Abstract
Phytoplankton pigment signatures from a cruise in 2005 are herein presented and used as a chemotaxonomic tool for phytoplankton diversity in the Svalbard marine archipelago. Studies from these waters have until recently reported only a few groups of phytoplankton, and while this paper is the first to show that the diversity around Svalbard includes all major phytoplankton pigment groups, the results are seen in relation to other similar studies from the Arctic. We present two potentially important marker pigments: prasinoxanthin, originating from prasinophytes, and gyroxanthin-diester, possibly originating from the temperate- and bloom-forming coccolithophore Emiliania huxleyi. Pigment identification by HPLC revealed a significant amount of Chlorophyll b-containing chlorophyceae, euglenophyceae and prasinophyceae. Prasinoxanthin was present at 50% of the examined stations, typically at Chl a maximum (15–25 m depth), in both Atlantic and Arctic water masses. Gyroxanthin-diester, in contrast to prasinoxanthin, was found only in Atlantic water masses and at low concentrations. Our data may be important for the identification and verification of remotely sensed images of different pigment groups of phytoplankton and their corresponding biomass, typically estimated from Chl a. Remotely sensed presence of coccoliths, indicating E. huxleyi at sea surface, is discussed in relation to water masses and pigment signatures at sea surface and Chl a maximum depths.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Barents Sea and water masses around the Svalbard archipelago are situated on a shelf with an average depth of about 230 m, an area of about 1.4 million km2 and a water volume of about 322 thousand km3. During winter, the sea is largely covered by an annual ice sheet, of which a major part is first-year ice (Sakshaug and Kovacs 2009). The Barents Sea consists mainly of two water masses, Atlantic water (AW) characterized by temperature >3°C and salinity >34.90 and Arctic water (ArW) characterized by temperature <0°C and salinity between 34.3 and 34.8 (Ingvaldsen and Loeng 2009). Until 10 years ago, the common perception was that the phytoplankton classes Bacillariophyceae (diatoms), Coccolithophycae (previously known as prymnesiophytes, Silva et al. 2007) and Dinophyceae (dinoflagellates) dominated the waters around Svalbard. In contrast, recent studies in the western Canadian Arctic showed that a high fraction of the phytoplankton biomass consisted of picoeukaryotes (2–3 mm cell size) and was dominated by small prasinophytes (Booth and Horner 1997; Booth and Smith 1997; Lovejoy et al. 2007). It is worthwhile to note that Throndsen and Kristiansen (1991), using dilution culture assays, found that the prasinophyte Micromonas pusilla (Butcher, Manton et Parke) was numerically abundant in European Arctic waters (Barents Sea, Greenland Sea and Svalbard region), later confirmed by Not et al. (2005) and Lovejoy et al. (2007). Hence, re-examination of phytoplankton diversity is in order.
Marine phytoplankton have a large variety of light-harvesting and photoprotective carotenoids (Jeffrey et al. 1997). Light-harvesting pigments (LHP) comprise chlorophylls (Chl); Chl a, Chl b, Chl c 1, c 2 and c 3; phycobiliproteins and all carotenoids that do not have a photoprotective role. Photoprotective carotenoids (PPC) include diadinoxanthin, diatoxanthin and zeaxanthin (Rodríguez et al. 2006). The different phytoplankton classes can be divided into three major pigment taxa based on 13 different pigment groups (PG): These major pigment taxa are the Chl c-containing chromophytes represented in PG 1–5 and 10–12, the Chl b-containing chlorophytes represented in PG 6–9 and lastly, the cyanobacteria in PG 13 (Table 1, Johnsen and Sakshaug 2007). The combined use of different pigment-specific taxonomical markers, for example Chl b and prasinoxanthin, can be used to identify different pigment groups in a given class (such as prasinoxanthin-containing prasinophytes), discriminating them from other chlorophytes (Jeffrey et al. 1997; Johnsen and Sakshaug 2007).
The objective in this study was to investigate pigment diversity and differences in the phytoplankton composition between the Atlantic water and the Arctic water masses surrounding the Svalbard archipelago.
We present group-specific pigment markers indicating phytoplankton groups that might have been overlooked or lost during sampling using traditional sampling methods like phytoplankton nets, detection methods as light microscopy (LM) and the use of formalin and lugol-based fixatives with the corresponding decay of small and fragile flagellates.
Materials and methods
Field work was carried out from the 21st to the 28th of August 2005 from the research vessel Jan Mayen in the coastal waters west and north of Svalbard. Water samples (2–9 replicates for each depth) were collected from sea surface and the depth of the Chl a maximum at 12 stations (Fig. 1). An in situ Chl a fluorescence detector (Seapoint chlorophyll fluorometer) attached to a CTD rig (Seabird 911 Plus) was used to identify the depth of the Chl a maximum, from which samples were taken with Niskin water sample bottles (5 l). Surface samples were taken using a plastic bucket (10 l) from the same location as the CTD was lowered. Samples were collected for (1) microscopic examination of phytoplankton cells, (2) Chl a concentration, determined on-board, and (3) full pigment analysis by high performance liquid chromatography (HPLC) carried out in the laboratory after return from the cruise. For light microscopy (LM), the water samples (100 ml) were preserved in Lugol (0.15 ml) and stored in a refrigerator at 4°C. Samples were later examined using a Leica DMIRB/E Das epifluorescence microscope with a Sony DFW-×700 colour digital camera attached, in order to verify major phytoplankton classes and genera.
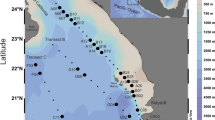
Map of the Svalbard archipelago with the SST (sea surface temperature) and the 12 water sampling stations. Stations 1–4 and 10–12 correspond to AW and stations 5–9 correspond to ArW as defined by Ingvaldsen and Loeng (2009). Mean SST of August 2005. Data courtesy of Feldman GC, McClain CR, Ocean Color Web, MODIS, NASA Goddard Space Flight Center. Eds. Kuring N, Bailey S. W, Accessed 16 Oct 2008
For on-board Chl a determination and HPLC pigment analysis, seawater was filtered with a Gelman filtering unit on glass fibre filters (Whatman GF/F, 2.5 cm diameter, 0.7-μm effective pore size). This was done in dim light to avoid pigment degradation. Immediately after collection at sea, filters were extracted in 5 ml methanol (100%), mechanically crushed with a glass rod for optimal extraction efficiency and stored in the dark for 24 h at 4°C to avoid pigment degradation. The extracts were then refiltered and analysed in a Beckman DU-60 spectrophotometer, using a 1 cm glass cuvette. The Chl a concentration was calculated according to the equation provided by Mackinney (1941).
For HPLC pigment analysis, the seawater sample volume ranged from 1 to 2 l, depending on phytoplankton abundance. Filters were wrapped in aluminium foil and stored in a dry shipper filled with liquid nitrogen (−196°C) for transportation and later transferred to a biological freezer (−80°C), where the HPLC filters were stored for 6 months prior to extraction. For HPLC analysis, 1.5 ml of 4°C methanol was added to the frozen filters in glass test tubes, shaken for 10 s in a vortex mixer and then left to extract in a dark 4°C refrigerator for 24 h. Subsequently, the extracts were refiltered into 2-ml dark-coloured HPLC glass vials through a 2-ml syringe with a GHP Acrodisc 13-mm membrane filter with 0.2-μm pore size. Pigments were analysed by a Hewlett Packard 1100 Series HPLC system equipped with a quaternary pump system, injector, auto sampler, Waters Symmetry C8 column (150 × 4.6 mm, 3.5 μm particle size) and a diode array absorbance detector (400–700 nm) connected to a PC. The auto sampler drew 77 μl from each sample vial and 23 μl water, which was mixed automatically 5 times before injection. The water was added to increase the polarity in order to improve the separation of Chl c’s. In the mobile phase, solvent 1 was a mixture of methanol:acetonitrile:aqueous pyridine (0.25 M pyridine) in the ratio 50: 25: 25 (vol·vol−1), solvent 2 was acetonitrile:acetone in the ratio 80: 20 (vol·vol−1) and solvent 3 (methanol) was used for cleaning the system before and after the analysis. Analytic separation was performed using the method of Zapata et al. (2000).
Following HPLC analyses, the replicates from each depth were averaged and the mean value was used for further calculations of the different pigment concentrations. The number of replicates used for calculating the mean of μg/ l−1 pigments ranged from 2 to 9 between and within stations, using the number of replicates containing a specific pigment. The standard deviation (SD) and ± coefficient of variation (CV%) were calculated, and SD and CV % are presented as mean values of all the stations together. The detected pigment signatures were compared with confirmed pigment standards isolated and kept at TBS (Trondheim Biological Station). The phytoplankton groups were classified according to Johnsen and Sakshaug (2007, Table 1).
Results and discussion
The CTD profiles at the 12 stations indicated in Fig. 1 show that the two major water masses in the survey area were of AW and ArW origin. Stations 1–4 and 10–12 all had a temperature that corresponded to dominance of AW (Fig. 2a), while the temperature at stations 5–9 corresponded to dominance of ArW (Fig. 2b). The polar front was situated between stations 9 and 10 (Fig. 1). The salinity in the surface layers was lower than the characteristics of both AW and ArW, except for stations 10–12. The lowered salinity indicated an influence from local water masses due to fresh water run-off from land or, most likely, sea ice melting.
Chl a measurements were only available for stations 1–7 and showed concentrations from 0.27 to 2.18 mg m−3, indicating an overall low biomass.
The following section (chemotaxonomy) presents the pigment groups (PG, numbered 1–13 in Johnsen and Sakshaug 2007) found in the water masses surrounding the Svalbard archipelago (Table 1, 2; Figs. 1, 2, 3, 4, 5). In the next section (gyroxanthin-diester as a potential pigment marker), we discuss the existence of gyroxanthin-diester in E. huxleyi and the possibility that this pigment is a marker for E. huxleyi in Arctic waters.
a HPLC chromatogram from field sample (station 11 (16 m)) containing gyroxanthin-diester (Peak 12). The Chl a peak in the field sample has been matched in retention time with the Chl a peak in the standard of Karlodinium veneficum, and therefore all the pigment peaks for the field sample appears 0.98 min earlier in the chromatogram. Each peak represents one pigment, the peak number with the pigment in parentheses: 1 (Chl c 3 ), 2 (Chl c 2 ), 3 (But-fuco), 6 (Fucoxanthin), 7 (19hex-fuco/4khex-fuco), 8 (Diadinoxanthin), 10 (Alloxanthin), 11 (Diatoxanthin), 12 (gyroxanthin-diester), 13 (Chl b) and 14 (Chl a) b HPLC chromatogram from a culture of Karlodinium veneficum, gyroxanthin-diester (peak 12) has the retention time; 31 min. Each peak represents one pigment, the peak number with the pigment in parentheses: 1 (Chl c 3 ), 2 (Chl c 2 ), 3 (But-fuco), 6 (Fucoxanthin), 7 (19hex-fuco/4khex-fuco), 8 (Diadinoxanthin), 9 (Zeaxanthin), 12 (gyroxanthin-diester), 14 (Chl a). c HPLC chromatogram from a culture of Emiliania huxleyi with gyroxanthin-diester (peak 12) at the retention time; 29 min. The Chl a peak in the field sample has been matched in retention time with the Chl a peak in the standard of Karlodinium veneficum, and therefore all the pigment peaks for the Emiliania huxleyi culture appear 1.8 min later in the chromatogram. Each peak represents one pigment, the peak number with the pigment in parentheses: 1 (Chl c 3 ), 2 (Chl c 2 ) 3 (But-fuco), 6 (Fucoxanthin), 7 (19hex-fuco/4khex-fuco), 8 (Diadinoxanthin), 11 (Diatoxanthin), 14 (Chl a). When growing E. huxleyi under different growth conditions and corresponding physiological status, the pigment ratios may differ significantly. The chromatogram shown is from a stock culture in stationary growth phase and with untypical large amounts of gyroxanthin-diester. d Absorbance spectra of gyroxanthin-diester standard (K. veneficum as source) compared with detected gyroxanthin-diester (average of 6 parallels, SD was 0.35 at 446 nm) from station 11
a Chromatogram from HPLC of field sample (station 11 (16 m)) containing prasinoxanthin and Micromonas pusilla. The Chl a peak in the field sample has been matched in retention time with the Chl a peak in the standard of Micromonas pusilla, and therefore all the pigment peaks for the field sample appear 2.65 min earlier in the chromatogram. Each peak represents one pigment, the peak number with the pigment in parentheses: 15 (MgDvP), 2 (Chl c 2 ), 16 (Prasinoxanthin -like), 17 (Neoxanthin), 18 (Prasinoxanthin), 19 (Violaxanthin), 16 (Prasinoxanthi -like), 13 (Chl b), 14 (Chl a). b Absorbance spectra of prasinoxanthin standard (M. pusilla as source) compared with detected prasinoxanthin from station 10 (24 m)
a Distribution of total Chl c and Chl b (μg/l) of Chromophyta (Sum of: Chl c1+ c2 + c3) and Chlorophyta (Chl b) in surface samples from Atlantic water (AW) and Arctic water (ArW). b Distribution of total Chl c and Chl b (μg/l) of Chromophyta (Sum of: Chl c1+ c2 + c3 and Chlorophyta (Chl b) in samples at Chl a max depth from AW and ArW
The third section (pigment ratios in chlorophytes and chromophytes) describes the distribution of major pigment groups of phytoplankton divided into chlorophytes (PG 6–9) and chromophytes (PG 1–5 and 10–12). The difference between light-harvesting pigments relative to Chl a is also discussed.
Chemotaxonomy
The pigment distribution from the 12 stations showed a more diverse pigment group composition in AW than ArW (Table 2). Pigments from PG 1 (diatoms) were found at all stations. However, the chrysophytes (PG 10) and the pavlovophyceae (PG 5) have the same pigment signature as diatoms (PG 1) and would therefore contribute to the total concentration ([concentration]) of Chl c 1 + c 2 and fucoxanthin. LM analyses also revealed that diatoms (PG 1) were present at all stations. Living cells from AW station 3, and ArW stations 5, 6 and 7 were examined in LM on the research vessel before fixation was carried out. Thirty-nine different diatom species, 22 different species of dinophyceae and three species of chrysophyceae were identified. Skeletonema costatum and pennate diatoms dominated station at 3, whereas stations 5, 6 and 7 were dominated by Dinobryum balticum and Chaetoceros ssp. (pers. comm. von Quillfeldt). S. costatum is known as a biological marker for warm Atlantic water (temperate species). The identification of this species in the surface at station 1 (LM) and station 3 indicates recent influx of AW from the West Spitsbergen Current (WSC) into Isfjorden. The finding of S. costatum in western parts of Spitsbergen is not unique; in 2005, several biological indications of influx of warm saline AW were registered in Isfjorden in addition to S. costatum, including the presence of the blue mussel (Mytilus edulis), mass occurrence of Atlantic cod (Gadus morhua) and Atlantic salmon (Salmo salar, Berge et al. 2005).
PG 2 (Dinophyceae I), with the marker pigment peridinin (0.12–0.62 μg l−1), was found at all the stations influenced by AW but only at two stations (8–9) influenced by ArW.
PG 3 (Dinophyceae II) and PG 4 (Coccolithophyceae), both containing Chl c 3 and acyl-oxy-fucoxanthins, were found at stations influenced by AW. Gyroxanthin-diester (0.04–0.05 μg l−1) and Chl c 3 (0.3–0.5 μg l−1), belonging to PG 3, were detected in AW at stations 11 and 12 and had the same HPLC absorption characteristics as the gyroxanthin-diester standard isolated from the source organism Karlodinium veneficum (aka Gymnodinium galatheanum, Johnsen and Sakshaug 1993, Fig. 3a,b and d). Images from LM of preserved cells from the Chl a max depths revealed diatoms only, which is in accordance with Quillfeldt (1997) who stated that marine environments as far north as stations 1–12 are mainly dominated by diatoms when investigated with this method. Also in the surface samples, LM showed diatoms only for all stations except for station 11 and 12 with coccoliths indicating the presence of E. huxleyi.
PG 6 (Prasinophyceae I) with the marker pigment prasinoxanthin (0.10–0.47 μg l−1) was detected from both AW and ArW stations. This pigment showed the same HPLC absorption characteristics as the prasinoxanthin standard isolated from the source organism Micromonas pusilla (Fig. 4a–b). Prasinophytes belonging to PG 6 are believed to be dominated by M. pusilla in our survey area, and they have been observed as the most abundant phototroph, based on epifluorescence analysis, in the Arctic Ocean (Chukchi Sea to Makarov Basin, Booth and Horner 1997). This species has also been reported as the dominant phytoplankton in Norwegian Arctic seas in late summer (Not et al. 2005). Recent studies by Lovejoy et al. (2007) have shown that Micromonas sp. is abundant in the Arctic basin and that it differs in genotype from Micromonas elsewhere in the world oceans, indicating that Micromonas has a pan-Arctic distribution.
From the chlorophyte Chl b-containing division, PG 6 was identified along with PG 8 (Euglenophyceae) through its marker pigment neoxanthin (0.06–0.21 μg l−1). The presence of PG 7 (Prasinophyceae) and 9 (Chlorophyceae) cannot be excluded, but lutein (marker for PG 7 and 9) was not identified in the samples. Thus, the Chl b-containing chlorophytes were represented by at least two of the four pigment groups (PG 6–9, Table 1). From the chromophytes, pigments representative of PG 2–4, 11–12 and the pigment signatures corresponding to PG 1, 5 and 10 were found. Since PG 1, 5 and 10 all have the same pigment composition (Table 1), they can only be separated by the fraction of light absorbed by PSII using in vivo absorption and fluorescence excitation spectra (see Johnsen and Sakshaug 2007).
Samples from AW stations 1, 4 and 6 displayed higher concentration of Chl b than Chl c (c 1,c 2 and c 3) at surface (Fig. 5a) and at Chl a maximum depth (Fig. 5b). The rest of the stations had higher concentrations of Chl c than Chl b. This shows that there are significant amounts of cells other than diatoms in the waters surrounding the Svalbard archipelago, with the highest in the Atlantic water.
Violaxanthin (0.03–0.09 μg l−1), Chl c 1 and Chl c 2 representing PG 11 (Raphidophyceae) were detected at stations 1, 10 and 11, indicating the presence of Raphidophyceae in the AW. Alloxanthin and Chl c2 were also detected, indicating the presence of PG 12 (Cryptophyceae) in AW. Also, zeaxanthin was detected representing PG 13 (Cyanophyceae) in AW at Chl a max.
AW had the most diverse pigment group composition with pigment markers from all the 13 pigment groups described in Johnsen and Sakshaug (2007, Table 2). ArW had pigment markers from 7 of the pigment groups (Table 2). The difference in diversity was most likely due to the different origins of ArW and AW, with temperate species being transported with the North Atlantic current, and cold-loving species coming southwards with the Arctic water.
Gyroxanthin-diester as a potential pigment marker
Gyroxanthin-diester has been used as a harmful algal bloom marker pigment for the dinoflagellate genera Karlodinium and Karenia (Bjørnland and Tangen 1979; Fiksdahl 1983; Johnsen and Sakshaug 1993; Millie et al. 1995, 1997; Bjørnland et al. 2000, 2003; Örnólfsdóttir et al. 2003). Karenia and Karlodinium have preferences for high temperatures and brackish water (Johnsen et al. 1997) and are thus unlikely the source of the gyroxanthin-diester and Chl c 3 detected in this survey. Recent studies have revealed that gyroxanthin-diester may have a broader distribution than just pigment group 3 (Dinophyceae II, Garcés et al. 2006). Gyroxanthin-diester-like pigments have occurred in samples from four haptophytes, E. huxleyi, Chrysochromulina leadbeateri, C. hirta and Imantonia rotunda, and two pelagophytes, Pelagococcus subviridis and Aureococcus anophagefferens (Zapata 2005). It is unlikely that the gyroxanthin-diester detected in our field samples originated from the two pelagophytes, because A. anophagefferens has not yet been detected in our study area or in the water masses surrounding Norway (Throndsen et al. 2003). P. subviridis has been detected at Haltenbanken, but not in water masses close to the coast (Throndsen et al. 2003). The four haptophytes that may contain gyroxanthin-diester are found both in coastal waters and in the open ocean and are thus possible sources. E. huxleyi is the most common of these, and it may dominate blooms in coastal waters. It has appeared over several years in huge concentrations in Skagerrak, in the Norwegian coastal current and in the southern Barents Sea region (Throndsen et al. 2003; Smyth et al. 2004; Naustvoll and Hansen 2006; Hovland 2007; Johnsen et al. 2009). The xanthophyll composition in E. huxleyi includes 19′-hexanoyloxyfucoxanthin (Hex-fuco), which was detected in water samples from stations 11 and 12 (Table 2, Zapata 2005). The sea surface temperature (SST) image (Fig. 1) shows the influence of warm AW just off the coast of West Spitsbergen, the same area where gyroxanthin-diester was detected at station 11. The calcite image (Fig. 6) shows concentrations up to 0.001 mol calcite m−3 associated with AW in the same area as stations 11 and 12, suggesting the presence of E. huxleyi. The source of this calcite could be the coccolith waters occurring in the southern Barents Sea where a calcite concentration of >0.005 mol m−3 is typical for this area and season in recent years (Fig. 6, Smyth et al. 2004). In accordance with Hegseth and Sundfjord (2008), who found living specimens of E. huxleyi north-east of Svalbard, LM from stations 11 and 12 indicated the presence of E. huxleyi and corresponded with the presence of gyroxanthin-diester and Chl c 3. E. huxleyi is thus the most likely source when gyroxanthin-diester and Chl c 3 are detected in these waters and can possibly be used to confirm the presence of living specimens of this species in the high Arctic. This would be of interest for remote sensing applications tracing different pigment groups of phytoplankton by means of the next generation of hyperspectral remote sensing of ocean colour. Also, by verifying satellite data with in situ sampling and pigment investigation, existing satellite algorithms can be refined. E. huxleyi blooms are known for having potentially a great impact on the carbon flux, from the ocean–atmosphere interface all the way down to the sea floor. Improved pigment-specific remote sensing data on the extent and concentration of such blooms would provide a much needed foundation to estimate their carbon flux impact.
Calcite concentration (monthly mean August 2005, mol m−3) map of stations 11 and 12 where gyroxanthin-diester was detected indicating [coccoliths] from E. huxleyi and the water masses surrounding the Svalbard archipelago. Stations 11 and 12 were situated in AW with [Chl a] ranging between 1–4 mg Chl a m−3. Traces of gyroxanthin-diester (0.04–0.05 μg m−3) indicated that a small fraction of the biomass was related to E. huxleyi. The polar front between stations 9 and 10 is an approximation, as is the stipulated ice edge north of Svalbard. Data courtesy of white pixels contain no valid data, mostly due to clouds and ice cover. Feldman GC, McClain CR, Ocean Color Web, MODIS, NASA Goddard Space Flight Center. Eds. Kuring N, Bailey S. W, Accessed 16 Oct 2008
In order to confirm that E. huxleyi is a possible producer of gyroxanthin-diester, we reinvestigated older HPLC data from laboratory cultures of E. huxleyi. Gyroxanthin-diester had been detected in several of the old data; mostly in small concentrations, but in some samples significant concentrations were found (Fig. 3c). In a trial to recreate the result with high concentrations, cultures of E. huxleyi were grown under different light, nutrient and temperature conditions and sampled from early exponential to the decaying phase. After analysing with HPLC, gyroxanthin-diester was not found in these laboratory cultures. We do not suspect the cloned cultures from the older data to have been contaminated, as they are routinely checked. Over several years and thousands of generations in the laboratory, they may however have mutated several times, modifying the characteristics of the originally harvested cells. This might be one reason why we were not able to recreate the high concentration of gyroxanthin-diester as well as the possibility that we were not able to reconstruct the right environmental condition.
Pigment ratios in chlorophytes and chromophytes
In addition to Chl a, twelve light-harvesting pigments (LHC and Chls) were present in the field samples (Table 2). The ratio (w:w) of [Chl b]: [Chl a] for all the 12 sampling stations had a mean value of 0.28, indicating that a significant amount of phytoplankton biomass present was chlorophytes (PG 6–9, Table 3). Typically, in prasinophytes, the Chl b to Chl a ratio ranges from 0.39 in low light (LL) to 0.69 in high light (HL) (G. Johnsen unpublished). The AW Station 2 (Chl a max) and 4 (Chl a max) had especially high ratios of chlorophytes with Chl b to Chl a ratios of 0.92 and 0.53, respectively. The [Chl c]: [Chl a] ratio had a mean value at 0.37 (Table 3) indicating chromophytes (PG 1–5 and 10–12), but also here station 2 (Chl a max) had a high ratio (1.18) compared to the other stations. The [LHC]: [Chl a] had a mean value of 0.88 (Table 3), station 2 (Chl a max) had a ratio of 3.33 indicating high amounts of other light-harvesting pigments than Chl a. Finally, the [LHP]: [Chl a] varied from 0.70–5.37 with a mean value at 1.50 and mean CV of ±8% (Table 3), meaning that the concentration of light-harvesting pigments was 1.5 times higher than that of Chl a.
Based on the results from this study, we conclude that gyroxanthin-diester is potentially a new pigment marker for E. huxleyi in the high Arctic, where few or no other gyroxanthin-diester containing species normally exist. Further investigation is needed to conclude on this. Furthermore, the water masses surrounding the Svalbard archipelago are shown to have a diverse pool of phytoplankton classes. Atlantic water is the most diverse, in which all 13 phytoplankton pigment groups were detected, while the pigment markers for 7 phytoplankton groups were detected in Arctic water. In particular, significant amounts of Chl b-containing chlorophyceae, euglenophyceae and prasinophyceae I and II were identified by pigment chemotaxonomy in this study, indicating that the pigment group diversity of phytoplankton in the waters surrounding the Svalbard archipelago has previously been underestimated.
References
Berge J, Johnsen G, Nilsen F, Gulliksen B, Slagstad D (2005) Ocean temperature oscilliations enable reappearance of blue mussels Mytilus edulis in Svalbard after a 1000 year absence. Mar Ecol Prog Ser 303:167–175
Bjørnland T, Tangen K (1979) Pigmentation and morphology of a marine Gyrodinium (Dinophyceae) with a major carotenoid different from peridinin and fucoxanthin. J Phycol 5:457–463
Bjørnland T, Fiksdahl A, Skjtne T, Krane J, Liaaen-Jensen S (2000) Gyroxanthin- the first allenic acetylenic carotenoid. Tetrahedron 56:9047–9056
Bjørnland T, Haxo FT, Liaaen-Jensen S (2003) Carotenoids of the florida red tide Karenia brevis. Biochem Syst Ecol 31:1147–1162
Booth B, Horner RA (1997) Microalgae on the Arctic Ocean section, 1994: species abundance and biomass. Deep-Sea Res II 44:1607–1622
Booth B, Smith W (1997) Autothrophic flagellates and diatoms in the Northeast water polynya, Greenland: sumer 1993. J Mar Syst 10:241–246
Fiksdahl A (1983) Chemical and physical studies on marine carotenoids- chemosystematic contribution. Dissertation, University of Trondheim
Garcés E, Fernandez M, Penna A, Van Lenning K, Gutierrez A, Camp J, Zapata M (2006) Characterization of NW Mediterranean Karlodinium SPP. (Dinophyceae) strains using morphological, molecular, chemical and physiological methodologies. J Phycol 42:1096–1112
Hegseth EN, Sundfjord A (2008) Intrusion and blooming of Atlantic phytoplankton species in the high Arctic. J Mar Syst 74:108–111
Hovland EK (2007) Tracing coccolithophorid blooms in the Barents Sea using MODIS level-3 imagery. MSc thesis, University of Science and Technology, Trondheim
Ingvaldsen R, Loeng H (2009) Physical oceanography. In: Sakshaug E, Johnsen G, Kovacs K (eds) Ecosystem barents sea, 2nd edn. Tapir Academic Press, Trondheim, pp 35–66
Jeffrey SW, Mantoura RFC, Wright SW (1997) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO Publishing, Paris
Johnsen G, Sakshaug E (1993) Bio- optical characteristics and photoadaptive responses in the toxic and bloom-forming dinoflagellates Gyrodinium aureolum, Gymnodinium galatheanum, and two strains of Proroventrum minimum. J Phycol 29:627–642
Johnsen G, Sakshaug E (2007) Bio-optical characteristics of PSII and PSI in 33 species (13 Pigment groups) of marine phytoplankton, and the relevance for pulse- amplitude- modulated and fast- repetition-rate fluorometry. J Phycol 43:1236–1251
Johnsen G, Volent Z, Tangen K, Sakshaug E (1997) Time series of harmful and benign phytoplankton blooms in northwest European waters using the Seawatch buoy system. In: Kahru M, Brown C (eds) Monitoring Algal Blooms: New techniques for detecting large-scale environmental change, 1st edn. Springer Verlag, New York, pp 115–143
Johnsen G, Volent Z, Sigernes F, Sakshaug E (2009) Remote sensing in the Barents Sea. In: Sakshaug E, Johnsen G, Kovacs K (eds) Ecosystem barents sea, 2nd edn. Tapir Academic Press, Trondheim, pp 141–168
Lovejoy C, Vincent WF, Bonilla S, Roy S, Martineau M-J, Terrado R, Potvin M, Massana R, Pedrós-Alió C (2007) Distribution, phylogeny and growth of cold-adapted picoprasinophytes in arctic seas. J Phycol 43:78–89
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Millie DF, Kirkpatric GJ, Vinyard TB (1995) Relating photosynthetic pigments and in vivo optical density spectra to irradiance for the Florida red-tide dinoflagellate Gymnodinium breve. Mar Ecol Prog Ser 120:65–75
Millie DF, Schofield OM, Kirkpatric GJ, Johnsen G, Tester PA, Vinyard TB (1997) Detection of harmful algal blooms using photopigments and absorption signatures: a case study of the Florida red tide dinoflagellate, Gymnodinium breve. Limnol Oceanogr 42:1240–1251
Naustvoll L, Hansen J (2006) Fysikk (sirkulasjon, vannmasser og klima). In: Iversen S, Fossum P, Gjøsæter H, Skogen M, Toresen R (eds) Havets Ressurser og Miljø. IMR, Bergen, pp 28–30
Not F, Massana R, Latasa M, Marie D, Colson C, Eikrem W, Pedrós-Alió C, Vaulot D, Simon N (2005) Late summer community composition and abundance of photosynthetic picoeukaryotes in the Norwegian and Barents seas. Limnol Oceanogr 50:1677–1686
Örnólfsdóttir EB, Pinckney J, Tester PA (2003) Quantification of the relative abundance of the toxic dinoflagellate, Karenia Brevis (Dinophyta), using unique photopigments. J Phycol 39:457–499
Quillfeldt CH (1997) Distribution of diatoms in the Northeast water polynya, Greenland. J Mar Syst 10:211–240
Rodríguez F, Chauton M, Johnsen G, Andresen K, Olsen LM, Zapata M (2006) Photoacclimation in phytoplankton: implications for biomass estimates, pigment functionality and chemotaxonomy. Mar Biol 148:963–971
Sakshaug E, Kovacs K (2009) Introduction. In: Sakshaug E, Johnsen G, Kovacs K (eds) Ecosystem Barents Sea 2nd edn. Tapir Academic Press, Trondheim, pp 9–32
Silva PC, Throndsen J, Eikrem W (2007) Revisiting the nomenclature of haptophytes. Phycologia 46:471–475
Smyth TJ, Tyrrell T, Tarrant B (2004) Time series of coccolithophore activity in the Barents Sea, from twenty years of satellite imagery. Geophys Res Lett doi:10.1029/2004GL019735
Throndsen J, Kristiansen S (1991) Micromonas pusilla (Prasinophyceae) as part of picoplankton and nanoplankton communities of the Barents Sea. Polar Res 10:201–208
Throndsen J, Hasle GR, Tangen K (2003) Norsk Kystplankton Flora. Almater forlag AS, Norway
Zapata M (2005) Recent advances in pigment analysis as applied to picophytoplankton. Vie Et Milieu 55:233–248
Zapata M, Rodríguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed-phase C8 column and pyridine containing mobile phases. Mar Ecol Prog Ser 195:29–45
Acknowledgments
We thank Kjersti Andresen for assistance in the practical work with the HPLC and analysis of the results and Pål Christian Sandtrø for help with the figures. This work was financed by NTNU and Statoil in the Subsea hyperspectral imager project no: 40105400.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pettersen, R., Johnsen, G., Berge, J. et al. Phytoplankton chemotaxonomy in waters around the Svalbard archipelago reveals high amounts of Chl b and presence of gyroxanthin-diester. Polar Biol 34, 627–635 (2011). https://doi.org/10.1007/s00300-010-0917-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0917-6