulates that a reduction in the number of CoTS predators through overfishing enhances CoTS survival and outbreak potential (Endean 1969; McCallum 1987, 1990). These claims followed the first documented outbreak on the Great Barrier Reef (GBR) in Australia, coinciding with exhaustive fishing-induced depletion of the giant triton, Charonia tritonis, a predator of adult CoTS (Endean 1973). However, the effectiveness of predation by C. tritonis on CoTS remains unresolved (Ormond et al. 1990; Motti et al. 2022), given that reported consumption rates are low, < 1 wk−1 (Endean 1969; Birkeland 1989b), and their historical (cf. pre-harvest) densities are unknown (Hall et al. 2017). The ‘predator-removal’ hypothesis has since expanded to consider the impact of large reef fishes, including many targeted by commercial fisheries (Ormond et al. 1990; Sweatman 1997; Kroon et al. 2020). Multiple studies have suggested that reefs exploited by fisheries, and therefore potentially lacking CoTS predators, may experience more severe and more frequent CoTS outbreaks relative to protected areas (Dulvy et al. 2004; Sweatman 2008; McCook et al. 2010; Mellin et al. 2016; Vanhatalo et al. 2017; Westcott et al. 2020; Kroon et al. 2021). This is supported by the increase in prevalence of injured and damaged CoTS within protected reef zones (Caballes et al. 2022). Despite the increasing number of fish species identified as potential CoTS predators (Cowan et al. 2017a; Kroon et al. 2020), mechanistic understanding of how fisheries-exploited species directly interact with CoTS is still lacking (Pratchett et al. 2021).
Until recently, research on the potential for predator regulation of CoTS has primarily focused on the adult life stage (Endean 1969; Pearson and Endean 1969; McCallum et al. 1989; Hall et al. 2017; Kroon et al. 2020, 2021). However, early life-history stages, including small planktonic larvae and benthic juveniles, are likely to be highly susceptible to predation relative to adults and represent a typical population bottleneck of most marine broadcast spawning invertebrates (Gosselin and Qian 1997). CoTS gametes and larvae may experience significant predation pressure from planktivorous fishes (Cowan et al. 2016a, 2017b, 2020), and predation-induced mortality rates of newly settled CoTS are suggested to be high (Keesing and Halford 1992a). Indeed, variation in outbreak intensity with fishing protection may be occurring through multiple and potentially indirect interactions with early life-history stages rather than adults (Sweatman 2008; Kroon et al. 2020). Importantly, CoTS juveniles are exposed to predators for much longer (i.e. months to years) in comparison with gametes and larvae (i.e. days to weeks) (Deaker et al. 2020; Wilmes et al. 2020a). Consequently, even small variations in predator-induced mortality of CoTS during this juvenile life stage can accumulate to substantially change the likelihood of outbreaks (Keesing and Halford 1992b; Morello et al. 2014; Keesing et al. 2018; Wilmes et al. 2018). Predation and variations in survival during the early life-history stages of the CoTS population cycle remain critical knowledge gaps but have the potential to significantly inform understanding of outbreak initiation mechanisms.
Coral rubble is the primary settlement habitat of CoTS (Zann et al. 1987; Wilmes et al. 2020b) and is home to a wide range of crustaceans, molluscs, echinoderms, fishes and worms, which occupy all trophic guilds (Glynn and Enochs 2011; Cortés et al. 2017). Trophodynamics within the rubble cryptofauna are largely unresolved, yet a variety of cryptic predators are common (reviewed in Wolfe et al. 2021). Rubble-dwelling predators play potentially important roles in the structuring of coral reef communities from the bottom-up, particularly through predation on vulnerable early life-history stages of organisms that recruit to rubble (Glynn 2006, 2013). Empirical data on relevant predation rates of these communities are sparse, making it difficult to quantify the magnitude of their role in population and recruitment bottlenecks, and thus, greater reef food webs.
Identification of CoTS predators common in rubble has thus far occurred opportunistically and less often on the juvenile stage (reviewed in Cowan et al. 2017a). Invertebrate communities in rubble habitats may contribute significantly to juvenile CoTS mortality (Keesing and Halford 1992a; Keesing et al. 1996, 2018), but there is poor understanding of the identity of specific predator species. Notably, the fireworm Pherecardia striata and harlequin shrimp Hymenocera picta have been observed feeding on juvenile CoTS across reefs of the Eastern Pacific (Glynn 1984). In Panama, where these predators are found in high abundance, CoTS are relatively scarce and have not exhibited outbreaks (Glynn 1982). In a laboratory context, the peppermint shrimp Lysmata vitatta has demonstrated capacity to consume CoTS juveniles (Balu et al. 2021), and the presence of polychaete worms and trapeziid crabs limited CoTS settlement and metamorphosis (Cowan et al. 2016b). In all, these initial findings provide strong evidence for the importance of invertebrate predation, yet a comprehensive evaluation of juvenile CoTS predators during this rubble-dwelling life stage has never been conducted (Wilmes et al. 2018).
Here, we assessed whether common rubble-dwelling taxa consume juvenile CoTS in a series of feeding trials. First, predators collected from coral rubble were offered one juvenile to determine whether they could indeed consume CoTS. Second, for two of the most likely predators in these initial trials, we examined their capacity to detect and consume CoTS in natural rubble mesocosms. From these experiments, we identify key novel predators of CoTS juveniles and discuss the implications of juvenile predation in context of CoTS population outbreaks on coral reefs.
Methods
Juvenile CoTS rearing
The taxonomy of the Pacific species of Acanthaster is uncertain (Haszprunar and Spies 2014), so we refer to the species on the GBR as Acanthaster sp. Adult CoTS were collected in the Cairns Region, Queensland, Australia and shipped live to the National Marine Science Centre in Coffs Harbour, New South Wales, Australia, where they were maintained in flow-through seawater at the approximate temperature of the collection habitat (25–27 °C). CoTS were spawned in December 2021 by dissecting the gonads from multiple males and females. Ovaries were rinsed in 1 μm filtered seawater (FSW) and steeped in 10–5 M 1-methyl adenine in FSW to induce ovulation. Sperm was collected directly from gonads using a pipette. Eggs and sperm that had been checked for quality microscopically were combined for fertilisation in a 1-L beaker at a sperm-to-egg ratio of approximately 100:1. Once greater than 95% fertilisation was confirmed, the sperm were rinsed away using FSW.
Larvae were reared in a 300-L culture container in FSW at 25–27 °C that was changed daily. The larvae were fed the cryptomonad algae Proteomonas sulcata at a density of approximately 1–5 × 104 cells mL−1 as needed. After 18–22 d, competent larvae were settled onto polycarbonate plates containing a culture of crustose coralline algae (CCA) and mixed algal biofilm. Juveniles were reared on these plates in flow-through seawater at approximately 25–27 °C. In February 2022, CoTS and CCA were transported to Heron Island Research Station where they were housed in two 6-L flow-through aquaria (mean 27.3 °C ± 0.1, n = 60) throughout the experiment.
Predator candidate collection
Potential CoTS predators were collected on SCUBA across coral rubble patches of Heron Reef, Australia. Collections were conducted between 2 and 12 m depth, as this covers the depth range wherein CoTS settlement and juvenile distributions have been documented in situ (Wilmes et al. 2020b; Doll et al. 2021). Rubble communities were sampled using multiple collection methods to obtain adequate replication and ensure representation of a variety of taxa. First, RUbble Biodiversity Samplers (RUBS), which are 3D-printed models used to standardise biodiversity sampling in rubble, were regularly deployed in rubble patches using standard protocol (Wolfe and Mumby 2020). Additionally, a series of plastic mesh baskets (4 L) were filled with rubble and buried in depressions at these sites. Both RUBS and rubble-filled baskets were collected after a minimum of 4 d and redeployed periodically to sustain rubble community collections. Upon retrieval, RUBS and rubble-filled baskets were lifted from their depressions in the rubble and sealed immediately in individual plastic bags underwater. Each sample was then returned to the laboratory and extensively searched for predator candidates by using probes, forceps and pressurised seawater delivered from wash bottles to dislodge individuals. In tandem with these passive collection methods, active searches for larger mobile taxa were conducted. This involved searches among rubble patches, manually overturning rubble pieces and collecting conspicuous individuals using small hand nets or by hand. Based on field collections, density of confirmed CoTS predators was approximated as either individuals per dive hour for manually collected species or individuals per L for species collected passively (see Table 1). We note that manual and passive collections were concentrated in rubble sites with known, and often specific, predator communities so the density estimates here may not reflect total abundance of these species across broader habitats and reef zones.
The potential for collected individuals to be predatory was determined from extensive literature searches and reports of diet for each species. Specifically, species reported as herbivores in the literature or those with body sizes much smaller than the minimum CoTS size (0.8 mm) were not used in experiments, while those considered scavengers, generalists or predators were used. Selected species were housed in communal 6-L flow-through aquaria with natural rubble supplied with seawater to provide food and shelter until use in predation trials. Larger individuals were separated to ensure predation among predator candidates did not occur within housing tanks and were fed chopped bait prawns every few days. Individuals were starved for ~ 24 h before use in experiments.
Predation trials
To determine whether a predator candidate collected from rubble ate CoTS, single specimens were placed with an individual juvenile CoTS in initial feeding trials. Predator candidates were photographed (Olympus TG6) for identification and measured (nearest mm) before being placed in individual 800-mL containers with flow-through seawater (~ 0.3 L min−1). No shelter was provided. One juvenile CoTS was then randomly selected from the housing tank, photographed under a dissecting microscope (Olympus SZ61, Dino-Eye AM7025X) and placed into each predator tank. Juvenile size (maximum diameter, mm) was quantified using ImageJ and ranged from 0.8 to 3.8 mm in diameter across predation trials (mean 1.67 ± 0.02).
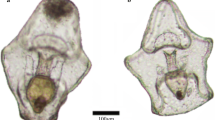
Feeding trials lasted for a maximum of 3 d with tanks checked once per day. When the juvenile CoTS was not readily visible, the tank was thoroughly searched before all water contents were filtered through a 200-µm mesh and further rinsed with freshwater. If the juvenile was still absent, the predator candidate was examined and rinsed with freshwater to ensure the CoTS juvenile was not on the predator itself, which occurred in several instances. CoTS were deemed consumed when not found after this extensive search process was repeated several times. At the end of the trial period, CoTS were scored as either not consumed, injured (partial predation) or consumed. CoTS that were found were scored as injured if they had visible portions of their body missing following the trial (see Fig. 1L, M).
Phylogenetic relatedness of 104 CoTS predator candidates—blue labels denote confirmed predator taxa. Featured predator candidates A the fireworm Eurythoe complanata, B Peristernia reincarnata, C the marbled shrimp Saron marmoratus, D the red decorator crab Schizophrys aspera, E Thalamita admete, F Thalamitoides tridens, G Chlorodiella nigra, H Actaeodes hirsutissimus, I Etisus anaglyptus and J Neoliomera insularis. K Shows healthy, intact juvenile CoTS, while L and M each show 1 juvenile CoTS damaged by predators with arrow indicating the site of injury. Scale bars in panels A–J equate to 10 mm, and 2 mm in panels K–M
Rubble mesocosms
Several species emerged as successful predators of CoTS in the initial experimental feeding trials. To address the potential of these predators to detect and consume juvenile CoTS among rubble, we conducted a series of feeding trials in tanks filled with natural coral rubble. Two decapods, Schizophrys aspera (Majidae) and Thalamita admete (Portunidae), were used given their consistency in initial feeding trials (see Results section) and their high abundance in our rubble sites (see Table 1).
To establish the rubble mesocosms, 1-L plastic mesh baskets were filled with rubble in situ and buried in rubble patches at ~ 4 m for at least 4 d to colonise a natural rubble community (rubble water displacement volume = 288 mL ± 10, n = 18). Baskets were collected on SCUBA and sealed in individual plastic bags for transportation back to the laboratory, as above. The contents of each rubble-filled basket were then placed in 6-L flow-through tanks (supplied with 1.15 L ± 0.06 seawater min−1) and left to stabilise for at least 6 h before trials commenced. Each tank was stocked with 30 juvenile CoTS (1.02–3.78 mm) and one predator, either S. aspera or T. admete (n = 6 per predator). Control tanks without predators were also conducted to capture any background CoTS mortality caused by the wider rubble community and to account for potential error in searching and handling procedures (n = 6). Rubble pieces in all treatments were covered with a range of sessile biota including CCA, turf algae, macroalgae, sponges and ascidians, as typical of shallow reef rubble (Wolfe et al. 2021). This microhabitat complexity served to amplify the cryptic nature of juvenile CoTS, which fed on CCA throughout the experiment, as evidenced by feeding scars on rubble pieces.
After 4 d, tanks were searched extensively for CoTS. Each rubble piece was removed and rinsed with freshwater at least 3 times to remove all visible CoTS and other rubble fauna, a common method for extracting rubble fauna (Takada et al. 2007; Stella et al. 2011; Wolfe et al. 2021). Given the complex morphology of some rubble pieces, it was necessary to break certain pieces apart to investigate crevices and holes within which CoTS may have been lodged. All CoTS were retained in a separate dish for each treatment, and all other individuals > 1 mm (the approximate size of CoTS used) from each rubble community were counted and classified to phylum under a dissecting microscope to indicate alternative prey and potential species’ interactions within mesocosms. All CoTS were photographed and measured, as above.
Data analysis
A phylogenetic tree of all predator candidates was constructed at the highest possible taxonomic resolution using the open-access interactive Tree Of Life (iTOL) tool (Letunic and Bork 2021). Fisher’s exact test was used to compare the incidence of feeding trial outcomes (relative proportion of not consumed, injured or consumed) among predator candidates with at least one observed instance of CoTS consumption or injury using the fisher.test function from the stats package in R version 4.0.0 (R Core Team 2020). Fisher’s exact test was selected to address small sample sizes, and the p value was computed using Monte Carlo simulation, given that the contingency table dimensions were larger than 2 × 2 (Patefield 1981). Pairwise comparisons of feeding trial results between species were extracted using the fisher.multcomp function in the RVAideMemoire package (Hervé 2022), and resulting p-values adjusted using Benjamini & Hochberg’s methods (Benjamini and Hochberg 1995). Inspection of pairwise comparisons was used to aggregate similar species into groups based on the relative frequencies of each outcome (I = incidental, C = consumers or P = partial consumers).
The proportions of CoTS that experienced predation (i.e. injured or consumed) in rubble mesocosm trials were compared across treatments using binomial regression implemented with the lme4 package (Bates et al. 2015). Here, we grouped the number of consumed and injured CoTS as partial predation was only observed in T. admete treatments, and both outcomes are ultimately representative of predatory interactions. Post hoc pairwise comparisons between treatments were conducted using Tukey’s HSD from the multcomp package (Hothorn et al. 2008).
Results
Predation trials
A total of 104 distinct taxa from 41 families and 74 genera (Fig. 1, Table S1) were collected and used across feeding trials (n = 404). Where possible, taxa were identified to species level, but 59 individuals were only identified to genus (Table S1). Feeding trials were replicated 1–19 times per species (mean = 3.9 ± 0.4 SE), with low replication for some owing to rarity of collection (Table 1, Table S1). Of the taxa tested, 26 species were found to have consumed or injured CoTS on at least one occasion (Fig. 1). Confirmed predators were overwhelmingly decapod crustaceans (85%), with greatest representation of species from the Portunidae and Xanthidae (Fig. 1A–J, Table 1). Two species of Annelida and of Mollusca were also found to consume juvenile CoTS. The remaining predator candidates (n = 78) that did not display capacity to consume CoTS across feeding trials (Table S1) were comprised of species from the Platyhelminthes (n = 7), Nemertea (n = 3), Annelida (n = 6), Mollusca (n = 12), Arthropoda (n = 43) and Chordata (n = 7).
Significant differences were found between relative proportions of feeding trial outcomes for confirmed predators (p < 0.001). Visual inspection of outcomes and pairwise comparisons revealed three distinct groupings. One class of predators, which we refer to as incidental, only partially or entirely consumed CoTS juveniles on 1 or 2 occasions (Table 1, Fig. 2). Predators in this class were comprised of worms, Eurythoe complanata (Fig. 1A) and Lepidonotus cristatus, gastropods, Latirus polygonus and Peristernia reincarnata (Fig. 1B), and several decapods, including species of the Alpheidae, Calcinidae and Hippolytidae (e.g. Saron marmoratus, Fig. 1C), as well as Xanthidae (e.g. Chlorodiella nigra, Actaeodes hirsutissimus and Neoliomera insularis, Fig. 1G, H and J, respectively). The incidental interactions of these predators stand in contrast to outcomes for Schizophrys aspera (Fig. 1D), a decorator crab that consumed CoTS in 89% of feeding trials (Fig. 2). We categorise this predator as an active consumer. A third group of predators, we refer to as partial consumers, less frequently consumed entire juvenile CoTS and most often inflicted arm and body damage of varying severity (Fig. 1K–M, Fig. 2). The partial predator archetype was most often associated with species of Portunidae (e.g. Thalamita admete and Thalamitoides tridens, Fig. 1E, F), as well as two xanthid crabs, Cyclodius ungulatus and Etisus anaglyptus (Fig. 1I, Table 1, Fig. 2).
Rubble mesocosms
Of the 30 juvenile CoTS added to control tanks (i.e. no added predator), we recovered > 98% (29–30 ind.). Both Schizophrys aspera (p < 0.001) and Thalamita admete (p < 0.05) consumed significantly more CoTS compared to the control, and these two predator treatments also differed (p < 0.001) (Fig. 3A, Table S2). When T. admete was present, we found ~ 93% CoTS intact, suggesting that 2 ± 0.5 CoTS were preyed on (Fig. 3A). Of these, one-third were injured rather than fully consumed. In S. aspera mesocosms, we recovered 21% of CoTS (i.e. 23.7 ± 1.6 ind. consumed). Taking background loss or mortality rates from control tanks into consideration (0.13 ± 0.06 CoTS d−1), T. admete and S. aspera results translate to daily consumption rates of 0.37 ± 0.14 and 5.78 ± 0.41 CoTS d−1, respectively. CoTS size did not vary substantially among treatments (mean 1.94–2.15 mm).
A proportion of CoTS (± SE) preyed on in experimental rubble mesocosms and B mean abundance (± SE) of rubble-dwelling taxa (> 1 mm) after 96-h exposure to either control conditions, a Thalamita admete or Schizophrys aspera individual (n = 6 per treatment). Significant differences between treatments are indicated as *p < 0.05 and ***p < 0.001
Post-trial rubble communities across all mesocosms treatments consisted primarily of individuals from the Arthropoda and Annelida (Fig. 3B). Fewer molluscs were found in control tanks, while lower total numbers of rubble fauna were documented following feeding trials with T. admete. The greatest abundance of rubble fauna after the experiment was found in tanks with S. aspera.
Discussion
A crucial first step in resolving the contribution of predator–prey interactions to population dynamics is identifying key predator species. Here, we investigated the capacity of 104 species to consume CoTS in their early post-settlement life stage. We focused our attention on predators found in coral rubble, as this is where juvenile CoTS predominantly settle and begin their early benthic life stage as herbivores (Zann et al. 1987; Wilmes et al. 2020b). There are currently > 90 species identified to consume CoTS across various life phases, most of them reef fishes and just 24 coral reef invertebrates (Cowan et al. 2017a; Kroon et al. 2020; Balu et al. 2021). Our results more than double this list with observations of 26 new invertebrate species that demonstrated varying capacity to consume CoTS in laboratory feeding assays.
By far the most consistent predator was the red decorator crab, Schizophrys aspera. Juvenile CoTS were consumed in their entirety in nearly all S. aspera feeding trials, including in the rubble mesocosm setting highlighting their ability to locate and consume CoTS among natural rubble and over a diversity of other prey options. Interestingly, rates of consumption by individual S. aspera in rubble mesocosm trials (approximately 5.8 CoTS d−1) are comparable to reported whole-of-community predation rates (5.05 CoTS d−1) for similar size–age cohorts (Keesing and Halford 1992a), although CoTS densities between studies potentially differed. The feeding rates of S. aspera documented here were lower than that measured of planktivores feeding on CoTS larvae (Cowan et al. 2016a), although larval duration of CoTS is short (days to weeks) compared to the juvenile phase (months to years), meaning the cumulative impact of predators on juveniles through time would be the critical bottleneck in CoTS ontogeny. Additionally, S. aspera consumption rates far exceed those reported on adult CoTS by Charonia tritonis (< 1 CoTS wk−1), a predator that has received considerable attention (Pearson and Endean 1969; Hall et al. 2017). These results help document how mortality rates of CoTS change during ontogeny (Wilmes et al. 2018) and emphasise the importance of predatory interactions during early life-history stages, when their susceptibility as prey is high.
The discovery of S. aspera as the foremost predator of juvenile CoTS in coral rubble at Heron Reef is not altogether unexpected. Decorator crabs have been implicated as important predators of echinoderms (Wicksten 1980; Mladenov 1983; Bonaviri et al. 2012; Clemente et al. 2013; Fagerli et al. 2014), including asteroids (Ling and Johnson 2013). While these predator–prey interactions occur between different species in different ecosystems, majoid crabs seem to be important echinoderm specialists. Decorator crabs are named given their propensity for covering their exoskeletons in a variety of taxa, including algae, sponges, ascidians and even hydroids, for camouflage (Wicksten 1980; Guinot and Wicksten 2015) and predator deterrence (Stachowicz and Hay 1999). We hypothesise that these decorating habits may confer some level of tolerance to toxins common in echinoderm species, including the saponins and plancitoxins found in CoTS (Howden et al. 1975; Shiomi et al. 1988), which make them unpalatable to many predators (Lucas et al. 1979; Shiomi et al. 1990, 2004). Four other decorator crab species, including the epialtids, Menaethius monoceros (n = 4, 1 observed incidence of consumption), Tiarinia cornigera (n = 8) and Tiarinia sp (n = 3), and the inachid, Camposcia retusa (n = 1), were also trialled in feeding assays. However, these species did not show the same consistency in consuming CoTS as S. aspera, a formidable predator deserving of further attention. In all, the demonstrated CoTS predation capacity of S. aspera should prompt further consideration of its distribution and abundance, especially relative to known outbreak initiation zones and between fished and unfished reefs.
A secondary group of predators, comprised primarily of portunid crabs, were distinct in their ability to cause injury more often than consume whole juvenile CoTS. While more opportunistic in nature, a larger number of species displayed this predation style as compared to S. aspera. Outcomes of rubble mesocosm trials with the portunid Thalamita admete exemplified this predation strategy, wherein predatory interactions occurred less frequently than in S. aspera trials and often led to injury rather than whole CoTS consumption. Lower overall abundance of other rubble taxa during these trials also suggests that CoTS are not the preferred food for this predator when other options are present. Conversely, S. aspera had the highest abundance of rubble fauna post-experiment, suggesting that CoTS may be a preferred food item.
Other predators have been noted to inflict injuries on CoTS, including the harlequin shrimp Hymenocera picta (Glynn 1982), and more recently in laboratory experiments with the peppermint shrimp Lysmata vittata (Balu et al. 2021). Indeed, a large proportion of CoTS juveniles and adults in situ are found to have injuries (Glynn 1984; McCallum et al. 1989; Messmer et al. 2017; Budden et al. 2019; Wilmes et al. 2019; Caballes et al. 2022). The likelihood of CoTS recovery from these dismembered states needs further attention as partial predation may, in some cases, result in the regeneration of multiple individuals (Lawrence and Vasquez 1996) or alternatively cause mortality depending on severity (Deaker et al. 2021). Surviving individuals with sustained injuries may display arrested growth trajectories, delayed transitions between life stages and reduced reproductive output, as recognised for other echinoderms (Lawrence and Larrain 1994; Zajac 1995; Lawrence and Vasquez 1996; Bingham et al. 2000; Maginnis 2006; Lindsay 2010; Budden et al. 2019; Deaker et al. 2021). Therefore, despite partial consumers not causing immediate CoTS mortality, the population-level consequences of partial predation may be substantial.
The remaining predators identified in this study were classified as incidental, given that CoTS were only consumed or injured on 1 or 2 occasions across feeding trials. For some of these species, such as the xanthid crabs Luniella spinipes, Actaeodes hirsutissimus, Atergatis floridus and Neoliomera insularis, this classification may be an artefact of low sample size, caused by constraints on our ability to collect individuals in the focal habitat of this study. Despite their potential to consume or injure CoTS, the low numbers of these xanthids found across our study sites suggest that their impacts on CoTS populations may be limited. Yet, 15 other xanthid species exhibited no predation (see Table S1), while two species, Etisus anaglyptus and Cyclodius ungulatus, grouped within the partial predators owing to a greater sample size and higher frequency of predator interactions with CoTS. Further research is required to demonstrate whether the species tested here can consistently consume or injure CoTS and to quantify their abundance in rubble communities in other locations.
Other incidental predators, including reef shrimp Athanas parvus and Saron marmoratus, did not generally demonstrate interest in consuming CoTS despite adequate replication of feeding trials. For these species, it is unlikely that they have meaningful interactions with CoTS in situ. This contrasts other reef shrimp (H. picta and L. vittata) known to consume CoTS (Glynn 1982; Balu et al. 2021), although we note these species are from distinct genera that were not found in rubble here. Similarly, the one incident of CoTS consumption by the fireworm Eurythoe complanata identified here contrasts the voracity of another identified amphinomid predator of CoTS, Pherecardia striata (Glynn 1984). However, P. striata predation occurs primarily on already injured CoTS, which may also motivate E. complanata but was not investigated here. Indeed, this would result in secondary predation of the partially consumed CoTS from interactions between CoTS and species of the Portunidae, which would, in turn, limit juvenile regeneration. These indirect predator interactions and the potential for facilitation among predatory species warrant further enquiry, especially given 75% of taxa (78 species) tested here did not show interest in whole starfish.
Future research could explore how the predator–prey interactions described here may change or persist across the broader CoTS ontogeny and age-size classes, including juveniles that have made the switch to a corallivorous diet and adults. We expect vulnerability to predation to change across these size and diet transitions, which can occur as early as 140–190 d post-settlement (Neil et al. 2022) but may be delayed when no coral is available (Deaker et al. 2020). Indeed, even within the herbivorous juvenile period, predation pressure may decrease significantly as body size increases, as documented in both experimental and field studies (Keesing et al. 2018; Balu et al. 2021).
We note that no juvenile CoTS were found in our searches. Given our predator collections were aligned with the time of year that CoTS juveniles are expected to be settled (Doll et al. 2021) and concentrated within the ~ 8 m depth range considered the “hotspot” of juvenile densities (Wilmes et al. 2020b), we expected to encounter some CoTS. However, the demonstrated ability of S. aspera, and to a lesser extent T. admete, to consume CoTS among natural rubble make it possible that predator populations had already reduced CoTS juvenile densities in their immediate surroundings. Heron Reef, a protected no-take zone, has experienced relatively little CoTS impact in comparison with other reefs on the GBR (Pratchett et al. 2014; Matthews et al. 2020). Indeed, protected reefs may have fewer or less severe CoTS outbreaks (Sweatman 2008; Kroon et al. 2021), which seems to be reflected at our study sites. This pattern for no-take zones is likely driven by higher predation from fishes and invertebrates in rubble (as found here) combined. Our ability to detect and collect high numbers of these predators in their cryptic habitats indicates they are indeed common in rubble on Heron Reef.
Broader information on the distribution of these novel predators remains limited but may begin to inform whether these predators have measurable influence over CoTS populations at broader reef scales, especially for S. aspera. It seems critical to incorporate the novel predators identified here in surveys on reefs open and closed to fishing to address mechanistically the potential for CoTS outbreaks to be suppressed in protected areas (Sweatman 2008; Kroon et al. 2021). Whether cryptic decapods benefit from no-take zones or are conversely at greater risk of predation themselves owing to higher fish densities requires attention, especially now in the context of trophic links to CoTS populations. In all, this study offers preliminary information that will contribute to resolving the role that predators play in regulating CoTS populations across life stages.
References
Babcock RC, Milton DA, Pratchett MS (2016) Relationships between size and reproductive output in the crown-of-thorns starfish. Mar Biol 163:234
Balu V, Messmer V, Logan M, Hayashida-Boyles AL, Uthicke S (2021) Is predation of juvenile crown-of-thorns seastars (Acanthaster cf. solaris) by peppermint shrimp (Lysmata vittata) dependent on age, size, or diet? Coral Reefs 40:641–649
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (methodol) 57:289–300
Bingham BL, Burr J, Wounded Head H (2000) Causes and consequences of arm damage in the sea star Leptasterias hexactis. Can J Zool 78:596–605
Birkeland C (1989a) The Faustian traits of the crown-of-thorns starfish. Am Sci 77:154–163
Birkeland C (1989b) The influence of echinoderms on coral reef communities. In: Jangoux M, Lawrence JM (eds) Echinoderm studies, vol 3. Balkema. Rotterdam, The Netherlands, pp 1–77
Birkeland C, Lucas J (1990) Acanthaster planci: major management problem of coral reefs. CRC Press, Boca Raton, Florida
Bonaviri C, Gianguzza P, Pipitone C, Hereu B (2012) Micropredation on sea urchins as a potential stabilizing process for rocky reefs. J Sea Res 73:18–23
Bozec YM, Hock K, Mason RAB, Baird ME, Castro-Sanguino C, Condie SA, Puotinen M, Thompson A, Mumby PJ (2021) Cumulative impacts across Australia’s Great Barrier Reef: a mechanistic evaluation. Ecol Monogr 92:e01494
Brodie J, Fabricius K, De’ath G, Okaji K (2005) Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar Pollut Bull 51:266–278
Budden C, Butler I, Wolfe K, Deaker D, Sweatman H, Byrne M (2019) Effect of sublethal predation on reproductive output of the crown-of-thorns starfish Acanthaster sp., with an overview of arm damage. Mar Ecol Prog Ser 629:103–116
Caballes CF, Byrne M, Messmer V, Pratchett MS (2021) Temporal variability in gametogenesis and spawning patterns of crown-of-thorns starfish within the outbreak initiation zone in the northern Great Barrier Reef. Mar Biol 168:13
Caballes CF, Messmer V, Raymundo ML, Pratchett MS (2022) Prevalence and severity of sublethal injuries in crown-of-thorns starfish relative to marine reserves in the Great Barrier Reef. Aquat Conserv Mar Freshwat Ecosyst 32:993–1004
Carrier TJ, Wolfe K, Lopez K, Gall M, Janies DA, Byrne M, Reitzel AM (2018) Diet-induced shifts in the crown-of-thorns (Acanthaster sp.) larval microbiome. Marine Biology 165:157
Clemente S, Hernández JC, Montaño-Moctezuma G, Russell MP, Ebert TA (2013) Predators of juvenile sea urchins and the effect of habitat refuges. Mar Biol 160:579–590
Cortés J, Enochs IC, Sibaja-Cordero J, Hernández L, Alvarado JJ, Breedy O, Cruz-Barraza JA, Esquivel-Garrote O, Fernández-García C, Hermosillo A, Kaiser KL, Medina-Rosas P, Morales-Ramirez A, Pacheco C, Pérez-Matus A, Reyes-Bonilla H, Riosmena-Rodriguez R, Sanchez-Noguera C, Wieters EA, Zapata FA (2017) Marine biodiversity of Eastern Tropical Pacific coral reefs. In: Glynn PW, Manzello DP, Enochs IC (eds) Coral reefs of the eastern tropical Pacific, vol 8. Springer. Dordrecht, The Netherlands, pp 203–250
Cowan Z-L, Dworjanyn SA, Caballes CF, Pratchett MS (2016a) Predation on crown-of-thorns starfish larvae by damselfishes. Coral Reefs 35:1253–1262
Cowan Z-L, Dworjanyn SA, Caballes C, Pratchett M (2016) Benthic predators influence microhabitat preferences and settlement success of crown-of-thorns starfish (Acanthaster cf solaris). Diversity 8:27
Cowan Z-L, Pratchett M, Messmer V, Ling S (2017) Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity 9:7
Cowan Z-L, Ling SD, Dworjanyn SA, Caballes CF, Pratchett MS (2017b) Interspecific variation in potential importance of planktivorous damselfishes as predators of Acanthaster sp. eggs. Coral Reefs 36:653–661
Cowan Z-L, Ling SD, Caballes CF, Dworjanyn SA, Pratchett MS (2020) Crown-of-thorns starfish larvae are vulnerable to predation even in the presence of alternative prey. Coral Reefs 39:293–303
Dana TF, Newman WA, Fager EW (1972) Acanthaster aggregations: interpreted as primarily responses to natural phenomena. Pac Sci 26:355–372
Deaker DJ, Mos B, Lin HA, Lawson C, Budden C, Dworjanyn SA, Byrne M (2020) Diet flexibility and growth of the early herbivorous juvenile crown-of-thorns sea star, implications for its boom-bust population dynamics. PLoS ONE 15:e0236142
Deaker DJ, Mos B, Lawson C, Dworjanyn SA, Budden C, Byrne M (2021) Coral defences: the perilous transition of juvenile crown-of-thorns starfish to corallivory. Mar Ecol Prog Ser 665:115–125
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci USA 109:17995–17999
Doll PC, Messmer V, Uthicke S, Doyle JR, Caballes CF, Pratchett MS (2021) DNA-based detection and patterns of larval settlement of the corallivorous crown-of-thorns sea star (Acanthaster sp.). Biol Bull 241:271–285
Dulvy NK, Freckleton RP, Polunin NVC (2004) Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol Lett 7:410–416
Endean RG (1973) Population explosions of Acanthaster planci and associated destruction of hermatypic corals in the Indo-West Pacific region. In: Jones OA, Endean RG (eds) Biology and Geology of Coral Reefs. Academic Press, New York, USA, pp 389–438
Endean RG (1969) Report on investigations made into aspects of the current Acanthaster planci (crown of thorns) infestations of certain reefs of the Great Barrier Reef. Queensland Department of Primary Industries (Fisheries Branch), Brisbane, Australia
Fabricius KE, Okaji K, De’ath G (2010) Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 29:593–605
Fagerli CW, Norderhaug KM, Christie H, Pedersen MF, Fredriksen S (2014) Predators of the destructive sea urchin Strongylocentrotus droebachiensis on the Norwegian coast. Mar Ecol Prog Ser 502:207–218
Glynn PW (1984) An amphinomid worm predator of the crown-of-thorns sea star and general predation on asteroids in eastern and western pacific coral reefs. Bull Mar Sci 35:54–71
Glynn PW, Enochs IC (2011) Invertebrates and their roles in coral reef ecosystems. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, The Netherlands, pp 273–325
Glynn P (1982) Acanthaster population regulation by a shrimp and a worm. In: Gomez ED, Birkeland CE, Buddemeier RW, Johannes RE, Marsh JA Jr, Tsuda RT (eds) Proceedings of the 4th International Coral Reef Symposium, Manilla, Philippines, pp 607–612
Glynn P (2006) Fish utilization of simulated coral reef frameworks versus eroded rubble substrates off Panama, eastern Pacific. In: Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, pp 250–256
Glynn PW (2013) Fine-scale interspecific interactions on coral reefs: functional roles of small and cryptic metazoans. In: Research and discoveries: the revolution of science through scuba, Smithsonian Contributions to the Marine Sciences, pp 229 - 248
Gosselin LA, Qian PY (1997) Juvenile mortality in benthic marine invertebrates. Marine Ecology Progress Series 146:265–282
Guinot D, Wicksten ML (2015) Camouflage: carrying behaviour, decoration behaviour, and other modalities of concealment in Brachyura. In: Castro P, Davie PJF, Guinot D, Schram FR, von Vaupel Klein JC (eds) Treatise on Zoology - Anatomy, Taxonomy, Biology, The Crustacea; Part C-I-Decapoda: Brachyura (Part-1), vol 9. Brill. Leiden, The Netherlands, pp 583–638
Hall M, Motti C, Kroon F (2017) The potential role of the giant triton snail, Charonia tritonis (Gastropoda: Ranellidae) in mitigating populations of the crown-of-thorns starfish. Report to the National Environmental Science Programme. Reef and Rainforest Research Centre Limited, Cairns, Australia
Haszprunar G, Spies M (2014) An integrative approach to the taxonomy of the crown-of-thorns starfish species group (Asteroidea: Acanthaster): A review of names and comparison to recent molecular data. Zootaxa 3841:271–284
Hervé M (2022) RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R package version 0.9–81–2. https://CRAN.R-project.org/package=RVAideMemoire
Hock K, Wolff NH, Condie SA, Anthony RN, Mumby PJ (2014) Connectivity networks reveal the risks of crown-of-thorns starfish outbreaks on the Great Barrier Reef. J Appl Ecol 51:1188–1196
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Howden ME, Lucas J, McDuff M, Salathe R (1975) Chemical defences of Acanthaster planci. Crown-of-thorns Starfish Seminar Proceedings. Australian Government Publishing Service, Canberra, Australia, pp 67–79
Keesing JK, Halford AR (1992a) Field measurement of survival rates of juvenile Acanthaster planci: techniques and preliminary results. Mar Ecol Prog Ser 85:107–114
Keesing JK, Halford AR (1992b) Importance of postsettlement processes for the population dynamics of Acanthaster planci (L.). Aust J Mar Freshw Res 43:635–651
Keesing JK, Wiedermeyer WL, Okaji K, Halford AR, Hall KC, Cartwright CM (1996) Mortality rates of juvenile starfish Acanthaster planci and Nardoa spp. measured on the Great Barrier Reef, Australia and Okinawa. Japan Oceanologica Acta 19:441–448
Keesing JK, Halford AR, Hall KC (2018) Mortality rates of small juvenile crown-of-thorns starfish Acanthaster planci on the Great Barrier Reef: implications for population size and larval settlement thresholds for outbreaks. Mar Ecol Prog Ser 597:179–190
Keesing JK (1990) Feeding biology of the crown-of-thorns starfish, Acanthaster planci (Linnaeus). Doctoral Dissertation. James Cook University, Townsville, Australia
Kroon FJ, Lefevre CD, Doyle JR, Patel F, Milton G, Severati A, Kenway M, Johansson CL, Schnebert S, Thomas-Hall P, Bonin MC, Cameron DS, Westcott DA (2020) DNA-based identification of predators of the corallivorous crown-of-thorns starfish (Acanthaster cf. solaris) from fish faeces and gut contents. Scientific Reports 10:8184
Kroon FJ, Barneche DR, Emslie MJ (2021) Fish predators control outbreaks of crown-of-thorns starfish. Nat Commun 12:6986
Lawrence JM, Larrain A (1994) The cost of arm autotomy in the starfish Stichaster striatus. Mar Ecol Prog Ser 109:311–313
Lawrence JM, Vasquez J (1996) The effect of sublethal predation on the biology of echinoderms. Oceanol Acta 19:431–440
Letunic I, Bork P (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:293–296
Lindsay SM (2010) Frequency of injury and the ecology of regeneration in marine benthic invertebrates. Integr Comp Biol 50:479–493
Ling SD, Johnson C (2013) Native spider crab causes high incidence of sub-lethal injury to the introduced seastar Asterias amurensis. In: Echinoderms in a Changing World: Proceedings of the 13th International Echinoderm Conference, Hobart, Australia, pp 195–201
Lucas JS (1973) Reproductive and larval biology of Acanthaster planci (L.) in Great Barrier Reef Waters. Micronesica 9:197–203
Lucas JS, Hart RJ, Howden ME, Salathe R (1979) Saponins in eggs and larvae of Acanthaster planci (L.) (Asteroidea) as chemical defences against planktivorous fishes. J Exp Mar Biol Ecol 40:155–165
Maginnis TL (2006) The costs of autotomy and regeneration in animals: a review and framework for future research. Behav Ecol 17:857–872
Matthews SA, Mellin C, Pratchett MS (2020) Larval connectivity and water quality explain spatial distribution of crown-of-thorns starfish outbreaks across the Great Barrier Reef. Adv Mar Biol 87:223–258
McCallum HI (1987) Predator regulation of Acanthaster planci. J Theor Biol 127:207–220
McCallum H (1990) Effects of predation on Acanthaster: age-structured metapopulation models. In: Bradbury RH (ed) Acanthaster and the Coral Reef: A Theoretical Perspective. Springer, Berlin, Germany, pp 208–219
McCallum HI, Endean R, Cameron AM (1989) Sublethal damage to Acanthaster planci as an index of predation pressure. Mar Ecol Prog Ser 56:29–36
McCook LJ, Ayling T, Cappo M, Choat JH, Evans RD, De Freitas DM, Heupel M, Hughes TP, Jones GP, Mapstone B, Marsh H, Mills M, Molloy FJ, Pitcher CR, Pressey RL, Russ GR, Sutton S, Sweatman H, Tobin R, Wachenfeld DR, Williamson DH (2010) Adaptive management of the Great Barrier Reef: a globally significant demonstration of the benefits of networks of marine reserves. Proc Natl Acad Sci USA 107:18278–18285
Mellin C, MacNeil MA, Cheal AJ, Emslie MJ, Caley MJ (2016) Marine protected areas increase resilience among coral reef communities. Ecology Letters 19:629–637
Messmer V, Pratchett M, Chong-Seng K (2017) Variation in incidence and severity of injuries among crown-of-thorns starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef. Diversity 9:12
Mladenov PV (1983) Rate of arm regeneration and potential causes of arm loss in the feather star Florometra serratissima (Echinodermata: Crinoidea). Can J Zool 61:2873–2879
Moran PJ (1986) The Acanthaster phenomenon. Oceanogr Mar Biol Annu Rev 24:379–480
Morello EB, Plagányi ÉE, BabcockSweatmanHillaryPunt RCHRAE (2014) Model to manage and reduce crown-of-thorns starfish outbreaks. Mar Ecol Prog Ser 512:167–183
Motti CA, Cummins SF, Hall MR (2022) A review of the giant triton (Charonia tritonis), from exploitation to coral reef protector? Diversity 14:961
Neil RC, Gomez Cabrera M, Uthicke S (2022) Juvenile age and available coral species modulate transition probability from herbivory to corallivory in Acanthaster cf. solaris (crown-of-thorns seastar). Coral Reefs 41:843–848
Ormond R, Bradbury RH, Bainbridge S, Fabricius K, Keesing JK, De Vantier L, Medlay P, Steven A (1990) Test of a model of regulation of crown-of-thorns starfish by fish predators. In: Bradbury RH (ed) Acanthaster and the Coral Reef: A Theoretical Persepctive. Springer, Berlin, Germany, pp 189–207
Osborne K, Dolman AM, Burgess SC, Johns KA (2011) Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009). PLoS ONE 6:e17516
Patefield WM (1981) Algorithm AS 159: An efficient method of generating r x c tables with given row and column totals. Appl Stat 30:91–97
Pearson RG, Endean R (1969) A preliminary study of the coral reef predator Acanthaster planci (L.) (Asteroidea) on the Great Barrier Reef. Fish Notes 3:27–55
Pratchett MS, Caballes CF, Rivera-Posada JA, Sweatman H (2014) Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr Mar Biol Annu Rev 52:133–200
Pratchett M, Caballes C, Wilmes J, Matthews S, Mellin C, Sweatman H, Nadler L, Brodie J, Thompson C, Hoey J, Bos A, Byrne M, Messmer V, Fortunato S, Chen C, Buck A, Babcock R, Uthicke S (2017) Thirty years of research on crown-of-thorns starfish (1986–2016): Scientific advances and emerging opportunities. Diversity 9:41
Pratchett MS, Caballes CF, Cvitanovic C, Raymundo ML, Babcock RC, Bonin MC, Bozec YM, Burn D, Byrne M, Castro-Sanguino C, Chen CCM, Condie SA, Cowan Z-L, Deaker DJ, Desbiens A, Devantier LM, Doherty PJ, Doll PC, Doyle JR, Dworjanyn SA, Fabricius KE, Haywood MDE, Hock K, Hoggett AK, Hoj L, Keesing JK, Kenchington RA, Lang BJ, Ling SD, Matthews SA, McCallum HI, Mellin C, Mos B, Motti CA, Mumby PJ, Stump RJW, Uthicke S, Vail L, Wolfe K, Wilson SK (2021) Knowledge gaps in the biology, ecology, and management of the Pacific crown-of-thorns sea star Acanthaster sp. on Australia’s Great Barrier Reef. Biol Bull 241:330–346
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Shiomi K, Yamamoto S, Yamanaka H, Kikuchi T (1988) Purification and characterisation of a lethal factor in venom from the crown-of-thorns starfish (Acanthaster planci). Toxicon 26:1077–1983
Shiomi K, Yamamoto S, Yamanaka H, Kikuchi T, Konno K (1990) Liver damage by the crown-of-thorns starfish (Acanthaster planci) lethal factor. Toxicon 28:469–475
Shiomi K, Midorikawa S, Ishida M, Nagashima Y, Nagai H (2004) Plancitoxins, lethal factors from the crown-of-thorns starfish Acanthaster planci, are deoxyribonucleases II. Toxicon 44:499–506
Stachowicz JJ, Hay ME (1999) Reducing predation through chemically mediated camouflage: Indirect ffects of plant defenses on herbivores. Ecology 80:495–509
Stella J, Pratchett MS, Hutchings PA, Jones GP (2011) Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr Mar Biol Annu Rev 49:43–104
Sweatman H (2008) No-take reserves protect coral reefs from predatory starfish. Curr Biol 18:598–599
Sweatman H (1997) Commercial fishes as predators of adult Acanthaster planci. In: Lessios HA, Macintyre IG (eds) Proceedings of the 8th International Coral Reef Symposium, Panama City Panama, pp 617–620
Takada Y, Abe O, Shibuno T (2007) Colonization patterns of mobile cryptic animals into interstices of coral rubble. Mar Ecol Prog Ser 343:35–44
Trapon ML, Pratchett MS, Penin L (2011) Comparative effects of different disturbances in coral reef habitats in Moorea, French Polynesia. Journal of Marine Biology 2011:1–11
Uthicke S, Schaffelke B, Byrne M (2009) A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol Monogr 79:3–24
Vanhatalo J, Hosack GR, Sweatman H (2017) Spatiotemporal modelling of crown-of-thorns starfish outbreaks on the Great Barrier Reef to inform control strategies. J Appl Ecol 54:188–197
Vercelloni J, Caley MJ, Mengersen K (2017) Crown-of-thorns starfish undermine the resilience of coral populations on the Great Barrier Reef. Glob Ecol Biogeogr 26:846–853
Vine PJ (1973) Crown of thorns (Acanthaster planci) plagues: the natural causes theory. Atoll Res Bull 166:1–10
Westcott DA, Fletcher CS, Kroon FJ, Babcock RC, Plaganyi EE, Pratchett MS, Bonin MC (2020) Relative efficacy of three approaches to mitigate crown-of-thorns starfish outbreaks on Australia’s Great Barrier Reef. Sci Rep 10:12594
Wicksten MK (1980) Decorator Crabs. Sci Am 242:146–154
Wilmes JC, Caballes CF, Cowan Z-L, Hoey AS, Lang BJ, Messmer V, Pratchett MS (2018) Contributions of pre- versus post-settlement processes to fluctuating abundance of crown-of-thorns starfishes (Acanthaster spp.). Mar Pollut Bull 135:332–345
Wilmes JC, Hoey AS, Messmer V, Pratchett MS (2019) Incidence and severity of injuries among juvenile crown-of-thorns starfish on Australia’s Great Barrier Reef. Coral Reefs 38:1187–1195
Wilmes JC, Hoey AS, Pratchett MS (2020a) Contrasting size and fate of juvenile crown-of-thorns starfish linked to ontogenetic diet shifts. Proceedings of the Royal Society b: Biological Sciences 287:20201052
Wilmes JC, Schultz DJ, Hoey AS, Messmer V, Pratchett MS (2020b) Habitat associations of settlement-stage crown-of-thorns starfish on Australia’s Great Barrier Reef. Coral Reefs 39:1163–1174
Wolfe K, Mumby PJ (2020) RUbble Biodiversity Samplers: 3D-printed coral models to standardize biodiversity censuses. Methods Ecol Evol 11:1395–1400
Wolfe K, Graba-Landry A, Dworjanyn SA, Byrne M (2015a) Larval starvation to satiation: influence of nutrient regime on the success of Acanthaster planci. PLoS ONE 10:e0122010
Wolfe K, Graba-Landry A, Dworjanyn SA, Byrne M (2015b) Larval phenotypic plasticity in the boom-and-bust crown-of-thorns seastar, Acanthaster planci. Mar Ecol Prog Ser 539:179–189
Wolfe K, Graba-Landry A, Dworjanyn SA, Byrne M (2017) Superstars: Assessing nutrient thresholds for enhanced larval success of Acanthaster planci, a review of the evidence. Mar Pollut Bull 116:307–314
Wolfe K, Kenyon TM, Mumby PJ (2021) The biology and ecology of coral rubble and implications for the future of coral reefs. Coral Reefs 40:1769–1806
Zajac RN (1995) Sublethal predation on Polydora cornuta (Polychaeta: Spionidae): patterns of tissue loss in a field population, predator functional response and potential demographic impacts. Mar Biol 123:531–541
Zann L, Brodie J, Berryman C, Naqasima M (1987) Recruitment, ecology, growth and behavior of juvenile Acanthaster planci (L.) (Echinodermata: Asteroidea). Bull Mar Sci 41:561–575
Acknowledgements
We thank Neil Bruce, Marissa McNamara, Peter Davie, Jessica Stella and Anna Murray for their assistance with taxonomy. We also thank Tania Kenyon, Karen Eigeland and the staff of Heron Island Research Station for field support, as well as Ben Mos and staff and students at the National Marine Science Centre for assistance rearing CoTS. Funding was provided by the Australian Coral Reef Society Danielle Simmons Award (AD) and the COTS Control Innovation Programme, which is supported by the partnership between the Australian Government’s Reef Trust and the Great Barrier Reef Foundation (KW, AD, SD, SU and PJM)
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All experiments and field collections were conducted under Great Barrier Reef Marine Park Authority permit G20/44613.1 and the University of Queensland animal ethics approval 2019/AE000388.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Desbiens, A.A., Mumby, P.J., Dworjanyn, S. et al. Novel rubble-dwelling predators of herbivorous juvenile crown-of-thorns starfish (Acanthaster sp.). Coral Reefs 42, 579–591 (2023). https://doi.org/10.1007/s00338-023-02364-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02364-w