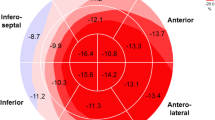
Abstract
Since cardiac sarcoidosis (CS) leads to substantial morbidity and sudden death, early diagnosis and appropriate management are crucial for patients with CS. Echocardiography used to be considered a useful diagnostic tool for patients with CS, but CS may clinically present as dilated cardiomyopathy (DCM). Our objective was to investigate whether a novel three-dimensional (3-D) speckle-tracking strain can identify patients with CS more accurately. We studied 23 CS patients with an ejection fraction (EF) of 46 ± 10 %, and 16 EF-matched patients with DCM (EF 45 ± 11 %). Global radial (GRS), circumferential (GCS) and longitudinal (GLS) strain was assessed using 3-D speckle-tracking system. GRS of patients with CS was significantly lower than that of patients with DCM (18.5 ± 8.4 vs. 28.5 ± 8.3 %, p < 0.01), but GCS and GLS in patients with CS and DCM were similar. GRS ≦ 21.1 could differentiate CS from DCM with a sensitivity of 70 %, specificity of 88 % and area under the curve of 0.79. An additional noteworthy findings was that, patients with CS showed more negative radial strain curves than did those with DCM (1.7 ± 2.3 vs. 0.1 ± 0.5, p < 0.01). In conclusion, 3-D speckle-tracking radial strain shows good potential to distinguish CS from DCM. Our observations can thus be expected to have clinical implications for management of CS patients.





Similar content being viewed by others
References
Newman LS, Rose CS, Maier LA (1997) Sarcoidosis. N Engl J Med 336(17):1224–1234
Deng JC, Baughman RP, Lynch JP 3rd (2002) Cardiac involvement in sarcoidosis. Semin Respir Crit Care Med 23(6):513–527
Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K (2005) Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol 95(1):143–146
Luk A, Metawee M, Ahn E, Gustafsson F, Ross H, Butany J (2009) Do clinical diagnoses correlate with pathological diagnoses in cardiac transplant patients? The importance of endomyocardial biopsy. Can J Cardiol 25(2):e48–e54
Nesser HJ, Mor-Avi V, Gorissen W, Weinert L, Steringer-Mascherbauer R, Niel J, Sugeng L, Lang RM (2009) Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: comparison with MRI. Eur Heart J 30(13):1565–1573
Tanaka H, Hara H, Adelstein EC, Schwartzman D, Saba S, Gorcsan J 3rd (2010) Comparative mechanical activation mapping of RV pacing to LBBB by 2D and 3D speckle tracking and association with response to resynchronization therapy. JACC Cardiovasc Imaging 3(5):461–471
Tanaka H, Hara H, Saba S, Gorcsan J 3rd (2010) Usefulness of three-dimensional speckle tracking strain to quantify dyssynchrony and the site of latest mechanical activation. Am J Cardiol 105(2):235–242
Thebault C, Donal E, Bernard A, Moreau O, Schnell F, Mabo P, Leclercq C (2011) Real-time three-dimensional speckle tracking echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Eur J Echocardiogr 12(1):26–32
Soejima K, Yada H (2009) The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol 20(5):578–583
Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H (1999) Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J 138(2 Pt 1):299–302
Fahy GJ, Marwick T, McCreery CJ, Quigley PJ, Maurer BJ (1996) Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 109(1):62–66
Uemura A, Morimoto S, Kato Y, Hiramitsu S, Ohtsuki M, Kato S, Sugiura A, Miyagishima K, Iwase M, Hishida H (2005) Relationship between basal thinning of the interventricular septum and atrioventricular block in patients with cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 22(1):63–65
Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ (2009) Detection of myocardial damage in patients with sarcoidosis. Circulation 120(20):1969–1977
Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Cheriex EC, Gorgels AP, Crijns HJ (2005) The additional value of gadolinium-enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis. Chest 128(3):1629–1637
Tadamura E, Yamamuro M, Kubo S, Kanao S, Saga T, Harada M, Ohba M, Hosokawa R, Kimura T, Kita T, Togashi K (2005) Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol 185(1):110–115
Patel AR, Klein MR, Chandra S, Spencer KT, Decara JM, Lang RM, Burke MC, Garrity ER, Hogarth DK, Archer SL, Sweiss NJ, Beshai JF (2011) Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail 13(11):1231–1237
Seo Y, Ishizu T, Enomoto Y, Sugimori H, Yamamoto M, Machino T, Kawamura R, Aonuma K (2009) Validation of 3-dimensional speckle tracking imaging to quantify regional myocardial deformation. Circ Cardiovasc Imaging 2(6):451–459
Cheong BY, Muthupillai R, Nemeth M, Lambert B, Dees D, Huber S, Castriotta R, Flamm SD (2009) The utility of delayed-enhancement magnetic resonance imaging for identifying nonischemic myocardial fibrosis in asymptomatic patients with biopsy-proven systemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 26(1):39–46
Kim JS, Judson MA, Donnino R, Gold M, Cooper LT Jr, Prystowsky EN, Prystowsky S (2009) Cardiac sarcoidosis. Am Heart J 157(1):9–21
Remme WJ, Swedberg K (2002) Comprehensive guidelines for the diagnosis and treatment of chronic heart failure. Task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur J Heart Fail 4(1):11–22
Ardehali H, Kasper EK, Baughman KL (2005) Diagnostic approach to the patient with cardiomyopathy: whom to biopsy. Am Heart J 149(1):7–12
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuji, T., Tanaka, H., Matsumoto, K. et al. Capability of three-dimensional speckle tracking radial strain for identification of patients with cardiac sarcoidosis. Int J Cardiovasc Imaging 29, 317–324 (2013). https://doi.org/10.1007/s10554-012-0104-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0104-7