Abstract
Marine Porifera (sponges) are known to produce several bioactive metabolites having a biotechnological potential, mostly derived from their bacterial symbionts; however, current knowledge on the production of metabolites such as enzymes and antibacterial molecules in sponges living in Antarctic environments is not fully exhaustive and needs further deepened investigation. The interest in discovering the broad spectrum of natural products potentially derived from species adapted to colonize extreme environments stimulates the research toward Antarctic sponge bioprospection. In this study, whole homogenates of Antarctic Demospongiae, belonging to five different species [Haliclona (Rhizoniera) sp., Haliclona (Rhizoniera) dancoi, Microxina sarai, Dendrilla antarctica, and Mycale acerata] were collected from Terra Nova Bay (Ross Sea) and examined for presence and activity of enzymes, including lysozyme, and antibacterial substances. Enzyme activities (leucine aminopeptidase, beta-glucosidase, and alkaline phosphatase) were measured using fluorogenic substrates; lysozyme content was determined on plates containing lyophilized Micrococcus lysodeikticus cell walls as a substrate. Homogenates were screened in microtiter plates for their antibacterial activity against Antarctic bacterial isolates, and the absorbance reduction was measured with a microplate reader. All homogenates exhibited proteolytic, glycolytic, and phosphatasic activities, lysozyme and antibacterial activities at near “in situ” temperature (5 °C), with some differences among the examined species. Results confirmed that Antarctic sponge homogenates are interesting sources of different bioactive substances, likely produced from associated bacterial symbionts, and that could have great potential to be used in medicine or industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within marine Antarctic benthic communities, Porifera represent one of the most important phyla in terms of spatial coverage, biomass, and species richness (Rios and Cristobo 2014). Sponges have a typical filter-feeding behavior, through which they are able to entrap and concentrate different microorganisms including prokaryotes and eukaryotes, which represent their trophic resources (Steinert et al. 2019). By ingestion, sponges can accumulate in their tissue contaminants, including bacterial pathogens; indeed, the production of secondary metabolites, such as enzymes or molecules with antimicrobial properties, represents a common defense mechanism in benthic invertebrates (Krug 2006).
Despite of the widespread distribution of Porifera in the Antarctic region, studies on these organisms in polar regions are still quite limited (Webster et al. 2004) and therefore their biotechnological potentialities have not been fully investigated (Lo Giudice and Rizzo 2018; Cárdenas et al. 2019; Savoca et al. 2019; Steinert et al. 2019; Papale et al. 2020). Antarctic Porifera, and more in general marine benthic invertebrates, represent a still inexhaustible source of natural compounds for biodiscovery; this topic has been recently reviewed (Avila and Angulo-Preckler 2021). Evaluating the enzymatic and antimicrobial activities of sponges is a critical step to discover the biotechnological potential of these organisms. Among marine invertebrates, sponges are largely studied in relation to the complex microbial communities that colonize their outer surfaces, ostia and oscula, as well as for the production of a wide range of molecules, including enzymes and secondary metabolites (Angulo-Precker et al. 2018; Parte et al. 2018; Avila and Angulo-Preckler 2021). Marine sponges host a large richness of associated microbial communities, acting as “hot-spots” of microbial diversity, and most sponge symbionts are taxonomically different from microbes inhabiting the surrounding waters (Taylor et al. 2013). Symbiotic microorganisms, which adhere to the sponge surface forming 3D-structure biofilms, are consortia of microbial species included into a mesohyl matrix of proteinaceous nature (Webster et al. 2001).
Many enzymes including hydrolases (such as proteases, lipases, a-amylase, cellulase, chitinase, glucosidase, invertase, pectinase, and xylanase) and oxidoreductases (laccase and superoxide dismutase) of microbial origin are usually found in sponges (Wang 2006; Andriyono et al. 2015; Parte et al. 2018); they can be produced by fungi (Poveda et al. 2018), yeasts (Vaca et al. 2013) or bacteria (Moreno-Pino et al. 2020; Ruocco et al. 2021) associated with sponges. Hydrolytic enzymes (such as alpha-amylase and alkaline phosphatase) were found in Gammaproteobacteria associated with the marine sponges Dysidea granulosa and Sigmadocia fibulata, as well as in the actinomycete Streptomyces sp. isolated from the sponge Ircinia sp. (Feby and Nair 2010; Krishnakumar et al. 2015). In Haliclona sp., amylase, protease and cellulase enzymes produced by bacterial isolates, and especially by Bacillus sp., were detected (Andriyono et al. 2015). Many of these molecules (proteases, alpha-amylases and carboxymethylcellulase, alkaline phosphatases, ureases, and acethylcolinesterases) have attracted interest for their wide applications in food and pharmaceutical production, wastewater treatment, leather and textile processing (Mohapatra et al. 2003; Wang 2006). In several Antarctic ecosystems, cold-active enzymes are also produced by free-living microorganisms and represent an important mechanism for microbial survival strategy (Cavicchioli et al. 2002; Marx et al. 2007; Duarte et al. 2018).
Sponge microbial symbionts are recognized to be a source of complex metabolites (Bibi et al. 2017; Brinkmann et al. 2017), some of which having a defense role. Chemical defense mechanisms were first detected in several marine Antarctic organisms belonging to Porifera, Cnidaria, Tunicata, Mollusca, and Echinodermata (McClintock and Baker 1997; Taboada Moreno 2012; Mehbub et al. 2014). In Antarctic sponges, molecules with toxic properties were produced by the species Cinachyra sp., Latrunculia sp., and Polymastia sp. (Battershill 1990). Defense molecules play a functional role in the response to predation and competition, acting also as antifoulants. Extracts of Antarctic sponges were found to be able to cause mortality in goldfish or inhibit the growth of allopatric microorganisms (McClintock and Gauthier 1992). Baker et al. (1993, 1994, 1995) reported the presence of diterpene metabolites such as dendrillin, picolinic acid, 9,11-dihydrogracilin A, and membranolide in the tissue of Dendrilla membranosa; in Latrunculia apicalis cytotoxic pigments such as discorhabdin were also isolated.
Porifera, which are the oldest metazoan phylum, possess an active innate immune system that consists of molecules structurally similar to those involved in the immune system in mammals (Müller et al. 1999). As a component of the first line of defense against bacteria or other foreign components, lysozyme is a naturally occurring bacteriolytic enzyme involved in the non-specific immune responses of both invertebrates and vertebrate organisms; it has a ubiquitous distribution among living organisms being present in mucus, lymphoid tissue, plasma and other body fluids and tissues (Van Herreweghe and Michiels 2012; Ferraboschi et al. 2021). The search for new molecules with antimicrobial activity against bacteria and pathogenic fungi is an important scientific challenge that assumes an added value in the light of the recently increased antibiotic resistance reports (WHO 2015; Vestergaard et al. 2019). Due to its ability to kill not only Gram-positive but also Gram-negative bacteria, lysozyme has been suggested as an alternative antibiotic (Ferraboschi et al. 2021).
A wide range of active substances, including molecules with antibiotic properties belonging to alkaloids, terpenoids, peptides, lectins, fatty acids and glycolipids, macrolides, have been reported from marine sponges (Laport et al. 2009). Reports documenting the production of antibacterial substances in Antarctic sponges are available (Taboada Moreno 2012; Angulo-Precker et al. 2018; and references cited herein). Antarctic bacteria also possess defensive chemistry mechanisms: the culture media of a strain of Pseudomonas aeruginosa isolated from the Antarctic sponge Isodictya setifera (Jayatilake et al. 1996) showed strong antibacterial activity and contained antibacterial active metabolites identified as a new diketopiperazine, cyclo-(L-proline-L-methionine) together with two phenazine alkaloids. From the southern Australian sponge Negombata sp. and an Antarctic Latrunculia sp. Ford and Capon (2000) isolated discorhabdin R, an antibacterial pyrroloiminoquinone alkaloid responsible for the antibacterial activity against Gram-positive bacteria (Staphylococcus aureus and Micrococcus luteus) and Gram-negative bacteria (Escherichia coli and Serratia marcescens). Potential antagonistic activity against fouling bacteria was observed in sponge-associated bacteria, suggesting epibiotic defense of host sponge (Thakur et al. 2004; Kanagasabhapathy et al. 2005; Duarte et al. 2018).
Within the research program “Antarctic Porifera: hot-spots for Prokaryotic diversity and biotechnological Potentialities—P3” funded by the Italian Research Program in Antarctica (PNRA16_00020) particular attention was given to the characterization of active secondary molecules present in sponges inhabiting the Tethys Bay (Terra Nova Bay, Ross Sea), to extend current knowledge on the biological properties of sponges. In this study, we refer about the first characterization of enzymatic, including lysozyme, and antibacterial activities of whole crude homogenates of Antarctic Demospongiae. To explore the biotechnological potentialities and ecological role of such organisms, different features were considered: enzymatic activity measurements were used to investigate the role of sponges as fouling organisms, while sponge lysozyme and antibacterial assays aimed at providing information on the chemical defense against sympatric bacteria and potential competitors.
Materials and methods
Collection of the sponge specimens
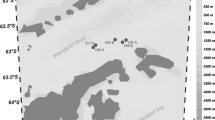
During the XXXIII and XXXIV Italian expeditions in Antarctica (Nov-2018 and Nov-2019, respectively) sponge specimens belonging to the Demospongiae (Porifera) species Haliclona (Rhizoniera) sp., H. dancoi, Microxina sarai, Mycale acerata, and D. antarctica were collected from the Tethys Bay (Terra Nova Bay, Antarctica) (Fig. 1). Sponges were collected by SCUBA at a depth ranging between 10 and 18 m. Sponge fragments were deposited at the Italian National Antarctic Museum (MNA, Section of Genoa, Italy). Sponge identification was performed as reported by Papale et al. (2020). The list of collected samples is reported in Table 1.
Terra Nova Bay (Antarctica). Map of the sampling sites, with indication of the retrieved sponge specimens. In particular all the sponges sampled during the XXXIII Antarctic campaign are reported in the left part of the figure, and were collected from the same sampling point, whereas the sponges collected during the XXXIV Antarctic campaign (marked with the acronym Sp) were retrieved from different sites
At the Mario Zucchelli Italian Station sampling activities were carried out with the necessary care to minimize human disturbance to the Tethys Bay area, and limit sponge collection to the minimum necessary to meet the scientific needs. Sponge collection was authorized by the PNRA project, conformably to the Antarctic Treaty legislation and the SCAR Code of Conduct for the Use of Animals for Scientific Purposes in Antarctica (SCAR 2011). The collected specimens were drained from seawater to avoid that the retained water could have altered the biochemical measurements and were frozen as whole organisms at − 20 °C for further analyses, which were performed upon their arrival in Italy. At the laboratory, the samples were thawed before their treatment; the organisms were dissected into the endoderm and ectoderm, then portions of endoderm (2 g) were taken with a sterile scalpel, diluted in sterile pre-filtered (0.22 µm pore size of the filters) seawater, in a volume of liquid 50 times the amount of sample, and homogenized in a Potter Elvehjem tissue grinder. After sedimentation, the obtained supernatant, representing the enzyme extract, was collected and treated differently according to the parameters (enzymes, lysozyme content, antibacterial activities) to be measured. The analyses were performed separately for each sample replicate. During the sample processing, samples were treated on ice keeping the temperature at around 4 °C to avoid sample degradation by temperature.
Enzymatic activities
For the determination of enzymatic activities on organic polymers (proteins, polysaccharides, organic phosphates) three replicates of each sponge homogenate were used in the analytical protocol based on fluorogenic substrates specific for the determination of proteolytic (leucine aminopeptidase), glycolytic (beta-glucosidase), and phosphatasic (alkaline phosphatase) activities, described in detail by Caruso (2010); this method involves the addition of increasing volumes of substrates (10, 50, 100 µL) to a 5 mL volume of the enzyme extract, according to a multiconcentration analytical procedure. Stock solutions in methyl cellosolve (5 mM concentration) of l-leucine-7-amido-4-methylcoumarin (Leu-MCA), 4-methylumbelliferyl-beta-d-glucopyranoside (MUF-beta-d-glu), and 4-methylumbelliferyl phosphate (MUF-phosphate) in methyl cellosolve (Merck, Milan, Italy) were used for the measurement of the activity rates of the enzymes leucine aminopeptidase, beta-glucosidase, and alkaline phosphatase, respectively. Methylcoumarine (MCA) and methylumbelliferone (MUF) at known concentrations (50, 100, 200, 400 nmol L−1 h−1) were used as the standards for Leu-MCA and for MUF-beta-d-glu and MUF-phosphate measurements, respectively. One negative control (blank) made of physiological saline without addition of the sponge homogenate was also included in the assay. Substrate concentrations were plotted versus the increase of fluorescence recorded between time 0 (immediately after the addition of the substrate) and after 2 h of incubation at + 4 °C; fluorescence measurements were performed at 380/440 nm and 365/455 nm excitation/emission wavelengths for LAP and for B-GLU/AP, respectively. The enzyme activity rate was obtained from the slope of the regression curve and expressed in mmol h−1 g−1, taking into account the initial dilution of the sponge sample.
Determination of lysozyme content
Lysozyme (muramidase, EC 3.2.1.17) is an enzyme able to hydrolyze the β-(1–4)-glucosidic linkage between N-acetyl-muraminic acid and N-acetyl-d-glucosamine residues present in the mucopolysaccharide cell wall of bacteria; the enzymatic assay relies on the measurement of the hydrolysis of the cell walls of M. lysodeikticus. The lysozyme activity was measured by the lysoplate method based on the radial diffusion method in 1% agarose plates containing 0.05% lyophilised M. lysodeikticus (Merck) as the substrate, dissolved in a 0.067 M, 0.1% NaCl, pH 6.3 phosphate buffer solution (Ossermann and Lawlor 1966). Briefly, wells were produced with a gel puncher (diameter 6 mm) in the agarose plate and 15 µL of each homogenate in triplicate were distributed into individual wells. One control well with 15 µL of physiological saline was also included. Plates were incubated at 5 °C for 48 h. Lysozyme activity was shown by the presence of lytic halos around the well. Halo diameter was proportional to the lysozyme content, and it was converted into Units of lysozyme per g of enzyme extract (U g−1 homogenate) using a standard of hen egg-white lysozyme (Merck). Known concentrations of egg-white lysozyme (0.001, 0.01, 0.1, and 1 mg) were used to create a standard calibration curve in which increasing concentrations of the lysozyme were plotted versus the diameter of lysis; the lysis diameter was converted into the U g−1 homogenate using the obtained regression curve.
Assay of antibacterial activity
Homogenate samples were tested to evaluate the possible presence of antibacterial activity against four bacterial strains (strains 2, 94, 101, and 238) previously isolated from Antarctic biofilms during the research project PNRA16_00105 (ANT-Biofilm) and identified by a biochemical miniaturized system (API 20NE strips, Biomerieux, Marcy l’Etoile, France) as Burkholderia cepacia (Caruso G., personal communication). The choice of these microorganisms was based on their frequent recovery within the biofilm bacterial flora covering the surface of some artificial structures anchored at the bottom; therefore, our assay was thought to ascertain whether bacterial biofilm isolates were also able to inhibit other bacterial strains associated with sponges, which shared with the biofilm strains a common autochthonous origin being both benthic microorganisms. Fresh cultures of each bacterial strain were prepared spreading a single inoculum onto Marine agar plates incubated at 5 °C for 5 days; single colonies were incubated overnight in sterile physiological solution (0.85% NaCl) and serial dilutions were performed to obtain per each strain a bacterial suspension of 108 cells mL−1 (standard inoculum). This final concentration was checked via Optical Density (Absorbance) measurement at 600 nm. The assay was conducted in three replicates in 96 wells-microtiter plates, using the protocol of antibacterial assay reported by Audoin et al. (2013). Wells were seeded with increasing volumes (20, 60, 80, and 100 µL) of sponge homogenates, to which a constant volume (100 µL) of bacterial inoculum was added. To adjust the final volume to 200 µL per well, the needed amount of sterile physiological saline was added. One control, consisting of 100 µL of the bacterial inoculum added with an equal volume of sterile physiological solution, was also included to each assay. Absorbance was spectrophotometrically measured at 600 nm at the start (Time zero, T0) and after 24 h of incubation (T1) of the plates at 5 °C.
Bacterial growth inhibition was calculated, per each sample volume, according to the following equation:
where Abssample was the difference T0−T1 recorded in the Absorbance of the sample and Abscontrol the difference T0−T1 recorded in the Absorbance of the control. The highest inhibition value shown by the different volumes of sponge homogenate was recorded as the percentage of inhibition.
Statistical analysis of the data
The results obtained per each examined parameter were expressed as mean values ± Standard Deviation per each experimental group. A normality test of the whole dataset was performed using the PAST version 4.0 software (Hammer et al. 2001) to verify whether data satisfied the assumption of normal (Gaussian) distribution prior to examine the occurrence of statistical differences among the sponge species by analysis of variance (ANOVA). A Kruskall-Wallis analysis (a non-parametric one-way ANOVA on ranks) was applied to those variables that were not normally distributed; a difference was considered significant at a p < 0.05 probability level.
The normal distribution of the antibacterial activities was verified by using the Shapiro–Wilk test and homoscedasticity was evaluated by using the Levene test. When both assumptions were verified then one-way ANOVA was applied to explore the occurrence of statistical differences between the inhibitory activities of sponge homogenates. When ANOVA outputs were significant, Tukey’s pairwise comparisons were applied as a post-hoc analysis to find those groups that were significantly different from each other.
To search for possible links between enzymatic (including lysozyme) and antibacterial activities in the examined sponge species, Pearson’s correlation coefficients and heatmaps were computed, using the PAST software.
Results
Enzymatic activities
The enzymatic activities measured in the sponge homogenates (Fig. 2) depicted a decreasing pattern in the order LAP > AP > B-GLU. LAP ranged from 0.023 to 28.36 mmol h−1 g−1, with a mean value of 9.56 ± 7.39 mmol h−1 g−1, B-GLU values were comprised between 0.035 and 6.66 mmol h−1 g−1, while its mean value was 0.87 ± 1.46 mmol h−1 g−1. AP ranged from 0.091 to 34.15 mmol h−1 g−1, with a mean value of 5.70 ± 7.69 mmol h−1 g−1. Haliclona (Rhizoniera) sp. exhibited the highest values of LAP; D. antarctica showed the maximum values of B-GLU and AP. Minimum hydrolytic activities were measured in M. acerata for all the enzymes.
Mean ± standard deviation (n = 3 replicates) of enzyme activity rates (in mmol−1 h−1 g−1) measured in the sponge homogenates. LAP leucine aminopeptidase (proteolytic activity), B-GLU Beta Glucosidase (glycolytic activity), AP alkaline phosphatase (phosphatasic activity). Different letters indicate significant differences among the values by ANOVA at a 5% p level
Normality test showed that LAP and AP distributions complied with the normality assumption, while B-GLU did not (Shapiro Wilk W24 = 0.747, p = 0.0281, data not shown in Table). ANOVA revealed significant (p < 0.01) differences in LAP and AP activity rates between M. acerata and all the other sponges, which did not differ significantly from one another, as well as differences between Haliclona sp. and D. antarctica for B-GLU activity rates.
Lysozyme content
Lysozyme values ranged from 0.187 to 1.06 U g−1 sponge homogenate (Fig. 3), with similarly lowest values recorded in M. acerata and H. dancoi and highest ones in Haliclona sp. and D. antarctica. The maximum lytic activity was displayed by the homogenate of M. sarai. Lysozyme values were normally distributed (Shapiro–Wilk W24 = 0.933, p = 0.6168, data not shown). ANOVA confirmed significant (p < 0.01) differences for M. sarai lysozyme compared to M. acerata and H. dancoi.
Antibacterial activity assay
The results of the antibacterial assay are shown in Table 2, where the percentage of inhibition exhibited by each sponge homogenate against the reference bacterial isolates is reported.
The inhibitory activity exhibited by sponge homogenates against the four bacterial tests (as percentage of inhibition) ranged from 26.35 ± 28.90% (D. antarctica against B. cepacia strain 2) to 77.75 ± 8.00% (M. acerata against B. cepacia strain 2). Globally, the highest values of inhibitory activity have been obtained by the homogenates of Haliclona (Rhizoniera) sp. and M. acerata against all the tested strains. H. dancoi and M. sarai homogenates resulted less effective, with a weak antibacterial activity (lower than 10%) exhibited against B. cepacia strain 101 (8.50 ± 0.00%) and B. cepacia strain 94 (1.13 ± 0.00%), respectively (Table 2, Fig. 4).
The Shapiro–Wilk and the Levene tests confirmed a normal distribution of the data, as shown by Shapiro–Wilk W24 values ranging from 0.799 to 0.875, p from 0.08053 to 0.2873, data not shown in Table). Significative differences have been evidenced by one-way ANOVA between inhibitory activity of H. dancoi and M. sarai and the other sponge homogenates, resulting in a significantly lower activity (p < 0.05).
Relationships among the assayed parameters
The relationship linking enzymatic (including lysozyme) and antibacterial activities in each sponge species was evaluated by a heatmap that allows to visualize potential associations among the assayed parameters; the statistical significance of the relationships was assessed by Pearson correlation analysis (Fig. 5). High enzymatic activity levels and high antibacterial effects of the sponge homogenate were observed in Haliclona sp. while in M. acerata these trends were uncoupled. No Pearson’s correlations were found between the enzymatic activity rates and the lysozyme content, as well as between the lysozyme content and the antibacterial activities, which rather appeared slightly inversely correlated (Pearson correlation r = − 0.25, n = 25, p = 0.6780). A possible reason to explain the lack of association between enzymatic activity rates and lysozyme relies on the different functional role played by these parameters (one mostly related to organic matter decomposition for LAP, B-GLU, and AP, whereas to host defense for lysozyme). Moreover, not all the enzymes have antibacterial properties, either antibacterial activities are not all mediated by lysozyme, but might involve other defense mechanisms/molecules, resulting in the low or inverse relationship between antibacterial activities and the levels of lysozyme.
Outputs of statistical analysis. Heatmap (on the left) and Pearson correlation analysis (on the right) carried out on the variables measured in the homogenates of each sponge (LAP leucine aminopeptidase, GLU beta-glucosidase, AP alkaline phosphatase; lysozyme content; antibacterial activity against four Burkholderia cepacia strains)
Discussion
Benthic organisms such as sponges have been recognized as a source of unique and diverse secondary metabolites (Faulkner 1984; Berne et al. 2016; Avila and Angulo-Preckler 2021) that have not only a pharmaceutical interest (in terms of anti-bacterial, anti-fungal, anti-viral, and anti-tumor properties) but also an ecological role, being involved in the prevention of fouling, defense against predators, competition for space.
This study investigated the active molecules produced by Antarctic sponges that could have biotechnological potential, focusing on their whole crude homogenates. The integrated approach adopted in this research allowed to acquire new data on sponges’ capability to colonize benthic habitats, thanks to their enzymatic and antibacterial activity profiles as well as to their defense mechanisms against potential sympatric bacteria, in relation to their lysozyme content.
Quantitative differences were found in the enzyme activity rates of the examined species, with high B-GLU and AP activity rates in D. antarctica. This suggested that the microbial community associated with this species was more effective to hydrolyze available organic substrates and provide monomers to the host. Conversely, M. acerata showed the lowest proteolytic, glycolytic, and phosphatasic activities.
To our knowledge, no data are available in literature on the presence of the same enzymes (LAP, B-GLU, and AP) assayed in this study in extracts of sponges or marine invertebrates. In a previous study, Berne et al. (2016) also reported that current knowledge on the potential effects of extracts of Antarctic marine sponges against carbohydrate metabolizing enzymes is still limited, except for one study (Shaaban et al. 2012) on marine sponge extracts from the Red Sea (genera Smenospongia, Callyspongia, Niphates, Stylissa) where inhibitory effects on amylase of a Callyspongia extract were observed. Berne et al. (2016) found that four of 24 ethanolic extracts of sponges (Isodictya lankesteri and Inflatella belli) showed inhibitory effects against alpha-amylase.
Compared to sponge extracts, a greater number of studies are available on enzymes produced by bacteria isolated from different species of Antarctic sponges. Due to their characteristics of remote and cold regions, Antarctic environments are extremely interesting study areas for the prospection of new microorganisms (such as bacteria, fungi, yeasts) producing cold enzymes (Danilovich et al. 2018). Interest in the bioprospection of Antarctic samples for the search of new enzymes relies on the possibility of exploiting such enzymes, active at a cold temperature, in biotechnological processes where low temperature is required. On the other hand, bacterial adaptation to the cold is mediated by the structural flexibility of enzymes at their active site, as well as high specific activity at low temperatures and low substrate affinity (Chattopadhyay 2006).
Protease-producing psychrophilic bacteria were isolated from several Antarctic environments such as soil, fresh and marine waters and sea sediments of King George Islands (Vazquez et al. 1995); amylase-producing bacteria have been reported from Antarctic samples, including marine sponges and invertebrates, sediments and soil biofilm (Ronzella Ottoni et al. 2020). Also, proteolytic and glycolytic enzymes are produced by bacterial strains isolated from black lichens of Galindez Island (Antarctica) (Borzova et al. 2021). Other than bacteria, also filamentous fungi, like Geomyces sp. isolated from marine sponges collected in King George Island, were recently found to be potential producers of pectinases (Poveda et al. 2018) that may be potentially suitable for biotechnological applications. New antimicrobial and enzymes with several hydrolytic, hemolytic, and bio-emulsifying activities were detected in bacterial isolates from the waters of Antarctic Peninsula and South Shetlands, confirming that bioprospecting in the Antarctic environment is still an open research field (Danilovich et al. 2018).
Antarctic Porifera, which represent a hot spot of biodiversity with their associated cold-adapted bacteria, have recently been reviewed as a source of biomolecules (Berne et al. 2016; Rizzo and Lo Giudice 2018). Also in invertebrates other than Porifera, enzymes adapted to extreme cold conditions have been reported (Rizzo and Lo Giudice 2018). The oligochaete Grania sp. has been the only benthic invertebrate from a polar marine environment in which intestinal bacteria belonging to Pseudomonas, Flavobacterium, and Psychrobacter genera, able to produce proteases, esterases, amylases, cellulases, and agarases, have been used as a source of hydrolytic enzymes (Herrera et al. 2017).
In addition, the production of defense molecules makes sponges interesting animal model organisms (Avila and Angulo-Preckler 2021). Our results showed that M. sarai was the species with the highest lysozyme content, followed by Haliclona sp. and D. antarctica, while M. acerata and H. dancoi exhibited the lowest lytic activity. Since the first Metchnikoff’s studies on phagocytosis dating back to 1892, it appeared clear that sponges can eliminate microorganisms through active humoral and cellular defense/immune mechanisms (Metchnikoff 1892). This is not surprising, being sponges exposed to large amounts of bacteria present in their surrounding aqueous milieu, which they also concentrate by their filter-feeding ability. Sponges were previously shown to possess pathogen recognition receptors for bacteria and fungi (Wiens et al. 2007). In Antarctic organisms, however, the modulating role of chemical defenses played by ecological factors need to be elucidated yet.
Most of the molecular and cellular studies on the immune mechanisms in sponges were performed in the demosponge Suberites domuncula (Müller et al. 1999). Thakur et al. (2005) demonstrated that this species possesses a gene that codes for lysozyme, which hydrolyzes the peptidoglycan, typical cell wall component of gram-positive bacteria; these authors demonstrated that sponge cells can react to peptidoglycan from S. aureus with a rapid activation of endocytosis, followed by the release of lysozyme. Lysozyme i-type found in S. domuncula was suggested to play not only a defensive role, but also a role in the digestion of captured bacteria, that are lysed in the mesohyl (Thakur et al. 2005); the involvement in digestive processes is a common capability suggested for filter-feeding invertebrates that feed on bacteria (Van Herreweghe and Michiels 2012). Therefore, the high content of lysozyme measured in our study in the species M. sarai, Haliclona sp. and D. antarctica indicated that these organisms was more able to digest bacteria than the other examined species, with a competitive advantage to their survival and growth.
Chemical defenses are a powerful tool that many benthic organisms possess to counteract microbial colonization and prevent possible infections from potential pathogens (Faulkner 1984). Several reports have documented the antimicrobial activity possessed by sponge extracts (McClintock and Gauthier 1992; Becerro et al. 2003; Kelman 2004; Graça et al. 2015). Peters et al. (2010) screened the hydrophilic and lipophilic extracts from 25 species of Antarctic Demosponges for their potential activities against 20 bacterial isolates (16 Gammaproteobacteria, one Flavobacterium, and three unidentified species) and one diatom species collected off the western Antarctic Peninsula; the lipophilic extracts were found to be more effective than the hydrophilic ones (96 versus 60%), showing higher activities against diatoms than bacteria. Similarly to the results obtained in our study, extracts of the Antarctic sponge Haliclona sp. caused growth inhibition in one strain of the gram-negative bacterium Klebsiella pneumoniae (McClintock and Gauthier 1992). Antimicrobial activity against B. cepacia complex bacteria was detected in sponge-associated Antarctic microbial communities by Papaleo et al., who found that volatile organic compounds were responsible for this activity (Papaleo et al. 2012, 2013). Extracts from deep-sea inhabiting Antarctic sponges belonging to the Latrunculia genus exhibited antibacterial activities against a wide range of bacteria, together with hemolytic and cytotoxic properties (Turk et al. 2013). The occurrence of antagonistic interactions among the cultivable bacteria associated with the Antarctic sponges Anoxycalyx joubini and Lissodendoryx nobilis collected from Terra Nova Bay (Ross-Sea) (Mangano et al. 2009), as well as of anti-biofilm inhibitory activity against Pseudomonas aeruginosa and Staphylococcus aureus (Rizzo et al. 2021) was also reported.
The lypophilic extracts of eighteen species of Antarctic shallow-water sponges collected from Deception Island showed antibacterial activity against both Antarctic and pathogenic bacteria. Particularly in Haliclona sp. a weak to moderate inhibition activity against sympatric bacteria (Micrococcus sp., Bacillus aquamaris and Paracoccus sp.) and bacterial pathogens (Ps. aeruginosa NCTC 10332T and E. coli O157:H7, ATCC 43888) was observed, while in M. acerata only a weak antibacterial activity against Micrococcus sp. and B. aquamaris was found (Angulo-Preckler et al. 2018).
In our study the sponge homogenates were assayed using the same concentrations of sponge homogenates per each of the assayed species and, within each species, per each replicate; this allowed to compare the antibacterial activities exhibited by the different sponge species and replicates. The results of the antibacterial test obtained from this study highlight how Antarctic sponges are among the most promising sources of bioactive molecules with antibacterial properties, especially Haliclona sp., D. antarctica, and M. acerata; consequently, their homogenates should deserve to be further characterized for their chemical composition. The availability of a range of enzymes provides to Haliclona sp. an important advantage for effective colonization and competition for nutrients in environments (like Antarctic waters), where they may be in limiting concentrations; in addition, the antibacterial activities represent an effective strategy that sponges can use to inhibit the growth of neighboring microorganisms.
Conclusion
This study shows that the examined sponges are a source of different marine bioactive compounds, likely produced from associated bacterial symbionts, that could have great potential to be used in medicine or industrial applications. The sponge homogenates showed significant variability in terms of both enzyme and antibacterial activities; M. acerata was the species characterized by the lowest enzyme activity rates, while exhibited high antibacterial activity. However, more information is needed to study the possible influence played by abiotic and biotic variables on the patterns of chemical defense. Future studies on these focal points should be addressed to continue the discovery of novel antimicrobial compounds of interest for natural product chemistry.
Data availability
The data will be made available upon reasonable request.
References
Andriyono S, Jalasena B, Tjahtjaningsih W, Pramono H (2015) Characterisation of symbiotic bacteria isolated from sponge Haliclona sp. In: The First Int. Conference on Life Sciences and Biotechnology 2015, Jember, September 28–29. http://seminar.unej.ac.id/index.php/icolib2015
Angulo-Preckler C, San Miguel O, García-Moreno C, Avila C (2018) Antibacterial defenses and palatability of shallow-water Antarctic sponges. Hydrobiologia 806:123–128. https://doi.org/10.1007/s10750-017-3346-5
Audoin C, Bonhomme D, Ivanisevic J, de la Cruz M, Cautain B, Monteiro MC, Reyes F, Rios L, Perez T, Thomas OP (2013) Balibalosides, an original family of glucosylated sesterterpenes produced by the Mediterranean sponge Oscarella balibaloi. Mar Drugs 11:1477–1489. https://doi.org/10.3390/md11051477
Avila C, Angulo-Precker C (2021) A minireview on biodiscovery in Antarctic marine benthic invertebrates. Front Mar Sci 8:86477. https://doi.org/10.3389/fmars.2021.686477
Baker BJ, Kopitzke W, Hamann M, McClintock JB (1993) Chemical ecology of Antarctic sponges from McMurdo Sound, Antarctica: chemical aspects. Antarctic J US 28:132–133
Baker BJ, Yoshida WY, McClintock JB (1994) Chemical constituents of four Antarctic sponges in McMurdo Sound, Antarctica. Antarctic J US 39:153–155
Baker BJ, Kopitzke RW, Yoshida WY, McClintock JB (1995) Chemical and ecological studies of the Antarctic sponge Dendrilla membranosa. J Nat Prod 58:1459–1462. https://doi.org/10.1021/np50123a020
Battershill CN (1990) The chemical ecology of Antarctic benthic marine invertebrates-initial observations. New Zealand Antarctic Rec 10:9–21
Becerro MA, Thacker RW, Turon X, Uriz MJ, Paul VJ (2003) Biogeography of sponge chemical ecology: comparisons of tropical and temperate defenses. Oecologia 135:91–101. https://doi.org/10.1007/s00442-002-1138-7
Berne S, Kalauz M, Lapat M et al (2016) Screening of the Antarctic marine sponges (Porifera) as a source of bioactive compounds. Polar Biol 39:947–959. https://doi.org/10.1007/s00300-015-1835-4
Bibi F, Faheem M, Azhar EI, Yasir M, Alvi SA, Kamal MA, Ullah I, Naseer MI (2017) Bacteria from marine sponges: a source of new drugs. Curr Drug Metab 18:11–15. https://doi.org/10.2174/1389200217666161013090610
Borzova NV, Gladka GV, Gudzenko OV, Hovorukha VM, Tashyrev OB (2021) Enzymatic activity of psychrotolerant Antarctic bacteria. Mikrobiol Z 83:3–11. https://doi.org/10.15407/microbiolj83.02.003
Brinkmann C, Marker A, Kurtböke D (2017) An overview on marine sponge-symbiotic bacteria as unexhausted sources for natural product discovery. Diversity 9:40. https://doi.org/10.3390/d9040040
Cárdenas CA, Font A, Steinert G, Rondon R, González-Aravena M (2019) Temporal stability of bacterial communities in Antarctic sponges. Front Microbiol 10:2699. https://doi.org/10.3389/fmicb.2019.02699
Caruso G (2010) Leucine aminopeptidase, beta-glucosidase and alkaline phosphatase activity rates and their significance in nutrient cycles in some coastal Mediterranean sites. Mar Drugs 8:916–940. https://doi.org/10.3390/md8040916
Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR (2002) Low-temperature extremophiles and their applications. Curr Opin Biotechnol 13:253–261. https://doi.org/10.1016/S0958-1669(02)00317-8
Chattopadhyay MK (2006) Mechanism of bacterial adaptation to low temperature. J Biosci 31:157–165. https://doi.org/10.1007/BF02705244
Danilovich ME, Sánchez LA, Acosta F et al (2018) Antarctic bioprospecting: in pursuit of microorganisms producing new antimicrobials and enzymes. Polar Biol 41:1417–1433. https://doi.org/10.1007/s00300-018-2295-4
Duarte AWF, dos Santos JA, Vianna MV, Vieira JMF, Mallagutti VH, Inforsato FJ, Wentzel LCP, Lario LD, Rodrigues A, Pagnocca FC, Pessoa AJ, Durães Sette L (2018) Cold-adapted enzymes produced by fungi from terrestrial and marine Antarctic environments. Crit Rev Biotechnol 38:600–619. https://doi.org/10.1080/07388551.2017.1379468
Faulkner DJ (1984) Marine natural products: metabolites of marine invertebrates. Nat Prod Rep 1:551–598. https://doi.org/10.1039/NP9840100551
Feby A, Nair S (2010) Sponge-associated bacteria of Lakshadweep coral reefs, India: resource for extracellular hydrolytic enzymes. Adv Biosci Biotechnol 1:330–337. https://doi.org/10.4236/abb.2010.14043
Ferraboschi P, Ciceri S, Grisenti P (2021) Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics 10:1534. https://doi.org/10.3390/antibiotics10121534
Ford J, Capon RJ (2000) Discorhabdin R: a new antibacterial pyrroloiminoquinone from two latrunculiid marine sponges, Latrunculia sp. and Negombata sp. J Nat Prod 63:1527–1528. https://doi.org/10.1021/np000220q
Graça AP, Viana F, Bondoso J, Correia MI, Gomes L, Humanes M, Reis A, Xavier JR, Gaspar H, Lage OM (2015) The antimicrobial activity of heterotrophic bacteria isolated from the marine sponge Erylus deficiens (Astrophorida, Geodiidae). Front Microbiol 6:389. https://doi.org/10.3389/fmicb.2015.00389
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software package for education and data analysis. Paleontol Electronica 4:1–9
Herrera LM, García-Laviña CX, Marizcurrena JJ et al (2017) Hydrolytic enzyme-producing microbes in the Antarctic oligochaete Grania sp. (Annelida). Polar Biol 40:947–953. https://doi.org/10.1007/s00300-016-2012-0
Jayatilake GS, Thornton MP, Leonard AC, Grimwade JE, Baker BJ (1996) Metabolites from an Antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J Natl Prod 59:293–296. https://doi.org/10.1021/np960095b
Kanagasabhapathy M, Sasaki H, Nakajima K, Nagata K, Nagata S (2005) Inhibitory activities of surface associated bacteria isolated from the marine sponge Pseudoceratina purpurea. Microbes Environ 20:178–185. https://doi.org/10.1264/jsme2.20.178
Kelman D (2004) Antimicrobial activity of sponges and corals. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, Berlin, pp 243–258. https://doi.org/10.1007/978-3-662-06414-6_12
Krishnakumar S, Bai VDM, Premkumar J (2015) Production of alpha amylase by salt-tolerant Actinomycete Streptomyces sp.-SBU3 isolated from marine sponge. Indian J Geo-Marine Sci 44:583–588
Krug PJ (2006) Defense of benthic invertebrates against surface colonization by larvae: a chemical arms race. Prog Mol Subcell Biol 42:1–53. https://doi.org/10.1007/3-540-30016-3_1
Laport MS, Santos OCS, Muricy G (2009) Marine sponges: Potential sources of new antimicrobial drugs. Curr Pharm Biotechnol 10:86–105. https://doi.org/10.2174/138920109787048625
Lo Giudice A, Rizzo C (2018) Bacteria associated with marine benthic invertebrates from polar environments: unexplored frontiers for biodiscovery? Diversity 10:80. https://doi.org/10.3390/d10030080
Mangano S, Michaud L, Caruso C, Brilli M, Bruni V, Fani R, Lo Giudice A (2009) Antagonistic interactions between psychrotrophic cultivable bacteria isolated from Antarctic sponges: a preliminary analysis. Res Microbiol 160:27–37. https://doi.org/10.1016/j.resmic.2008.09.013
Marx JC, Collins T, D’Amico S, Feller G, Gerday C (2007) Cold-adapted enzymes from marine Antarctic microorganisms. Mar Biotechnol 9:293–304. https://doi.org/10.1007/s10126-006-6103-6108
McClintock JB, Baker BJ (1997) A review of the chemical ecology of Antarctic marine invertebrates. Am Zool 37:329–342. https://doi.org/10.1093/icb/37.4.329
McClintock J, Gauthier J (1992) Antimicrobial activities of Antarctic sponges. Antarctic Sci 4:179–183. https://doi.org/10.1017/S0954102092000270
Mehbub MF, Lei J, Franco C, Zhang W (2014) Marine sponge derived natural products between 2001 and 2010: trends and opportunities for discovery of bioactives. Mar Drugs 12:4539–4577. https://doi.org/10.3390/md12084539
Metchnikoff E (1892) Leçons sur la pathologie comparée de l’inflammation: faites à l’Institut Pasteur en avril et mai 1891. Masson, Paris
Mohapatra BR, Bapuji M, Sree A (2003) Production of industrial enzymes (amylase, carboxymethylcellulase and protease) by bacteria isolated from marine sedentary organisms. Acta Biotechnol 23:75–84. https://doi.org/10.1002/abio.200390011
Moreno-Pino M, Cristi A, Gillooly JF, Trefault N (2020) Characterizing the microbiomes of Antarctic sponges: a functional metagenomic approach. Sci Rep 10:645. https://doi.org/10.1038/s41598-020-57464-2
Müller WEG, Blumbach B, Müller IM (1999) Relationships between potential immune molecules in the lowest Metazoan Phylum (Porifera) and those in Vertebrates. Transplantation 68:1215–1227. https://doi.org/10.1097/00007890-199911150-00001
Ossermann EF, Lawlor DP (1966) Serum and urinary lysozyme (Muramidase) in monocytic and monomyelocytic leukemia. J Exp Med 124:921–951. https://doi.org/10.1084/jem.124.5.921
Papale M, Rizzo C, Fani R, Bertolino M, Costa G, Paytuví-Gallart A, Schiaparelli S, Michaud L, Azzaro M, Lo Giudice A (2020) Exploring the diversity and metabolic profiles of bacterial communities associated with Antarctic sponges (Terra Nova Bay, Ross Sea). Front Ecol Evol 8:268. https://doi.org/10.3389/fevo.2020.00268
Papaleo MC, Fondi M, Maida I, Perrin E, Lo Giudice A, Michaud L, Mangano S, Bartolucci G, Romoli R, Fani R (2012) Sponge-associated microbial Antarctic communities exhibiting antimicrobial activity against Burkholderia cepacia complex bacteria. Biotechnol Adv 30:272–293. https://doi.org/10.1016/j.biotechadv.2011.06.011
Papaleo MC, Romoli R, Bartolucci G, Maida I, Perrin E, Fondi M, Orlandini V, Mengoni A, Emiliani G, Tutino ML, Parrilli E, de Pascale D, Michaud L, Lo Giudice A, Fani R (2013) Bioactive volatile organic compounds from Antarctic (sponges) bacteria. New Biotechnol 30:824–838. https://doi.org/10.1016/j.nbt.2013.03.011
Parte S, Sirisha VL, D’Souza JS (2018) Biotechnological applications of marine enzymes from algae, bacteria, fungi, and sponges. In: Kim S-K, Toldrà F (eds) Advances in Food and Nutrition Research. Marine enzymes biotechnology: Production and industrial applications, Part III- Application of marine enzymes. Academic Press, Cambridge, Chapter 4, pp 75–106
Peters KJ, Amsler CD, McClintock JB, Baker BJ (2010) Potential chemical defenses of Antarctic sponges against sympatric microorganisms. Polar Biol 33:649–658. https://doi.org/10.1007/s00300-009-0741-z
Poveda G, Gil-Duran C, Vaca I, Levicán G, Chávez R (2018) Cold-active pectinolytic activity produced by filamentous fungi associated with Antarctic marine sponges. Biol Res 51:28. https://doi.org/10.1186/s40659-018-0177-4
Rios P, Cristobo J (2014) Antarctic Porifera database from the Spanish benthic expeditions. Zookeys 401:1–10. https://doi.org/10.3897/zookeys.401.5522
Rizzo C, Lo Giudice A (2018) Marine Invertebrates: underexplored sources of bacteria producing biologically active molecules. Diversity 10:52. https://doi.org/10.3390/d10030052
Rizzo C, Zammuto V, Lo Giudice A, Rizzo MG, Spanò A, Laganà P, Martinez M, Guglielmino S, Gugliandolo C (2021) Antibiofilm activity of Antarctic sponge-associated bacteria against Pseudomonas aeruginosa and Staphylococcus aureus. J Mar Sci Eng 9:243. https://doi.org/10.3390/jmse9030243
Ronzella Ottoni J, Rodrigues e Silva T, de Oliveira VM, Zambrano Passarini MR (2020) Characterization of amylase produced by cold-adapted bacteria from Antarctic samples. Biocatal Agric Biotechnol 23:101452. https://doi.org/10.1016/j.bcab.2019.101452
Ruocco N, Esposito R, Bertolino M, Zazo G, Sonnessa M, Andreani F, Coppola D, Giordano D, Nuzzo G, Lauritano C, Fontana A, Ianora A, Verde C, Costantini M (2021) A metataxonomic approach reveals diversified bacterial communities in Antarctic sponges. Mar Drugs 19:173. https://doi.org/10.3390/md19030173
Savoca S, Lo Giudice A, Papale M, Mangano S, Caruso C, Spanò N, Michaud L, Rizzo C (2019) Antarctic sponges from the Terra Nova Bay (Ross Sea) host a diversified bacterial community. Sci Rep 9:16135. https://doi.org/10.1038/s41598-019-52491-0
Scientific Committee on Antarctic Research (SCAR) (2011) Code of conduct for the use of animals for scientific purposes in Antarctica. In: XXXIV ATCM (Antarctic Treaty Consultative Meeting), Buenos Aires June 20th–July 1st, 2011. http://www.scar.org/treaty/atcmxxxiv/ATCM34_ip053_e.pdf
Shaaban M, Abd-Alla HI, Hassan AZ, Aly HF, Ghani MA (2012) Chemical characterization, antioxidant and inhibitory effects of some marine sponges against carbohydrate metabolizing enzymes. Org Med Chem Lett 16:30. https://doi.org/10.1186/2191-2858-2-30
Steinert G, Wemheuer B, Janussen D, Erpenbeck D, Daniel R, Simon M, Brinkhoff T, Schupp PJ (2019) Prokaryotic diversity and community patterns in Antarctic continental shelf sponges. Front Mar Sci 6:297. https://doi.org/10.3389/fmars.2019.00297
Taboada Moreno S (2012) Antarctic marine benthic invertebrates: chemical ecology, bioactivity and biodiversity. PhD Thesis, Universitat de Barcelona, Spain, pp 1–373
Taylor M, Tsai P, Simister R, Deines P, Botte E, Ericson G, Schmitt S, Webster NS (2013) Sponge-specific’ bacteria are widespread (but rare) in diverse marine environments. ISME J 7:438–443. https://doi.org/10.1038/ismej.2012.111
Thakur NL, Anil AC, Müller WEG (2004) Culturable epibacteria of the marine sponge Ircinia fusca: temporal variations and their possible role in the epibacterial defense of the host. Aquat Microb Ecol 37:295–304. https://doi.org/10.3354/ame037295
Thakur NL, Perovic-Ottstadt S, Batel R, Korzhev M, Diehl-Seifert B, Müller IM, Müller WEG (2005) Innate immune defense of the sponge Suberites domuncula against gram-positive bacteria: induction of lysozyme and AdaPTin. Mar Biol 146:271–282. https://doi.org/10.1007/s00227-004-1438-z
Turk T, Ambrožič Avguštin J, Batista U, Strugar G, Kosmina R, Čivović S, Janussen D, Kauferstein S, Mebs D, Sepčić K (2013) Biological activities of ethanolic extracts from deep-sea Antarctic marine sponges. Mar Drugs 11:1126–1139. https://doi.org/10.3390/md11041126
Vaca I, Faúndez C, Maza F, Paillavil B, Hernández V, Acosta F, Levicán G, Martínez C, Chávez R (2013) Cultivable psychrotolerant yeasts associated with Antarctic marine sponges. World J Microbiol Biotechnol 29:183–189. https://doi.org/10.1007/s11274-012-1159-2
Van Herreweghe JM, Michiels CW (2012) Invertebrate lysozymes: diversity and distribution, molecular mechanism and in vivo function. J Biosci 37:327–348. https://doi.org/10.1007/s12038-012-9201-y
Vazquez SC, Rios Merino LN, MacCormack WP, Fraile ER (1995) Protease-producing psychrotrophic bacteria isolated from Antarctica. Polar Biol 15:131–135. https://doi.org/10.1007/BF00241051
Vestergaard M, Frees D, Ingmer H (2019) Antibiotic resistance and the MRSA problem. Microbiol Spectr 7:2. https://doi.org/10.1128/microbiolspec.GPP3-0057-2018
Wang G (2006) Diversity and biotechnological potential of the sponge-associated microbial consortia. J Ind Microbiol Biotechnol 33:545–551. https://doi.org/10.1007/s10295-006-0123-2
Webster NS, Wilson KJ, Blackall LL et al (2001) Phylogenetic diversity of bacteria associated with the marine sponge Rhopaloeides odorabile. Appl Environ Microbiol 67:4538–4545. https://doi.org/10.1128/AEM.67.1.434-444.2001
Webster NS, Negri AP, Munro MM, Battershill CN (2004) Diverse microbial communities inhabit Antarctic sponges. Environ Microbiol 6:288–300. https://doi.org/10.1111/j.1462-2920.2004.00570.x
WHO (World Health Organization) (2015) Global action plan on antimicrobial resistance. World Health Organization, Geneva, pp 1–28
Wiens M, Korzhev M, Perović-Ottstadt S, Luthringer B, Brandt D, Klein S, Müller WEG (2007) Toll-like receptors are part of the innate immune defense system of sponges (Demospongiae: Porifera). Mol Biol Evol 24:792–804. https://doi.org/10.1093/molbev/msl208
Acknowledgements
The authors wish to thank Dr. Marco Bertolino and Prof. Stefano Schiaparelli for sponge identification.
Funding
This research was funded by PNRA (National Research Program in Antarctica), Italian Ministry of Education and Research, in the framework of the projects “Antarctic Porifera: hot-spots for Prokaryotic diversity and biotechnological Potentialities (P3)” Grant number PNRA16_00020 and “Microbial colonization of benthic ANTarctic environments: responses of microbial abundances, diversity, activities and larval settlement to natural or anthropogenic disturbances and search for secondary metabolites (ANT-Biofilm)” Grant number PNRA16_00105. These projects contributed equally with a percentage of 50%.
Author information
Authors and Affiliations
Contributions
GC, MP, and ALG conceived the research; MP, CR, and MA performed the investigation; GC, MP, PL conducted the experiments; methodology, validation, GC, ALG; investigation, MP and CR; resources, GC and ALG; data curation, GC, RC, and PL; writing-original draft preparation, all the Authors wrote the manuscript; editing, all the Authors; supervision, GC; funding acquisition, GC and ALG. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caruso, G., Papale, M., Azzaro, M. et al. Antarctic Porifera homogenates as a source of enzymes and antibacterial substances: first results. Polar Biol 45, 895–907 (2022). https://doi.org/10.1007/s00300-022-03042-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03042-3








