Abstract
Objectives
The study aimed to investigate the alterations of myocardial deformation responding to long-standing pressure overload and the effects of focal myocardial fibrosis using feature-tracking cardiac magnetic resonance (FT-CMR) in patients with resistant hypertension (RH).
Methods
Consecutive RH patients were prospectively recruited and underwent CMR at a single institution. FT-CMR analyses based on cine images were applied to measure left ventricular (LV) peak systolic global longitudinal (GLS), radial (GRS), and circumferential strain (GCS). Functional and morphological CMR variables, and late gadolinium enhancement (LGE) imaging were also obtained.
Results
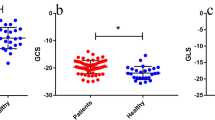
A total of 50 RH patients (63 ± 12 years, 32 men) and 18 normotensive controls (57 ± 8 years, 12 men) were studied. RH patients had a higher average systolic blood pressure than controls (166 ± 21 mmHg vs. 116 ± 8 mmHg, p < 0.001) with the intake of 5 ± 1 antihypertensive drugs. RH patients showed increased LV mass index (78 ± 15 g/m2 vs. 61 ± 9 g/m2, p < 0.001), decreased GLS (− 16 ± 3% vs. − 19 ± 2%, p = 0.001) and GRS (41 ± 12% vs. 48 ± 8%, p = 0.037), and GCS was reduced by trend (− 17 ± 4% vs. − 19 ± 4%, p = 0.078). Twenty-one (42%) RH patients demonstrated a LV focal myocardial fibrosis (LGE +). LGE + RH patients had higher LV mass index (85 ± 14 g/m2 vs. 73 ± 15 g/m2, p = 0.007) and attenuated GRS (37 ± 12% vs. 44 ± 12%, p = 0.048) compared to LGE − RH patients, whereas GLS (p = 0.146) and GCS (p = 0.961) were similar.
Conclusion
Attenuation of LV GLS and GRS, and GCS decline by tendency, might be adaptative changes responding to chronic pressure overload. There is a high incidence of focal myocardial fibrosis in RH patients, which is associated with reduced LV GRS.
Clinical relevance statement
Feature-tracking CMR-derived myocardial strain offers insights into the influence of long-standing pressure overload and of a myocardial fibrotic process on cardiac deformation in patients with resistant hypertension.
Key Points
• Variations of left ventricular strain are attributable to the degree of myocardial impairment in resistant hypertensive patients.
• Focal myocardial fibrosis of the left ventricle is associated with attenuated global radial strain.
• Feature-tracking CMR provides additional information on the attenuation of myocardial deformation responding to long-standing high blood pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among other cardiovascular risk factors hypertension remains a major cause of cardiovascular mortality worldwide [1]. Resistant hypertension (RH) is defined as above-goal elevated blood pressure despite the concurrent use of three or more different antihypertensive medications including a diuretic [2]. RH is associated with a higher risk of adverse cardiovascular events compared to controlled hypertension, and might be accompanied by extensive target organ damage, including left ventricular hypertrophy (LVH) [3, 4]. Myocardial fibrosis is a major determinant of hypertrophied myocardium and potentially associated with cardiovascular events, including heart failure and sudden death [5].
A recent work offers an overview of speckle-tracking echocardiography (STE) in assessing LV dysfunction in hypertension [6]. The explanation that attenuated longitudinal function and preserved circumferential and radial function are due to compensatory mechanisms has received reasonable attention, whereas longitudinal function is not always the earliest indicator in all circumstances, all three directions of function may decline in response to disease progress [6]. Although STE is the most available technique to quantify myocardial deformation, several weaknesses do exist. Reproducibility of acquisition planes is limited, which can influence particularly the evaluation of circumferential and radial strain [7]. The novel technique of feature-tracking cardiac magnetic resonance (FT-CMR), despite suffering from through-plane motion effects and having a lower spatial and temporal resolution than STE, has a better performance in measuring longitudinal, radial, and circumferential strain [7, 8]. Furthermore, the majority of the patient populations included in the previous echocardiographic literature had controlled mild to moderate hypertension [6].
Therefore, the main purpose of this study was to investigate the alterations of myocardial deformation responding to long-standing pressure overload and to elucidate the degree of myocardial impairment using FT-CMR. The secondary objective was to identify the potential effects of focal myocardial fibrosis in RH patients.
Materials and methods
Study population
The prospective study was approved by the local research ethics committee and complied with the Declaration of Helsinki. All participants gave written informed consent. This study recruited consecutive RH patients at a single institution and included 16 patients who were recruited in a previously publication [9]. The initial publication reported the effects of a renal denervation procedure on LV mass, myocardial strain, and diastolic function in RH patients [9].
The enrollment of criteria and diagnostic definitions have been detailed previously [9]. Briefly, RH patients were diagnosed according to the current guideline: blood pressure ≥ 140/90 mmHg despite the intake of at least 3 antihypertensive drugs in full dosages including a diuretic [2, 10]. The demographic and anthropometric characteristics were collected accordingly. Main exclusion criteria were as follows: (1) severe renal failure (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2), (2) significant stenosis and prior stenting or dilatation of renal arteries, (3) myocardial infarction < 6 months before planned renal denervation, (4) diabetes mellitus type I, and (5) persisting atrial fibrillation [9]. All patients underwent an office and 24-h ambulant blood pressure monitoring (ABPM). In addition, 18 healthy individuals, who underwent CMR scans for this particular research purpose, were enrolled to serve as a control group and had no known cardiovascular or systematic diseases.
CMR acquisition
CMR was performed on a 1.5-T scanner equipped with a 5-channel cardiac-phased array receiver coil (Achieva, Philips Medical Systems). Standard retrospectively gated, ECG-triggered steady-state free-precession cine images (25 phases per cardiac cycle) were acquired in short- and long-axis (2-, 3-, and 4-chamber) views using a breath-hold technique with the following typical parameters: acquired voxel size 1.98 × 1.80 × 6 mm3, reconstructed voxel size 1.36 × 1.36 × 6 mm3, gap 4 mm, 9–10 slices for full LV coverage, echo time = 1.67 ms, time to repetition = 3.34 ms, flip angle = 60°, parallel acquisition technique = SENSE [factor 2]). Ten minutes after a bolus injection of 0.2 mmol/kg gadoteric acid (Dotarem®, Guerbet) at a rate of 2.5 mL/s late gadolinium enhancement (LGE) images were acquired using an end-diastolic phase-sensitive inversion-recovery sequence in short-axis direction covering the entire heart and in 2-, 3-, and 4-chamber views.
CMR data analysis
CMR images were post-processed independently and blindly using a commercially available software (CVi42, Circle Cardiovascular Imaging Inc.). CMR parameters are given as the mean of two investigators and are indexed to body surface area (BSA). For LV volume and mass evaluation, the endo- and epicardial contours were delineated in systole and diastole in a stack of short-axis cine slices covering the whole LV with inclusion of the papillary muscles as part of the LV volume [11]. For right ventricular (RV) volume evaluation, the endocardial contours were delineated in systole and diastole in a stack of short-axis cine slices covering the whole RV [11]. Left (LA) and right atrial (RA) volumes and LV ejection fraction (EF) were calculated based on the biplane area-length method [12], measurements excluded pulmonary veins and atrial appendage. LVH was defined as LV mass index > 81 g/m2 for men and > 61 g/m2 for women [13]. Focal myocardial fibrosis (LGE +) was identified and assessed visually using short- and long-axis LGE images. LV myocardial strain analysis was performed with cine images using the feature-tracking software (Segment, version 2.1.R.6108, Medviso), through computing interframe deformation fields using an endocardial tracking strategy based on non-rigid image registration [14, 15]. LV peak systolic global longitudinal (GLS), radial (GRS), and circumferential strain (GCS) were measured on the long-axis (2-, 3-, and 4-chamber) and three short-axis (apical, mid, and basal) slices by manual delineation of the endo- and epicardial contours at end-diastole. Endo- and epicardial contours were automatically propagated by the software throughout the cardiac cycle to calculate myocardial strain.
Statistical analysis
All statistical analyses were performed using SPSS (version 28.0, IBM) and GraphPad Prism (version 9.2.0). All continuous data were checked for normality using the D’Agostino-Pearson omnibus normality test. Numerical variables are presented as the mean ± SD. Differences of continuous data between the groups were performed using the independent samples t-test or Wilcoxon signed rank-test as appropriate. Categorical data are presented as absolute numbers (percentage) and were compared using χ2 test or Fischer’s exact test as appropriate. Multivariate linear regression analyses were conducted to identify the independent associations of clinical and CMR-derived parameters with strain. p < 0.05 was regarded as statistically significant.
Results
Clinical characteristics
A total of 50 consecutive RH patients (63 ± 12 years, 32 men) and 18 normotensive controls (57 ± 8 years, 12 men) were eventually enrolled. A flowchart of the study is presented in Figure S1. Cardiovascular risk factors and antihypertensive medication of RH patients are detailed in Table 1. There were no statistical differences in gender distribution (p = 0.839) and age (p = 0.055) between RH patients and normotensive controls. RH patients had higher BSA (p = 0.004) and body mass index (BMI) (p < 0.001) than controls. Office systolic blood pressure (SBP) (166 ± 21 mmHg) and diastolic blood pressure (DBP) (91 ± 17 mmHg) were elevated in RH patients despite the intake of 5 ± 1 antihypertensive drugs. The mean of 24-h ABPM, SBP, and DBP of the patient group were 149 ± 18 mmHg and 84 ± 16 mmHg, respectively (Table 2).
CMR findings
CMR findings of the study subjects are summarized in Table 2. RH patients showed similar LVEF (62 ± 9% vs. 64 ± 7%, p = 0.276) and LV/LA volumes, but markedly increased LV mass index (78 ± 15 g/m2 vs. 61 ± 9 g/m2, p < 0.001) compared to controls. In the patient group, 28 (56%) had LVH. No differences were observed between the groups regarding RV function and volumes. Feature-tracking analyses showed attenuated LV GLS (− 16 ± 3% vs. − 19 ± 2%, p = 0.001) and GRS (41 ± 12% vs. 48 ± 8%, p = 0.037) in RH patients. LV GCS had a downward tendency (− 17 ± 4% vs. − 19 ± 4%, p = 0.078) (Table 2 and Fig. 1).
Demographics, blood pressure, and CMR findings of LGE + and LGE − RH patients.
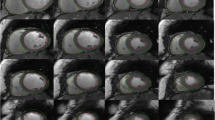
In 21 out of 50 (42%) RH patients, a focal myocardial fibrosis (LGE +) of the LV was detected. A total of 7 ischemic and 14 non-ischemic LGE patterns were visualized as shown in Fig. 2a. A schematic overview is given in Fig. 2b depicting the segmental distribution of focal myocardial fibrosis in LGE + RH patients. LGE areas were predominantly localized in the LV basal inferior and inferolateral segments, whereas midventricular anteroseptal and apical septal segments showed no focal myocardial fibrosis.
a LGE images depicting the focal myocardial fibrosis in LGE + RH patients. Short- and long-axis LGE images depicting an ischemic (red arrowheads) and non-ischemic (white arrowheads) pattern in 21 (42%) LGE + RH patients. b Schematic representation of fibrosis localization in LGE + RH patients. LGE areas were predominantly localized in the LV basal inferior (segment 4) and inferolateral (segment 5) segments, whereas midventricular anteroseptal (segment 8) and apical septal (segment 14) segments showed no focal myocardial fibrosis. LGE, late gadolinium enhancement; LV, left ventricular; RH, resistant hypertension
LGE − and LGE + RH patients had significantly higher BMI and office SBP than controls (all p < 0.001), increased LV mass index was found in both LGE − (p = 0.004) and LGE + (p < 0.001) RH patients. The two RH subgroups and controls had similar cardiac functional and anatomical parameters. Compared to normotensive controls, LV GLS was decreased in LGE + (− 15 ± 3% vs. − 19 ± 2%, p < 0.001) and LGE − RH patients (− 16 ± 3% vs. − 19 ± 2%, p = 0.015), GRS was decreased in LGE + RH (37 ± 12% vs. 48 ± 8%, p = 0.002), but not in LGE − RH patients (44 ± 12% vs. 48 ± 8%, p = 0.269) (Table 2). There were no statistical differences in LV GCS between controls and LGE − (p = 0.101) and LGE + (p = 0.127) RH patients, but a trend for attenuation.
There was a greater proportion of male patients in the LGE + RH group (p = 0.007) (Table 2). LGE + RH patients had higher BSA (2.16 ± 0.15 m2 vs. 2.01 ± 0.23 m2, p = 0.017) than LGE − RH patients. Age (p = 0.727) and BMI (p = 0.842) were similar. Office SBP (160 ± 18 mmHg vs. 164 ± 39 mmHg, p = 0.716) and DBP (91 ± 16 mmHg vs. 88 ± 25 mmHg, p = 0.629), ABPM SBP (148 ± 20 mmHg vs. 150 ± 18 mmHg, p = 0.857) and DBP (84 ± 16 mmHg vs. 84 ± 16 mmHg, p = 0.935) were similar. CMR revealed a higher LV mass index in LGE + RH patients with 85 ± 14 g/m2 than in LGE − RH patients with 73 ± 15 g/m2 (p = 0.007). Patients with LVH had a similar distribution between the LGE − and LGE + RH subgroups. No significant differences regarding cardiac function and volumes were observed (Table 2). Feature-tracking analyses showed that LGE + RH patients had attenuated LV GRS (37 ± 12% vs. 44 ± 12%, p = 0.048) compared to LGE − RH patients, whereas there were no differences in LV GLS (− 15 ± 3% vs. − 16 ± 3%, p = 0.146) and GCS (− 17 ± 5% vs. − 17 ± 4%, p = 0.961) (Fig. 1).
Associations of clinical and CMR-derived parameters with strain
Univariate regression analyses showed that LV stroke volume index (LVSVi) and LV mass index in RH patients were associated with LV GLS (R = − 0.443, p = 0.001 and R = 0.466, p < 0.001, respectively), GRS (R = 0.420, p = 0.003 and R = − 0.392, p = 0.005, respectively), and GCS (R = − 0.307, p = 0.03 and R = 0.289, p = 0.041, respectively) (Fig. 3). After adjustment for age, gender, BMI, and BP, multivariate regression analyses demonstrated that LV end-systolic volume index (LVESVi), LVSVi, and LV mass index were independently associated with LV GLS (β = 0.301, p = 0.002; β = − 0.689, p < 0.001 and β = 0.558, p < 0.001, respectively; model R2 = 0.713) and GRS (β = − 0.447; β = 0.616 and β = − 0.379, respectively, all p < 0.001; model R2 = 0.685). LVESVi and LVSVi were independently associated with LV GCS (β = 0.711, p < 0.001; β = − 0.413, p < 0.001, respectively; model R2 = 0.588) (Table 3). Multivariate regression analyses in normotensive controls showed that considering the covariates of age, gender, BMI, and BP, LVSVi was independently associated with LV GLS (β = − 0.521, p = 0.027, model R2 = 0.271), LVESVi was independently associated with LV GRS (β = − 0.675, p = 0.002, model R2 = 0.456), and LVESVi and LVSVi were independently associated with LV GCS (β = 0.722 and β = − 0.615, all p < 0.001; model R2 = 0.746) (Table 4).
The associations of LV mass index (a) and LVSVi (b) with LV global deformation parameters in RH patients. The gray shade indicates the 95% confidence interval. Increase in LV mass index and decrease in LVSVi were associated with decrease in longitudinal, radial, and circumferential strain. LV, left ventricular; LVSVi, left ventricular stroke volume index; RH, resistant hypertension
Discussion
This study analyzed cardiac morphology and function in RH patients compared to normotensive controls using CMR. The novel method of FT-CMR was used to determine LV global peak systolic strain and LGE imaging was used to investigate the influence of focal myocardial fibrosis on LV myocardial deformation. The main findings are (1) RH patients had significantly higher LV mass index and attenuated LV GLS and GRS in comparison to normotensive controls, whereas GCS was attenuated by trend; (2) 21 RH patients (42%) demonstrated a focal myocardial fibrosis (LGE +), predominantly localized in the basal inferior and inferolateral LV segments; (3) in the subgroup analysis, LGE + RH patients had a markedly reduced LV GLS compared to controls, attenuated GRS compared to LGE − RH patients and controls, and GCS was also attenuated by trend; and (4) LV mass index and stroke volume index were associated with multidirectional strain in RH patients.
Long-standing pressure overload causing strain alterations
In this study, a decrease of LV GLS and GRS was observed in RH patients compared to normotensive controls, GCS did not differ statistically, but showed a decreasing trend. LV strain is sensitive to and influenced by afterload alteration [16], and its altering patterns are determined by the fiber structure of the myocardium and its interaction with local wall stress [17]. Longitudinal strain represents the contraction of the subendocardial fibers, while circumferential shortening reflects the contraction of the subepicardial fibers, and both contribute to radial thickening [8]. LV subendocardial fibers are more vulnerable to increased wall stress, ischemia, and microvascular dysfunction, and thus longitudinal strain is prone to impairment at an early phase of hypertension even before hypertrophy has occurred [18,19,20] and is a sensitive marker for subclinical LV dysfunction [17, 21].
The alterations in radial and circumferential strain are more complex compared to longitudinal strain, especially with the progression of the given disease and the presence of LVH. Imbalzano et al detected reduced longitudinal strain by STE in hypertensive patients both with and without LVH, and those with LVH had reduced radial and increased circumferential strain [18]. Wang et al showed a reduction in all three strain components in patients with systolic heart failure, while patients with diastolic heart failure and preserved LVEF had reduced longitudinal and radial strain, but circumferential strain was preserved [21]. In the current cohort, longitudinal and radial strain was decreased and circumferential strain was preserved in analogy, and more than half (56%) of the patients had LVH due to persistent high-pressure overload. The anatomic differences of myocardial fibers may explain the potential robustness of circumferential strain in terms of clinically significant LV dysfunction [22]. Thus, different stages of hypertensive heart disease seem to be associated with different longitudinal, radial, and circumferential strain response, which may provide a possible explanation for the above discrepancy.
Of note, in the current study, there was a borderline difference of age between controls and RH patients; age-dependency may contribute to the compensation of age-related LV stiffness by radial strain [23]. However, a multivariate regression analysis after adjustment for age showed that RH remained independently associated with LV GLS and GRS, but not with GCS (Table S1).
Taken together, our results support the notion that the attenuation of longitudinal and radial strain in RH patients might constitute a LV adaptation as a response to a long-standing pressure overload. The tendential decrease of circumferential strain might be explained with the subepicardial layer being affected to a lesser degree in this RH patient cohort.
Prevalence of LGE in RH
Recently, an observational study reported that 145 (18%) of 786 patients with essential hypertension had non-ischemic LGE; they were more likely to be men and had greater LV mass and decreased strain [24]. Also, Wang et al detected 29.9% LGE + in their hypertension group [25]. In contrast, our cohort showed a higher prevalence of LGE (42%) with a predominantly non-ischemic pattern, suggesting that RH might be associated with a higher prevalence of LGE than controlled hypertension.
Myocardial fibrosis is a common end point of many cellular and noncellular pathological processes in hypertension; the severity and duration of hypertension might be responsible for the development of cardiac remodeling [26, 27]. In our study, LGE + RH patients had higher LV mass index; increased LV mass in remodeling is due to expanded extracellular interstitium and myocardial cell volume [28]. In the presence of an expanded interstitium, focal replacement fibrosis (non-ischemic LGE) is regarded as a result from the progression of interstitial fibrosis [24]. Increased collagen deposition in the extracellular interstitium induces stiffness and reduction of end-diastolic myofiber length, consequently inducing weakened contraction [29].
Differences in strain between LGE + and LGE − RH patients
Our results showed that reduction in longitudinal strain was observed in both LGE − and LGE + RH patients, compatible with an early decrease of longitudinal systolic function. However, while LGE − RH patients showed similar radial strain compared to controls, a worsening radial strain emerged in LGE + RH patients.
Generally, radial strain has been shown to have large ranges between studies and the variability of segmental strain remains rather high [8]. Nevertheless, radial strain can help to distinguish cardiac sarcoidosis from dilated cardiomyopathy [30], can predict clinical outcome in hypertrophic cardiomyopathy [31], and is more predictive for scar (defined with LGE) transmurality than longitudinal strain [32]. In fact, the underlying mechanisms responsible for worsening radial strain have not been completely defined yet. Radial strain represents the global myocardial function in the radial direction, which is influenced by the deformation of all myocardial layers. Thus, it seems reasonable to assume that once focal myocardial fibrosis visualizable by LGE has occurred it might contribute to the reduction of LV radial strain.
Earlier echocardiographic studies have investigated the effects of myocardial fibrosis on LV deformation through identifying the association of plasma markers of myocardial fibrosis with strain alterations. Kang et al found increasing tissue inhibitor of matrix metalloproteinase (TIMP)-1 in hypertensive patients with normal LVEF correlated with attenuation of longitudinal strain, whereas circumferential and radial strain were not attenuated [19]. Poulsen et al showed that hypertensive patients had decreased longitudinal strain and increased amino-terminal propeptide of procollagen type III, accompanied by an inverse correlation of the two parameters [20]. Plasma markers emerge in an early stage of a myocardial fibrotic process in mild to moderate hypertensive patients and indirectly reflect myocardial fibrosis, and may lack specificity in the case of concomitant fibrotic diseases (e.g., cardiac fibrosis combined with liver or kidney fibrosis) [33]. However, the patients in the current study rather suffered a late fibrotic process due to long-standing arterial hypertension. LGE-CMR is a visual approach to directly display focal myocardial fibrosis [34]. Identifying the strain differences in RH patients with and without focal myocardial fibrosis might provide data on the extent of myocardial layer impairment and offer insights into the influence of a long-standing pressure overload and a myocardial fibrotic process on cardiac deformation.
Limitations
The sample size in our study was small, which may have had an influence on the power to identify differences between study groups. However, all participants were recruited consecutively and prospectively according to the stringent selection criteria; future studies with larger populations are warranted to corroborate the consistency and reproducibility of our preliminary findings. Second, although some risk factors had been adjusted for multivariate regression analyses, several potential confounders, such as the dosages of antihypertensive drugs and sodium intake, may have an additional effect. Age-matching of controls and RH patients was not precise, but a multivariate analysis did not alter the results after adjustment for age. Another limitation is the lack of detailed information about the duration of hypertension. Nevertheless, all recruited patients were classified as RH according to the ESC guidelines and extensive diagnostics had been previously performed at our tertiary university medical center excluding secondary causes of hypertension. It can be assumed that hypertension has been developing over a long period of time during the aging process in the vast majority of the current elder cohort. Further, FT-CMR is performed mainly based on a block-matching algorithm, which requires a careful tuning of the search region and solving for displacements between short-distance regions [7]. Thus, radial strain being calculated over smaller regions between endo- and epicardium is less reliable than longitudinal and circumferential strain [7].
Conclusions
Our study revealed that attenuation of LV global longitudinal and radial strain as well as the tendency of circumferential strain attenuation might be consecutive adaptations responding to long-standing pressure overload in RH patients, and global circumferential strain attenuation only by tendency might be attributable to a still partially preserved subepicardial layer. Further, focal myocardial fibrosis has a high incidence in RH patients, presents primarily with a non-ischemic LGE pattern predominantly localized in the basal inferior and inferolateral LV segments, and is associated with reduced global radial strain. Therefore, FT-CMR-derived myocardial strain offers insights into the influence of long-standing pressure overload and of a myocardial fibrotic process on cardiac deformation in RH.
Abbreviations
- ABPM :
-
Ambulant blood pressure monitoring
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- CMR:
-
Cardiac magnetic resonance
- DBP:
-
Diastolic blood pressure
- EDVi:
-
End-diastolic volume index
- EF:
-
Ejection fraction
- ESVi:
-
End-systolic volume index
- FT-CMR:
-
Feature-tracking cardiac magnetic resonance
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- GRS:
-
Global radial strain
- LA:
-
Left atrial
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- LVH:
-
Left ventricular hypertrophy
- RA:
-
Right atrial
- RH:
-
Resistant hypertension
- RV:
-
Right ventricular
- SBP:
-
Systolic blood pressure
- SSFP:
-
Steady-state free-precession
- STE:
-
Speckle-tracking echocardiography
- SVi:
-
Stroke volume index
References
Roth GA, Mensah GA, Johnson CO et al (2020) Global burden of cardiovascular diseases and risk factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol 76:2982–3021
Williams B, Mancia G, Spiering W et al (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39:3021–3104
Kaplan NM (2005) Resistant hypertension. J Hypertens 23:1441–1444
Daugherty SL, Powers JD, Magid DJ et al (2012) Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 125:1635–1642
Weber KT, Brilla CG, Janicki JS (1993) Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res 27:341–348
Alsharari R, Oxborough D, Lip GYH, Shantsila A (2021) Myocardial strain imaging in resistant hypertension. Curr Hypertens Rep 23:24
Amzulescu MS, De Craene M, Langet H et al (2019) Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging 20:605–619
Claus P, Omar AMS, Pedrizzetti G, Sengupta PP, Nagel E (2015) Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging 8:1444–1460
Tahir E, Koops A, Warncke ML et al (2019) Effect of renal denervation procedure on left ventricular mass, myocardial strain and diastolic function by CMR on a 12-month follow-up. Jpn J Radiol 37:642–650
Mahfoud F, Urban D, Teller D et al (2014) Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J 35:2224–2231b
Schulz-Menger J, Bluemke DA, Bremerich J et al (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update : Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson 22:19
Le TT, Tan RS, De Deyn M et al (2016) Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J Cardiovasc Magn Reson 18:21
Olivotto I, Maron MS, Autore C et al (2008) Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 52:559–566
Morais P, Marchi A, Bogaert JA et al (2017) Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: assessment of variability in a real-life clinical setting. J Cardiovasc Magn Reson 19:24
Tahir E, Starekova J, Muellerleile K et al (2019) Impact of myocardial fibrosis on left ventricular function evaluated by feature-tracking myocardial strain cardiac magnetic resonance in competitive male triathletes with normal ejection fraction. Circ J 83:1553–1562
Donal E, Bergerot C, Thibault H et al (2009) Influence of afterload on left ventricular radial and longitudinal systolic functions: a two-dimensional strain imaging study. Eur J Echocardiogr 10:914–921
Baltabaeva A, Marciniak M, Bijnens B et al (2008) Regional left ventricular deformation and geometry analysis provides insights in myocardial remodelling in mild to moderate hypertension. Eur J Echocardiogr 9:501–508
Imbalzano E, Zito C, Carerj S et al (2011) Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography 28:649–657
Kang SJ, Lim HS, Choi BJ et al (2008) Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr 21:907–911
Poulsen SH, Andersen NH, Heickendorff L, Mogensen CE (2005) Relation between plasma amino-terminal propeptide of procollagen type III and left ventricular longitudinal strain in essential hypertension. Heart 91:624–629
Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF (2008) Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J 29:1283–1289
Mangion K, McComb C, Auger DA, Epstein FH, Berry C (2017) Magnetic resonance imaging of myocardial strain after acute ST-segment-elevation myocardial infarction: a systematic review. Circ Cardiovasc Imaging 10:e006498
Andre F, Steen H, Matheis P et al (2015) Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 17:25
Iyer NR, Le TT, Kui MSL et al (2022) Markers of focal and diffuse nonischemic myocardial fibrosis are associated with adverse cardiac remodeling and prognosis in patients with hypertension: the REMODEL study. Hypertension 79:1804–1813
Wang S, Hu H, Lu M et al (2017) Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in hypertension and associated with left ventricular remodeling. Eur Radiol 27:4620–4630
Hill JA, Olson EN (2008) Cardiac plasticity. N Engl J Med 358:1370–1380
Kuruvilla S, Janardhanan R, Antkowiak P et al (2015) Increased extracellular volume and altered mechanics are associated with LVH in hypertensive heart disease, not hypertension alone. JACC Cardiovasc Imaging 8:172–180
Rodrigues JC, Amadu AM, Ghosh Dastidar A et al (2017) ECG strain pattern in hypertension is associated with myocardial cellular expansion and diffuse interstitial fibrosis: a multi-parametric cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging 18:441–450
Pichler G, Redon J, Martinez F et al (2020) Cardiac magnetic resonance-derived fibrosis, strain and molecular biomarkers of fibrosis in hypertensive heart disease. J Hypertens 38:2036–2042
Tsuji T, Tanaka H, Matsumoto K et al (2013) Capability of three-dimensional speckle tracking radial strain for identification of patients with cardiac sarcoidosis. Int J Cardiovasc Imaging 29:317–324
Smith BM, Dorfman AL, Yu S et al (2014) Relation of strain by feature tracking and clinical outcome in children, adolescents, and young adults with hypertrophic cardiomyopathy. Am J Cardiol 114:1275–1280
Maret E, Todt T, Brudin L et al (2009) Functional measurements based on feature tracking of cine magnetic resonance images identify left ventricular segments with myocardial scar. Cardiovasc Ultrasound 7:53
Rubis P, Dziewiecka E, Szymanska M et al (2021) Lack of relationship between fibrosis-related biomarkers and cardiac magnetic resonance-assessed replacement and interstitial fibrosis in dilated cardiomyopathy. Cells 10:1295
Karamitsos TD, Arvanitaki A, Karvounis H, Neubauer S, Ferreira VM (2020) Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc Imaging 13:1221–1234
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Enver Tahir, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
All participants gave their written informed consent before being included in this study.
Ethical approval
The ethics committee of the general medical council approved the study.
Previous reports of the study cohort
The study population included 16 RH patients, who were included in a previously publication. The initial publication reported on the effects of a renal denervation procedure in RH patients. In brief, changes in LV mass, myocardial strain, and diastolic function were assessed before and on a 12-month follow-up after renal denervation.
Tahir E, Koops A, Warncke ML et al (2019) Effect of renal denervation procedure on left ventricular mass, myocardial strain and diastolic function by CMR on a 12-month follow-up. Jpn J Radiol 37:642-650.
Methodology
• prospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, H., Brunner, F.J., Özden, C. et al. Left ventricular myocardial strain responding to chronic pressure overload in patients with resistant hypertension evaluated by feature-tracking CMR. Eur Radiol 33, 6278–6289 (2023). https://doi.org/10.1007/s00330-023-09595-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09595-z