Abstract
Key message
Sodium nitroprusside mediates drought stress responses in tomatoes by modulating nitrosative and oxidative pathways, highlighting the interplay between nitric oxide, hydrogen sulfide, and antioxidant systems for enhanced drought tolerance.
Abstract
While nitric oxide (NO), a signalling molecule, enhances plant tolerance to abiotic stresses, its precise contribution to improving tomato tolerance to drought stress (DS) through modulating oxide-nitrosative processes is not yet fully understood. We aimed to examine the interaction of NO and nitrosative signaling, revealing how sodium nitroprusside (SNP) could mitigate the effects of DS on tomatoes. DS-seedlings endured 12% polyethylene glycol (PEG) in a 10% nutrient solution (NS) for 2 days, then transitioned to half-strength NS for 10 days alongside control plants. DS reduced total plant dry weight, chlorophyll a and b, Fv/Fm, leaf water potential (ΨI), and relative water content, but improved hydrogen peroxide (H2O2), proline, and NO content. The SNP reduced the DS-induced H2O2 generation by reducing thiol (–SH) and the carbonyl (–CO) groups. SNP increased not only NO but also the activity of l-cysteine desulfhydrase (L-DES), leading to the generation of H2S. Decreases in S-nitrosoglutathione reductase (GSNOR) and NADPH oxidase (NOX) suggest a potential regulatory mechanism in which S-nitrosylation [formation of S-nitrosothiol (SNO)] may influence protein function and signaling pathways during DS. Moreover, SNP improved ascorbate (AsA) and glutathione (GSH) and reduced oxidized glutathione (GSSG) levels in tomato plants under drought. Furthermore, the interaction of NO and H2S, mediated by L-DES activity, may serve as a vital cross-talk mechanism impacting plant responses to DS. Understanding these signaling interactions is crucial for developing innovative drought-tolerance strategies in crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomatoes, a cornerstone of global agriculture, face production challenges due to drought stress, which is a critical factor limiting their yield and quality (Ahanger et al. 2021). Understanding the adaptive mechanisms of tomato plants to drought stress is therefore essential. In particular, the role of NO in enhancing drought tolerance presents a promising avenue for research, offering potential strategies to bolster resilience in this vital crop. Drought stress presents a substantial challenge to agriculture, especially in regions marked by limited water sources for irrigation, including arid and semi-arid regions (Samimi et al. 2020). Its potential for adverse impacts extends to plant growth, photosynthetic activity and fruit production, ultimately resulting in economic losses and diminished crop yields (Ahluwalia et al. 2021; Seleiman et al. 2021). The escalating global population and the consequences of global warming, including increasing temperatures and changing precipitation patterns, have contributed to a continuous reduction in the available water for agricultural crop irrigation (Islam and Karim 2019). DS can harm plants by disrupting the delicate balance between reactive oxygen species (ROS) and compensatory antioxidants (Razi and Muneer 2021), potentially leading to physiological imbalances, but antioxidants are involved in modulating ROS levels and safeguarding the plant against oxidative damage (Jahan et al. 2023).
Biostimulators exhibit promising capability to alleviate the adverse consequences of different stressors, especially water stress. They achieve this by fostering plant growth and elevating the enzymes’ activities linked to stress response pathways, ultimately enhancing plant tolerance to drought (Jafari et al. 2022; Choudhary et al. 2024). Nitric oxide (NO) has been specifically focused in this research paper to control and regulate drought and water stress in tomato plants due to its unique role as a biostimulator. NO is a pivotal signaling molecule that not only enhances plant tolerance to abiotic stresses but also actively participates in various stress responses, including drought stress (DS). Its ability to modulate antioxidant enzyme activities helps in mitigating oxidative damage caused by reactive oxygen species (ROS), which is crucial during DS (Rezayian et al. 2020). Furthermore, the interplay between NO and hydrogen sulfide (H2S) in regulating plant physiology has been gaining attention, as this interaction influences mutual production and impacts downstream signaling pathways, potentially leading to enhanced stress tolerance (Marcolongo et al. 2019; Sun and Li 2022). Therefore, the focus on NO in this study is aligned with the current research trajectory that seeks to understand and harness its multifaceted role in improving plant resilience to drought, which is increasingly important in the context of global water scarcity and climate change (Seleiman et al. 2021).
The post-translational modifications (PTMs) induced by NO, such as S-nitrosylation, are essential for altering protein function and regulating stress responses, highlighting the sophisticated regulatory system of NO during stress conditions (Kour et al. 2023). S-nitrosylation, a critical PTM induced by nitric oxide (NO), plays a vital role in plant responses to drought stress (Nabi et al. 2019). This PTM involves the addition of an NO group to the thiol group of cysteine residues in proteins, leading to the formation of S-nitrosothiols (SNOs). S-nitrosylation can alter protein function, stability, and interaction with other biomolecules, thereby regulating a wide range of physiological processes (Wei et al. 2020). Additionally, S-nitrosylation influences the antioxidant defense system by modulating the activity of enzymes that scavenge ROS, thus protecting plants from oxidative damage caused by drought stress (Zhang and Liao 2022; Samanta et al. 2023). Numerous proteins are targeted by both NO and H2S to stimulate PTMs, including S-nitrosylation and persulfidation resulting from oxido-nitrosative processes (Wang et al. 2021). Notably, the interaction between NO and ROS contributes to dynamic oxido-nitrosative processes. This intricate network has been reported to involve the –CO group in protein carbonylation and regulatory enzymes such as GSNOR and NOX (Valderrama et al. 2019). The significance of S-nitrosylation in this research article lies in its potential to enhance the understanding of NO-mediated signaling pathways and their role in improving plant tolerance to drought stress.
While NO’s role as a signaling molecule in enhancing plant tolerance to various abiotic stresses has been well-documented (Gupta and Seth 2020, 2023; Mariyam et al. 2023; Prajapati et al. 2023), its specific effect on oxidative and nitrosative processes under drought stress in tomato plants remains less explored. Previous studies have highlighted the importance of NO in drought stress signaling and tolerance in plants, emphasizing its role in activating ROS scavenging enzymes and regulating antioxidative systems (Chavoushi et al. 2019; Jafari and Shahsavar 2022). However, the intricate mechanisms by which NO modulates oxidative and nitrosative processes, particularly in tomato plants under drought stress, have not been thoroughly investigated. Building on this, our study explores the cross-talk between NO and hydrogen sulfide (H2S), mediated by l-cysteine desulfhydrase (L-DES) activity, as a novel area of investigation. This cross-talk is proposed as a crucial mechanism impacting plant responses to drought stress. The interaction between NO and H2S may lead to modifications in protein function and signaling pathways, which are essential for the plant’s adaptation to water scarcity. The role of L-DES in mediating the production of H2S from l-cysteine has been recognized as a significant factor in plant stress physiology (Shen et al. 2019; Muñoz-Vargas et al. 2022). Furthermore, the interplay between NO and H2S has been shown to involve various post-translational modifications, including S-nitrosylation and persulfidation, which affect protein activity and cellular signaling under stress conditions (Aroca et al. 2018; Singh et al. 2022). These modifications are crucial for the regulation of stomatal closure, antioxidant defense, and hormonal signaling, which collectively contribute to the plant’s drought tolerance (Lau et al. 2021; Yasir et al. 2023).
Materials and methods
Cultivation practices and treatments
Tomato (Lycopersicum esculentum) cultivar “SC 2121” were grown in the greenhouse. The seeds of the tomato cultivar ‘SC 2121’ were sourced from Connect Anadolia seed company, based in Izmir, Turkey. This variety was selected for its early growth, suitability for field cultivation and table consumption, and desirable characteristics such as being round, red, and thin-shelled. After being surface sterilised with 1% sodium hypochlorite solution (v/v), tomato seeds were placed in a dish composed of cleaned sand and given water each day to germinate. The greenhouse maintained optimal environmental settings for the tomato, with nighttime temperatures within the range of 12–15 °C and daytime temperatures ranging between 20 and 30 °C. Throughout the experiment, the light period consistently lasted about 11 h, and the relative humidity was maintained between 60 and 70%. One week after germination and before stress treatment, the control (C; no PEG application) and DS seedlings were subjected to a pretreatment with 0.2 mM sodium nitroprusside (SNP), which is an NO donor. In addition, 0.1 mM cPTIO (NO scavenger) was also supplied through 10% Hoagland nutrient solution for 24 h. After 24 h of pretreatment with NO/cPTIO, to induce drought stress conditions mimicking drought stress in relevant groups of seedlings, DS seedlings were treated with a nutrient solution diluted by half, supplemented with 12% PEG for 48 h. This osmotic stress limits plants' ability to absorb water, modifying water deficiency stress. Then both control (C, no PEG application) and DS-plants were further grown in half-strength NS for 10 days.
Our study employed a randomized full-block design with three replications to assess the effects of drought stress on plant growth. Each replication consisted of three seedlings planted in individual 5 L containers. These containers were filled with a half-strength Hoagland nutrient solution to ensure consistent nutrient availability.
The details of these treatments are summarized in Fig. 1A, which is included to enhance comprehension of the experimental design. The treatments were as follows:
-
1.
Control Group: Seedlings were treated with a mock solution to serve as a baseline for comparison.
-
2.
SNP Treatment Group: Seedlings were subjected to the Control group and treated with sodium nitroprusside (SNP), a known donor of NO.
-
3.
SNP + cPTIO Treatment Group: Seedlings experienced Control group and received a combination of SNP and cPTIO, scavenger of NO.
-
4.
Drought Stress Group: Seedlings underwent drought conditions and were also treated with the mock solution.
-
5.
SNP Treatment Group: Seedlings were subjected to drought stress and treated with sodium nitroprusside (SNP), a known donor of NO.
-
6.
SNP + cPTIO Treatment Group: Seedlings experienced drought stress and received a combination of SNP and cPTIO, scavenger of NO.
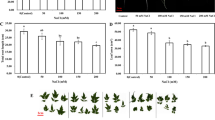
A Experimental protocol overview: One week post-germination, control (C) and drought-stressed (DS) seedlings received pretreatment with 0.2 mM sodium nitroprusside (SNP) and 0.1 mM cPTIO (NO scavenger) for 24 h. Subsequently, DS seedlings were exposed to 12% PEG for 48 h to induce drought stress, while control seedlings remained untreated. Both groups were then maintained in half-strength nutrient solution for 10 days. B, C Shoot (B) and root (C) dry matter (DM) measured in phenotyping experiments. NS stays for “not sprayed” (e.g.—the mock control; plants sprayed with distilled water). SNP- plants pretreated with 0.2 mM of sodium nitroprusside; cPTIO – plants pretreated with a scavenger of nitric oxide. Data is mean ± S.E. (n = 6–8). Bars assigned different low-case letters indicate a statistically significant difference at P ≤ 0.05
Each treatment was replicated three times, and within each replicate, there were three pots, each containing three plants. This resulted in a total of nine plants per treatment across all replicates. The pH level of the NS was adjusted to 5.5 using a small amount of solution of KOH (0.01 M).
At the end of the 10-day treatment period, three plants selected, one from each replicate, for each treatment were harvested to measure their dry weight. The plants were allowed to air-dry before undergoing a 10-min exposure to 105 °C in an oven before being maintained at 72 °C for an extra 72 h to achieve a constant dry weight. The two additional plants in each replicate (resulting in six plants per treatment) were uprooted to quantify the parameters listed below:
Measurement of chlorophylls and chlorophyll fluorescence
For chlorophyll measurement in plant samples, we employed the Strain and Svec (1966) method. This technique entails pigment extraction using a solvent, followed by absorbance measurement with a spectrophotometer. Homogenization of 1 g of leaf tissue was carried out in 5 mL of 90% acetone. The extracts were maintained in sealed tubes after filtration. Concerning chlorophyll a and chlorophyll b, corresponding absorbance measurements were recorded at 663.5 nm and 645 nm using a spectrophotometer, and chlorophyll concentrations were determined using the formula outlined by Lichtenthaler and Wellburn (1983).
The leaves of tomato underwent standard light and dark periods, a widely used technique for assessing levels of chlorophyll fluorescence. Our analysis benefited from the precise and reliable data provided by the MINI-PAM-II Photosynthesis Yield Analyzer (Model No. PYAA0664), manufactured by Heinz Walz GmbH, Germany, which features a 735 nm LED and PAR sensor among its advanced functionalities. Before acquiring the fluorescence measurements, the leaves were placed in the absence of light for thirty minutes. This darkness period was crucial for determining the maximum quantum efficiency of PSII, denoted as Fv/Fm. This specific value holds significance in the analysis of photosynthetic activity, representing the efficiency of energy conversion into chemical energy during photosynthesis in plants.
Quantification of leaf water potential (ΨI) and determination of relative water content (RWC)
The ΨI of the third uppermost leaf was measured utilizing a pressure chamber, adhering to standard protocols and employing a ΨI measuring device (USA-made PMS model 600). The process comprises the step of fixing a leaf from the plant onto a chamber and subsequently sealing it securely. The pressure is systematically raised until the first appearance of water droplets at the tip of the petiole. The corresponding pressure level recorded by the pressure chamber at this juncture is recorded as the ΨI of the leaf.
The RWC was computed using the Barrs and Weatherley (1962) technique. Initially, fresh leaves from the corresponding part of the plant were swiftly weighed to determine their FW (fresh weight). Subsequently, these leaves underwent a one-day incubation in vials filled with distilled water. Once the leaves were removed from the vials, the removal of any surface water was accomplished by gently wiping it off employing a paper towel to absorb excess moisture, and the leaf TW (turgid weight) was determined. The subsequent procedure included dehydrating the samples consistently in an oven set at 65 °C to determine the leaf DW (dry weight). The equation used for estimating the Relative Water Content (RWC) as per Barrs and Weatherley (1962) is as follows:
Processing and analysis of thermal images
Infrared imaging through a FLIR T540 thermal camera was utilized to monitor the plant canopy temperatures at their warmest during the daytime. The thermal camera, positioned on a tripod and calibrated for emissivity, recorded images of the complete canopy. Utilizing FLIR Tools software, the images underwent processing to quantify temperatures, and subsequently, the average temperature readings were computed. In order to guarantee a sample representative of the canopy, in total, eight readings were recorded, with two readings collected from every facets of the plant.
Determination of free proline content
To determine the leaf-free proline concentration, we followed the experimental approach detailed according to Bates et al. (1973) methodology. The procedure included extracting a half g leaf sample with a 3% of 10 mL sulfosalicylic acid solution. Next, a 2 mL portion of the filtrate was combined with 2 mL each of 2.5% acid-ninhydrin (w/v) and 60% glacial acetic acid (v/v) solutions. The resulting reaction solution was then subjected to heating for an hour in a water bath with a temperature set to 100 °C, following that, it was permitted to cool down. The cooled solution was supplemented with 4 mL toluene, and the mixture’s absorbance was subsequently measured at 520 nm. Subsequently, the absorbance data was employed to calculate free proline concentration. This quantification was achieved by referencing a standard graph constructed with known quantities of proline added.
Hydrogen peroxide (H2O2) content
The H2O2 concentration was calculated using the Sergiev et al. (1997) protocol. Centrifugation of the fresh samples was performed at 12,000×g at 4 °C for 15 min after being homogenised in TCA (0.1%). A 1 M potassium iodide, which is produced in phosphate buffer, was used to treat the supernatant. The mixture's absorbance was noted at 390 nm. This reading was then employed to compute the H2O2 concentration, referring to a standard graph constructed using established H2O2 concentration.
The extraction of protein
To measure the protein concentration, the Bradford (1976) procedure was employed. After being powdered using liquid nitrogen, the samples underwent homogenization in a 100 mM Tris–HCl extraction buffer (pH 8.0) prepared in a solution of 10% glycerol (v/v), 5 mM DTT, 1 mM EDTA, and 0.02% Triton X-100 (v/v). This extraction buffer helps to maintain the integrity of the proteins and prevent their denaturation during the extraction process. Subsequently, the supernatant, representing the liquid fraction, was carefully extracted after centrifugating at 17,000g for 20 min at 4 °C, effectively separating it from the cellular remnants. Following centrifugation, the supernatant was acquired for evaluating the protein concentration, along with other assessments of enzyme activity, protein carbonyl groups (–CO). and thiol groups (–SH). To determine the protein concentration, the Bradford (1976) method was used, which relies on the interaction with Coomassie Brilliant Blue dye to the protein molecules, leading to a shift in the dye’s absorbance spectrum. The sample optical density was recorded through absorbance measurements at 595 nm, and the protein concentration was computed using a calibration plot generated using known bovine serum albümin concentrations.
The quantification of protein –CO and –SH groups
For protein –CO content measurement in the extracted proteins, a solution comprising 6 M guanidine and 10 mM DNPH was formulated in 2 N HCl as solvent. The protein extract was subsequently subjected to incubation in this solution. Spectrophotometric analysis at a wavelength of 480 nm was employed for calculating the –CO contents, following the method detailed in the study by Levine et al. (1994). To determine the levels of –SH in the protein extracts, the increment in absorbance at 412 nm due to DTNB reduction in 0.05 M Tris–HCl (pH 8) was measured. This procedure was carried out following the approach detailed in the study by Ellman (1959).
Analysis of enzyme activity
The SOD activity was determined by the degree to which it inhibited the photoreduction of NBT (Van Rossum et al. 1997). The quantity of needed enzyme to achieve a 50% reduction in cytochrome c levels was quantified as SOD activity. The technique introduced by Fielding and Hall (1978) was used to determine the GPX activity. Its activity was evaluated by measuring absorbance increase at 470 nm due to the polymerization of guaacol into tetragacol when H2O2 is present. Spectrophotometer was used to measure the GSNOR activity at 25 °C by monitoring the NADH oxidation at 340 nm (Barroso et al. 2006). The initiation of the enzymatic process commenced with the addition of GSNO into the reaction mixture. Enzyme activity measurement relied on the utilization of NADH, enabling the measurement of its activity through the rate of either NADH consumption or NADH production. The assessment of NOX activity was conducted according to the principle of NADPH oxidation leading to the formation of NADP + , with absorbance at 340 nm. The NOX activity was assessed through tracking the decline in absorbance at 340 nm, as outlined by Ishida et al. (1987).
Measurement of NO and S-nitrosothiol (SNO)
Adhering to the procedure outlined by Kaur et al. (2015), the Griess reagent was employed to assess the NO concentration in a solution of 5% phosphoric acid. Fresh samples were homogenized and then underwent centrifugation at 12,000g at 4 °C for 10 min. To ascertain the NO concentration, the Griess reagent-supernatant mixture underwent a 10-min incubation at room temperature, shielded from light. During this incubation period, the Griess reagent was permitted to undergo a reaction with NO, producing a colored compound measurable with spectrophotometer. Post-incubation, the absorbance of the resulting solution was recorded at 540 nm. To quantify NO concentration, a calibration curve was generated using NaNO2.
The extraction of SNO was carried out following the methodology detailed by Gow et al. (2007). Leaf material was extracted using 50 mM phosphate buffer (pH 7.2) with the addition of 80 mM S-methyl methanethiosulfonate. Following centrifugation at 20,000g for 20 min at 4 °C, the resultant solutions were collected and mixed with cold acetone. Subsequent centrifugation and a 60-min incubation at 24 °C were performed before re-suspending the recovered pellets in the extraction buffer. The measurement of SNO was then carried out following the procedure outlined by Saville (1958). For a duration of 20 min, a 0.3 mL protein extract sample was combined with a solution consisting of 3.4% sulfanilamide (prepared in 0.4 M HCl) and 0.1% N-(1-naphthyl) ethylenediamine. This solution selectively blocked the reaction of specific –SH groups, facilitating the measurement of distinct subsets of –SH groups. Following a 20-min incubation at ambient temperature, the absorbance reading at 540 nm was recorded.
Measurement of AsA and GSH contents
For the analysis of AsA and GSH, a half g fresh leaf sample was utilized. The extraction process involved a 3 mL cold solution consisted of 5% meta-phosphoric acid and 1 M EDTA. After centrifugation, measurements for AsA and GSH were performed following the procedure detailed by Huang et al. (2005). The measurement of reduced glutathione (GSH) and glutathione disulfide (GSSG) levels utilized a procedure detailed by Yu et al. (2003). After neutralizing the sample solution with a 500 mM K-phosphate buffer, the GSH level was determined at 412 nm by monitoring changes in optical density values resulting from the reduction of 5,5'-dithiobis (2-nitrobenzoic acid). GSSG concentration was calculated by subtracting the 2-vinyl pyridine content from the GSH content.
Statistical analysis
A one-way ANOVA was conducted on the data to assess potential significant differences among various parameter settings. Mean values and standard errors were calculated and are visually illustrated in the figures. The determination of statistical significance for observed differences adhered to a significance level of 0.05%.
Results
SNP promotes plant growth under DS
Exposure to PEG-induced DS resulted in a significant reduction (P ≤ 0.05) of shoot and root dry mass of plants by 37% and 58%, respectively, in comparison to untreated control plants. However, application of 0.2 mM SNP, a donor of NO, significantly improved plant growth-related variables by 28% and 98% for shoot and root dry mass, respectively, when compared to plants experiencing DS alone (Fig. 1B, C), but the SNP-related results on improved plant growth parameters remained lower than those in C-plants.
To assess whether nitric oxide (NO) produced by SNP (sodium nitroprusside) had a mitigating effect on drought stress, we combined SNP treatment with the NO scavenger, cPTIO, in settings with normal conditions and drought stress. The addition of 0.1 mM cPTIO along with SNP treatment resulted in the loss of the positive effects of SNP on plant triats in drought-stressed plants. No changes in these characteristics were observed in control plants due to different treatments.
SNP promotes photosynthesis-related parameters under DS
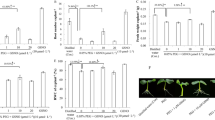
The levels of chlorophyll a and b, total chlorophyll, and photosystem II efficiency (Fv/Fm) were significantly reduced (P ≤ 0.05) in the DS tomato seedlings. Specifically, in comparison of C plants, the concentrations of chlorophyll a and b decreased by 54% and 30%, respectively. Furthermore, the total chlorophyll concentration and Fv/Fm in DS plants were decreased by 6142% and 27%, respectively. However, SNP treatment led to significant elevations in these photosynthesis-related parameters, with 72%, 39%, 56%, and 34% in chlorophyll a and b, total chlorophyll, and Fv/Fm, respectively (Fig. 2A–D), compared to plants exposed to DS. The administration of cPTIO reversed the positive impacts of SNP on these traits under drought conditions by scavenging NO, thus demonstrating that SNP increases the levels of NO, acting as a donor of NO to enhance these physiological responses in plants. Under control conditions, plants did not show any differences in these traits resulting from the various treatments.
Leaf photosynthetic characteristics of pepper plants under control and PEG-stimulated drought conditions. A, B Chlorophyll a and b content; C Total chlorophyll content; D Chlorophyll fluorescence Fv/Fm ratio. NS = mock control (plants sprayed with distilled water). SNP- plants pretreated with 0.2 mM of sodium nitroprusside; cPTIO – plants pretreated with a scavenger of nitric oxide. Data is mean ± S.E. (n = 6–8). Bars assigned different low-case letters indicate a statistically significant difference at P ≤ 0.05
Exogenous SNP treatment enhances proline content, transpirational cooling and improves leaf water relations
Plants affected by drought exhibited wilting signs (Fig. 3A), which were not observed in plants treated with SNP. The positive effect of SNP on alleviating wilting symptoms of DS was nullified by pretreatment with cPTIO. An infrared image was utilized for measuring the canopy temperatures of tomatoes that were subjected to various treatments. Compared to the control treatment, drought significantly raised leaf temperatures from 29.3 to 31.9 °C. However, SNP application significantly lowered the canopy temperature of DS plants to 29.9 °C (Fig. 3A), indicating an improvement in transpiration-induced cooling. However, the positive effect of NaHS in reducing canopy temperature was nullified by cPTIO, which is a scavenger of NO. This finding suggests that the lower canopy temperature observed with SNP treatment is possibly attributed to the NO application, which enhances the plants' ability to tolerate DS.
The water relations of tomato plants under PEG-stimulated drought conditions. A Plant phenotype and leaf temperature. B Leaf relative water content (RWC). C Leaf water potential (Ψl). NS = mock control (plants sprayed with distilled water). SNP- plants pretreated with 0.2 mM of sodium nitroprusside; cPTIO – plants pretreated with a scavenger of nitric oxide. Data is mean ± S.E. (n = 6–8). Bars assigned different low-case letters indicate a statistically significant difference at P ≤ 0.05
DS decreased tomato leaf RWC and ΨI to 35% and 125%, respectively, but increased the proline content of tomato plants to 120% (Figs. 3B–C, 4A). SNP, a NO donor, was employed to test whether exogenous NO is capable of altering how tomato seedlings respond to water deficiencies. When compared to drought-stressed plants alone, SNP elevated leaf RWC, ΨI and proline by 41%, 37% and 39%, respectively. None of the treatments had an effect on the C-plants. Plants supplied with cPTIO, an NO scavenger, together with SNP abolished the benefits of SNP on those traits. SNP produces NO and increases leaf water relation and proline while scavenging NO renders SNP ineffectual in increasing leaf water status.
Biochemical modifications in tomato plant under PEG-stimulated drought conditions. A Proline content; B Nitric oxide content (NO); C Hydrogen sulphide (H2S); D l-cysteine desulfhydrase (L-DES); E S-nitrosothiol (SNO) on fresh weight (FW) basis; F S-nitrosoglutathione reductase (GSNOR). NS = mock control (plants sprayed with distilled water). SNP- plants pretreated with 0.2 mM of sodium nitroprusside; cPTIO – plants pretreated with a scavenger of nitric oxide. Data is mean ± S.E. (n = 6–8). Bars assigned different low-case letters indicate a statistically significant difference at P ≤ 0.05
SNP modulates H2S, L-DES and nitrosative potential: crosstalk between H2S and NO signaling
The observed increases in endogenous NO and H2S levels, along with L-DES activity in drought-stressed plants treated with SNP. This suggests a synergistic action where NO may enhance H2S synthesis, contributing to the overall stress response. The elevation of endogenous NO and H2S, both signaling molecules, indicates their role in activating stress-responsive pathways. (Fig. 4B–D). Conversely, the reduction in SNO content and GSNOR activities upon SNP treatment alone suggests that NO signaling is tightly regulated with S-nitrosylation during stress responses. Under DS, the combined treatment of cPTIO and SNP increased the SNO content and GSNOR activity while decreasing the NO and H2S generation, as well as L-DES activity (Fig. 4E, F), highlighting the complexity of NO signaling and its interaction with H2S. This indicates that the presence of cPTIO alters the balance of NO and H2S, potentially through the modulation of S-nitrosylation processes. In contrast, under control conditions, the SNP application elevated the NO content, while the combined treatment of cPTIO and SNP decreased it. The H2S content, L-DES activity, SNO concentration, and GSNOR activity in control plants were not affected by any treatment, indicating the specificity of the responses observed under drought stress conditions and highlighting the complex regulatory mechanisms governing NO and H2S signaling pathways in tomato plants.
This intricate crosstalk between NO and H2S is crucial for the regulation of plant physiological processes, particularly under stress conditions. The mutual influence on each other’s production and the downstream signaling pathways they impact are central to the plant’s ability to adapt and survive under adverse conditions.
SNP modulates antioxidant enzymes and oxidative stress markers
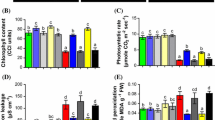
The GPX, NOX, and SOD activities and H2O2 levels exhibited a significant elevation in response to DS (Fig. 5A–D). In contrast to plants exposed to drought alone, treatment with SNP significantly (P ≤ 0.05) decreased NOX, SOD, and H2O2 levels by 41%, 27%, and 33%, respectively, but dramatically increased GPX activity by 49%. No notable changes in these parameters were noted in the control groups due to the different treatments. Scavenging NO buildup with cPTIO pre-treatment counteracted the effects of SNP on these traits.
Activities of key antioxidant enzymes and redox characteristics of tomato plants under control or PEG-stimulated drought conditions. A Glutathione peroxidase (GPX); B NADPH oxidase (NOX); C Superoxide dismutase (SOD); D Hydrogen peroxide (H2O2) on fresh weight (FW) basis; E Thiol (–SH); F Carbonyll (–CO). i NS = mock control (plants sprayed with distilled water). SNP- plants pretreated with 0.2 mM of sodium nitroprusside; cPTIO – plants pretreated with a scavenger of nitric oxide. Data is mean ± S.E. (n = 6–8). Bars assigned different low-case letters indicate a statistically significant difference at P ≤ 0.05
The levels of carbonyl (–CO) and thiols (–SH) were considerably increased by drought stress, but treatment with SNP reduced these levels (Fig. 5E, F). The control plants showed no significant changes in these traits when subjected to the different treatments. However, pre-treatment with cPTIO reversed the effects of SNP on these traits. The control plant variables remained unchanged following the different treatments.
SNP improves non-enzymatic antioxidants, and reduces oxidized glutathione under drought
Drought stress dramatically boosted leaf AsA and GSH concentrations, with a 134% increase in AsA and an 83% elevation in GSH compared to C plants (Fig. 6A, B). Additionally, supplementation with SNP further elevated AsA and GSH levels by 42% and 28%, respectively, compared to the levels observed in plants exposed to drought conditions. This shows that SNP may protect plants against oxidative stress due to DS.
Non-enzymatic antioxidants and oxidized glutathione of tomato plants under control or PEG-stimulated drought conditions. A Ascorbate (AsA); B Reduced glutathione (GSH); C Oxidized glutathione (GSSG) on fresh weight (FW) basis; D GSH/GSSG ratio. NS = mock control (plants sprayed with distilled water). SNP- plants pretreated with 0.2 mM of sodium nitroprusside; cPTIO– plants pretreated with a scavenger of nitric oxide. Data is mean ± S.E. (n = 6–8). Bars assigned different low-case letters indicate a statistically significant difference at P ≤ 0.05
Drought stress increased the quantity of oxidized glutathione (GSSG) by 226% when compared to C plants (P ≤ 0.05) (Fig. 6C). However, the use of SNP led to a notable reduction in GSSG content when compared to plants experiencing drought stress alone. Drought stress decreased the GSH/GSSG ratio in plants (Fig. 6D), but SNP application increased the ratio, indicating a potential positive effect on redox balance. The cPTIO fully reversed the increase in AsA and GSH caused by SNP under DS. This suggests that SNP provides NO, which in turn enhances AsA and GSH concentrations while reducing the concentration of GSSG. These results suggest that the defensive impacts of SNP on plants under drought stress may be attributed to this potential mechanism. Other treatments, on the other hand, had no effect on these compounds in plants that were not under stress, indicating that the observed alterations in these compounds were specific to the plant response to DS and the application of the treatments.
Discussion
SNP improves critical growth traits under DS
Drought stress has the potential to disturb the water balance in plants, leading to compromised physiological processes such as growth, leaf water status, and oxidative stress (Hosseini et al. 2019; Saja-Garbarz et al. 2021; Wahab et al. 2022). In response to DS, plants employ adaptive strategies, including the production of osmolytes like proline, to facilitate enhanced water uptake from the root zone, as proposed by Hosseinifard et al. (2022). In the face of DS, our study uncovered a notable decline in both leaf RWC and water potential (Ψl) among the affected plants. In response to drought conditions, there was a notable increase in proline content, indicating the plant’s effort to sustain its water status (Pandey and Shukla 2015; Hanif et al. 2021). However, the introduction of SNP resulted in enhanced leaf Ψl, RWC, and proline content of drought-stressed plants. This suggests that SNP has the potential to ameliorate water content in plants experiencing drought stress. Consistent with our findings, prior studies have indicated that SNP-induced alterations in osmoregulation in marjoram herb contributed to heightened proline content and improved water potential (Farouk and Al-Ghamdi 2021). One potential explanation for the observed improvement in plant growth following treatment with SNP under drought stress, as proposed by Faraji and Sepehri (2020) in wheat plants, is that SNP enhances proline content, this improves the plant water status under DS.
To clarify the function of NO in the increased DS tolerance of tomato treated with SNP, the plants received treatment with a NO scavenger, specifically cPTIO. The results showed that the cPTIO application inverted the beneficial impacts of SNP on plant growth under drought stress, indicating that NO generated by SNP is essential for the improved DS tolerance of tomato plants. The findings also show that the observed enhancement in plant growth induced by SNP during drought stress may be due to NO accumulation. Numerous studies have used cPTIO to elucidate the role of NO in boosting stress tolerance. For instance, Kataria et al. (2020) and Wei et al. (2022a) demonstrated the involvement of NO in improving the tolerance of soybean and tomato to salt stress by using cPTIO as a NO scavenger. Therefore, the use of cPTIO in this study is consistent with previous research and helps to confirm the role of NO in the observed enhancement in drought tolerance of tomato plants treated with SNP.
SNP enhances photosynthesis-associated traits under drought
In the presence of drought stress, crucial parameters for photosynthesis in tomato plants, such as Fv/Fm and chlorophyll content, experienced a negative impact. This aligns with prior studies that have consistently reported comparable reductions in chlorophyll content and Fv/Fm in various plant species facing drought conditions (Badr and Brüggemann 2020; Larouk et al. 2021). The imposition of DS can result in elevated levels of the enzyme chlorophyllase, subsequently impeding chlorophyll production (Altuntaş et al. 2020); this phenomenon may be a contributing factor in the observed decrease in chlorophyll levels under DS.
However, in the current study, the SNP treatment positively influenced photosynthetic attributes while mitigating the accumulation of H2O2, a potential threat to photosynthetic machinery. These outcomes align with previous research showing the beneficial impact of SNP on photosynthetic traits in wheat plants subjected to drought (Faraji and Sepehri 2020).
Under DS, SNP application in tomato plants acted as a donor of NO, resulting in a significant rise in NO content. The application of SNP in tomato plants subjected to DS resulted in a notable elevation in NO content, which is involved in promoting plant growth and enhancing stress tolerance. This was substantiated by the enhanced Fv/Fm and chlorophyll content, indicating the beneficial influence of NO on photosynthetic efficiency and overall plant health. To verify the involvement of NO in these beneficial effects, cPTIO, a NO scavenger, was administered alongside SNP. The findings showed that the cPTIO application inverted the beneficial effects of SNP on increases in Fv/Fm and chlorophyll content, providing more proof regarding NO’s participation in the action mechanism of SNP in enhancing plant growth and tolerance to DS.
SNP controls nitrosative stress in DS conditions
The tomato plants that were fed with SNP showed significant increases in NO and H2S concentrations. This demonstrates how NO activates H2S as a downstream signal mediator in declining oxidative stress caused by drought. This boosts the antioxidant defense system, hence increasing the tomato plant’s tolerance to drought. Under drought, NO was observed to build up in various plants, including Lotus japonicas (Signorelli et al. 2013), and maize (Majeed et al. 2020). Likewise, the accumulation of H2S has been documented in wheat (Li et al. 2021) and tomato (Siddiqui et al. 2021) under DS. Numerous studies have indicated that DS increases ROS formation and oxidative stress (Sohag et al. 2020; Zou et al. 2021). In the face of oxidative stress, plants can naturally generate both NO and H2S as components of their strategy for safeguarding against stress (Zhu et al. 2018). These signaling molecules have been demonstrated to safeguard plants against oxidative stress and to promote plant survival during drought conditions (Li et al. 2016; Antoniou et al. 2020). By upregulating the levels of NO and H2S levels, plants can initiate their antioxidant defense system, stimulate stress-induced gene expression, and modulate stomatal closure. These mechanisms are involved in reducing the adverse impacts of oxidative stress and other environmental stressors (Bhuyan et al. 2020; Gautam et al. 2022). Through the implementation of these adaptive strategies, plants enhance their ability to withstand and recover from various environmental stressors, thereby contributing to improved overall growth and survival.
Furthermore, SNP treatment during drought induced an increase in NO buildup and H2S synthesis. Exogenous SNP enhances NO buildup in safflower (Chavoushi et al. 2020) and maize (Majeed et al. 2020) under water stress. In addition, in a study by Alamri et al. (2020), it was noted that exposure to hazardous amounts of chromium significantly elevated the activity of L-DES and D-cysteine desulfhydrase (D-CD), leading to an elevation in H2S production in tomato plants. Similarly, in our investigation, the SNP application also enhanced the H2S content by enhancing the activity of L-DES. Both findings indicate that plants H2S production can be stimulated by various stressors, including Cr(VI) toxicity and DS, and that this production can be further elevated due to SNP application. These two signalling molecules have been shown to have overlapping and sometimes antagonistic functions in facilitating plant adaptation to abiotic stress (Kharbech et al. 2020b).
Crucially, it should be acknowledged that NO and H2S, along with their derivatives, have the capability to interact and modulate the signaling pathways of one another. This underscores the possibility of inter-molecular crosstalk and Its possible repercussions on downstream signaling processes (Singh et al. 2020). Numerous pertinent target proteins associated with these signaling molecules are susceptible to PTMs, including S-nitrosylation and persulfidation. These modifications alter the functioning of proteins, introducing a layer of complexity to cellular processes influenced by NO and H2S. (Corpas et al. 2021; Corpas and Palma 2023). The interplay between H2S and ROS/RNS in DS conditions holds considerable impact on plant growth and ability to thrive. H2S has demonstrated the ability to eliminate ROS/RNS, mitigating their detrimental impacts on plant cells (de Bont et al. 2022). A comprehensive understanding of the crosstalk between these molecular signals and their influence on PTMs can offer valuable perspectives on the intricate regulatory networks modulating plant stress responses.
S-nitrosylation is a prevalent cellular communication process involving reversible PTMs (Shi and Qiu 2020). Under drought conditions, there was a notable rise in the cellular SNO concentration in tomato, suggesting the presence of S-nitrosylation on R-SH, specifically targeting protein thiols. This finding aligns with observations made by Wang et al. (2022) and Wei et al. (2022b) in tomato plants subjected to salinity stress. However, SNP resulted in a decrease in SNO levels and an increase in NO concentrations in drought-exposed leaves, corroborating findings noted in tomato plants subjected to salt stress (Wang et al. 2022; Wei et al. 2022b). This complex interaction between SNO and NO signaling pathways in governing plant responses to DS indicates a nuanced regulatory mechanism. Various PTMs are involved in facilitating NO’s physiological functions, with protein S-nitrosylation, specifically the formation of an SNO through covalent bonding to the cysteine residue's reactive thiol group within a protein, being a prominent PTM (Wei et al. 2022b).
An important observation is that the NO generated through SNP application has the capacity to form covalent bonding between NO-derived species and other molecules, such as SNOs, influencing diverse plant signal transduction pathways and cellular activities (Sun et al. 2021). SNOs, originating from NO, tend to build up in plants during DS conditions, related to raised levels of oxidative and nitrosative damage (Corpas 2017). However, studies have shown that SNP can decrease SNO content in stressed-plants. For instance, Khan et al. (2019) reported that soybean plants under flooding stress and received SNP treatment showed a decline in SNO level. Furthermore, Kharbech et al. (2020a) reported a decrease in SNO level in maize plants that received SNP treatment under chromium stress. The decrease in SNO levels noted in plants that received SNP treatment under DS conditions might be ascribed to the interaction between H2S and SNOs. H2S has the ability to react with SNOs, forming substances, such as thionitrous acid, capable of decomposing into elemental sulfur and NO (Yuan et al. 2015; Benchoam et al. 2019). This reaction can lead to the transformation of SNOs into NO, consequently reducing the concentration of SNOs in the plant.
Drought stress induced an elevation in the GSNOR activity, an enzyme responsible for degrading SNOs and regulating their concentrations in plant cells. Similar observations have been documented in poplar plants exposed to chilling (Cheng et al. 2015) and heat stress (Cheng et al. 2018). The homeostasis of NO and SNOs is remarkably governed by GSNOR, which is elaborated in controlling actions mediated by reactive nitrogen species (RNS) (Badiani et al. 2022). In this study, DS-induced GSNOR activity resulted in an elevated level of NO. GSNOR believed to be the primary enzyme that regulates NO levels within cells, catalyzes the reduction of S-nitrosoglutathione (GSNO), a primary reservoir of NO, in an NADH-dependent process (Treffon and Vierling 2022).
Furthermore, the increased NO content triggered by DS was in tandem with heightened activity of L-DES and increased H2S production. The interaction between NO and H2S can result in the generation of new nitrosothiols, influencing protein activity and contributing to enhanced stress resilience (Singh et al. 2020). Conversely, elevating NO concentration due to SNP treatment can suppress the GSNOR activity, an enzyme facilitating the decomposition of S-nitrosoglutathione (GSNO) into NO and GSH (Tichá et al. 2016), resulting in NO accumulation. Additionally, SNP application increases both NO and H2S levels. In wheat under osmotic stress, exogenously applied NO was observed to enhance H2S production (Khan et al. 2017). These findings imply a complex interplay between NO and H2S signaling molecules in modulating plant responses to drought. Another study by Da-Silva et al. (2018) investigated the interplay between NO and H2S signaling pathways in regulating salinity tolerance in tomato.
SNP lowers H2O2 through modulating ROS-scavenging enzymes and protein –CO and –SH under DS
Drought stress triggers ROS accumulation in the cell of plants, disrupting the mitochondrial electron transport machinery (Yang et al. 2021). This ROS generation process has been associated with perturbed photosynthetic performance in plants experiencing stress. This results in a surplus accumulation of energy that cannot be efficiently used for plant development and growth (Hasanuzzaman et al. 2020). Multiple studies have demonstrated that NOX serves as a principal origin of ROS generation during water stress within a spectrum of plant species, such as rice (Jing et al. 2021) and maize (Demiralay et al. 2022). It is activated by various signaling pathways in response to DS, including abscisic acid (ABA) signaling, calcium (Ca2+) signaling, and MAPK signaling results in ROS generation in the vicinity of the stress stimulus (Verma et al. 2019; Takata et al. 2020). The current study revealed that DS caused an elevated H2O2 in tomatoes. However, this augmentation could be moderated by SNP treatment. Furthermore, NOX activity was observed to elevate in response to water stress, a trend that was attenuated by SNP treatment. This finding was consistent with a study on cobalt-stressed wheat after SNP treatment, where NOX activity was also found to decrease (Ozfidan-Konakci et al. 2020). Additionally, a notable decline in H2O2 and NOX activity may be a possible reason for the DS tolerance observed in tomato plants following SNP treatment. In contrast to the NOX enzyme, which contributes to the harmful effects of ROS, SOD activity acts to alleviate these effects by promoting the transformation of O2•‾ into less harmful H2O2 and O2 (Islam et al. 2022). The study observed an enhancement in SOD activity due to DS, but SNP treatment did not further increase this activity. Instead, SNP markedly decreased SOD activity in tomato leaves, indicating that SNP does not promote SOD activity to counteract ROS generation in the presence of drought. Additionally, GPX enzyme activity, responsible for detoxifying H2O2 using AsA and GSH as sources of electrons (Rajput et al. 2021), increased in response to DS and was further boosted by SNP treatment in tomato leaves. This suggests that SNP treatment enhances GPX activity, potentially contributing to the plant's competence to tolerate oxidative stress induced by DS. Moreover, the recorded decline in NOX activity with SNP treatment may help reduce H2O2 accumulation, further enhancing the tomato plant's resilience to oxidative damage. Overall, these findings propose that the regulation of the activities of both NOX and GPX due to SNP may present a promising approach for bolstering plant tolerance to oxidative stress induced by drought.
The ROS generation due to stress in the cell is anticipated to result in the regulation of redox-active –SH and –CO groups on proteins (Corpas et al. 2021). This process leads to protein oxidation and the production of –CO groups. Consequently, the measurement of protein –CO groups can serve as an indicator to assess the severity of oxidative damage imposed on proteins (Heinonen et al. 2021). The protein –CO and –SH concentrations within the leaf cells of tomato seedlings were assessed following DS treatment. The SNP application led to a notable decrease in the heightened levels of –CO and –SH groups in leaf cells due to DS. This suggests that SNP exhibits the capability to alleviate the detrimental impacts of DS on protein oxidation. Under DS conditions, plants, including maize plants, have been reported to exhibit an elevation in protein –CO concentration (Warrad et al. 2020), indicating a response to increased oxidative stress during water scarcity. However, the application of SNP has been reported to mitigate the alteration of –CO groups in tomato plants under DS (Nasibi et al. 2009), consistent with the present findings. This suggests that SNP is anticipated to have a favorable effect on mitigating protein oxidation in plants under DS.
SNP's beneficial effect on reduction in ROS, carbonyl, and thiol groups, and modulating the NOX, SOD, and GPX enzymes’ activities, is dependent on the presence of NO. When cPTIO, a scavenger of NO, was used, SNP's beneficial effect on reducing oxidative stress and modulating enzyme activity was abolished. This finding supports the hypothesis that SNP, as a source of NO, improves plant resilience to DS by regulating ROS and enzyme activities. cPTIO, a compound that scavenges NO, has been reported in studies to inverse the favorable impacts of NO on plant responses to DS. For instance, in one study, the application of cPTIO eliminated the advantageous impacts of NO on antioxidant enzyme activity and ROS buildup in wheat seedlings under DS conditions (Wu et al. 2017).
SNP upregulates non-enzymatic antioxidants under drought
Increases in the compounds that scavenge ROS and metabolites that maintain cellular redox buffering, such as GSH and AsA, represent significant adaptations that plants have evolved to cope with DS (Guo et al. 2020). One of the most crucial mechanisms for scavenging cellular H2O2 in plants involves two important metabolites, namely AsA and GSH, which have been previously reported by Kaya et al. (2020). The present findings strongly indicate that SNP treatment led to an elevation in GSH and AsA contents, contributing to the reduction of oxidative stress in drought-stressed plants. The elevation of GSH and AsA levels may have been pivotal in bolstering the antioxidant defense system, highlighting the potential of SNP to mitigate oxidative stress in plants under DS. Additionally, the decrease in GSSG levels induced by SNP is essential for ROS scavenging, maintaining the GSH redox state, and aiding in the detoxification of ROS. Hence, the lowering of GSSG levels following SNP treatment is considered a significant contributor to ROS scavenging and the alleviation of oxidative stress. Similar findings have demonstrated that SNP increased AsA and GSH levels while lowering GSSG in wheat plants under DS (Hasanuzzaman et al. 2018) and cobalt stress (Ozfidan-Konakci et al. 2020). In stress conditions, the increased ROS production can elevate GSSG levels, disrupting the GSH/GSSG ratio and possibly impairing cellular processes (Sachdev et al. 2021), as evidenced in plants under DS in our research. Failure to eliminate surplus ROS can result in oxidative damage (Mansoor et al. 2022). The current study suggests that SNP treatment leading to a higher ratio of GSH to GSSG can enhance plant resistance to DS. This finding is in agreement with earlier findings on wheat under salinity stress, which also reported a positive correlation between GSH/GSSG ratio and stress tolerance (Maslennikova et al. 2022). The increase in non-enzymatic antioxidant levels induced by SNP was reversed by cPTIO supplementation. This indicates that the increase in non-enzymatic antioxidants caused by SNP treatment is likely mediated by NO, as discussed in the review by Sharma et al. (2020). These results further support the role of NO as an important signaling molecule in plants' response to environmental stress.
Conclusions
The study provides valuable insights into the complex cross-talk between NO and nitrosative signaling pathways, elucidating the role of sodium nitroprusside (SNP) in enhancing tomato plant tolerance to DS. Our findings demonstrate that pre-treatment with SNP effectively mitigated the adverse effects of DS on tomato plants by modulating oxidative and nitrosative processes. This included a reduction in H2O2 generation, an increase in NO content, and the stimulation of L-DES activity leading to H2S production. Notably, the observed decrease in the activity of GSNOR suggests a potential regulatory mechanism involving S-nitrosylation, highlighting its significance in modulating protein function and signaling pathways under stress conditions. Furthermore, SNP positively influenced non-enzymatic antioxidants such as AsA and GSH, contributing to improved plant adaptive responses to DS. These findings emphasize the importance of exploring the dynamic interplay between nitrosative and oxidative signaling pathways for a comprehensive understanding of the regulatory mechanisms governing plant responses to DS. This knowledge opens avenues for innovative strategies aimed at enhancing drought tolerance in crops.
Future prospects
Future research endeavours could delve deeper into elucidating the specific molecular mechanisms underlying the observed interactions between NO and nitrosative signaling pathways, especially focusing on the intricate cross-talk mediated by S-nitrosylation. Additionally, exploring the downstream targets of S-nitrosylation and its impact on protein function could provide further insights into the regulatory network modulating plant responses to environmental challenges.
Furthermore, investigating the interplay between NO and H2S, as mediated by L-DES activity, warrants attention as it appears to represent a crucial cross-talk mechanism influencing plant adaptive responses. Unraveling the specific pathways and molecular targets influenced by this interplay could provide a more nuanced understanding of the synergistic effects between NO and H2S in enhancing drought tolerance.
Lastly, translating these findings into practical applications for crop improvement, such as the development of biofortified and stress-tolerant varieties, stands as an exciting prospect. This could involve the integration of SNP or related compounds into agricultural practices to enhance drought resilience and improve overall crop productivity in the face of changing environmental conditions.
Data availability
Data will be made available on request.
References
Ahanger MA, Qi M, Huang Z, Xu X, Begum N, Qin C, Zhang L (2021) Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotoxicol Environ Saf 216:112195. https://doi.org/10.1016/j.ecoenv.2021.112195
Ahluwalia O, Singh PC, Bhatia R (2021) A review on drought stress in plants: implications, mitigation and the role of plant growth promoting rhizobacteria. J Environ Sustain 5:100032. https://doi.org/10.1016/j.resenv.2021.100032
Alamri S, Ali HM, Khan MIR, Singh VP, Siddiqui MH (2020) Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol Biochem 155:20–34. https://doi.org/10.1016/j.plaphy.2020.07.003
Altuntas C, Demiralay M, Sezgin Muslu A, Terzi R (2020) Proline-stimulated signaling primarily targets the chlorophyll degradation pathway and photosynthesis associated processes to cope with short-term water deficit in maize. Photosynth Res 144:35–48. https://doi.org/10.1007/s11120-020-00727-w
Antoniou C, Xenofontos R, Chatzimichail G, Christou A, Kashfi K, Fotopoulos V (2020) Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomolecules 10(1):120. https://doi.org/10.3390/biom10010120
Aroca A, Gotor C, Romero LC (2018) Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front Plant Sci 9:404511. https://doi.org/10.3389/fpls.2018.01369
Badiani E, Pasqualini S, Ciaffi M, Paolacci AR, Sorgonà A, Badiani M (2022) Nitric oxide and reactive oxygen species interaction for stress signaling. In: Ahanger MA, Ahmad P (eds) Nitric oxide in plants: a molecule with dual roles. Wiley, New York, pp 118–147. https://doi.org/10.1002/9781119800156.ch7
Badr A, Brüggemann W (2020) Comparative analysis of drought stress response of maize genotypes using chlorophyll fluorescence measurements and leaf relative water content. Photosynthetica 58(2):38–645. https://doi.org/10.32615/ps.2020.014
Barroso JB, Corpas FJ, Carreras A, Rodríguez-Serrano M, Esteban FJ, Fernández-Ocaña A, Chaki M, Romero-Puertas MC, Valderrama R, Sandalio LM, del Río LA (2006) Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J Exp Bot 57:1785–1793. https://doi.org/10.1093/jxb/erj175
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15(3):413–428. https://doi.org/10.1071/BI9620413
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Benchoam D, Cuevasanta E, Möller MN, Alvarez B (2019) Hydrogen sulfide and persulfides oxidation by biologically relevant oxidizing species. Antioxidants 8(2):48. https://doi.org/10.3390/antiox8020048
Bhuyan MB, Hasanuzzaman M, Parvin K, Mohsin SM, Al Mahmud J, Nahar K, Fujita M (2020) Nitric oxide and hydrogen sulfide: two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul 90:409–424. https://doi.org/10.1007/s10725-020-00594-4
Bradford MM (1976) A rapid and sensitive method for the quantitation of micro gram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Chavoushi M, Najafi F, Salimi A, Angaji SA (2019) Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind Crops Prod 134:168–176. https://doi.org/10.1016/j.indcrop.2019.03.071
Chavoushi M, Najafi F, Salimi A, Angaji SA (2020) Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Sci Hortic 259:108823. https://doi.org/10.1016/j.scienta.2019.108823
Cheng T, Chen J, Ef A, Wang P, Wang G, Hu X, Shi J (2015) Quantitative proteomics analysis reveals that S-nitrosoglutathione reductase (GSNOR) and nitric oxide signaling enhance poplar defense against chilling stress. Planta 242:1361–1390. https://doi.org/10.1007/s00425-015-2374-5
Cheng T, Shi J, Dong Y, Ma Y, Peng Y, Hu X, Chen J (2018) Hydrogen sulfide enhances poplar tolerance to high-temperature stress by increasing S-nitrosoglutathione reductase (GSNOR) activity and reducing reactive oxygen/nitrogen damage. Plant Growth Regul 84:11–23. https://doi.org/10.1007/s10725-017-0316-x
Choudhary R, Rajput VD, Ghodake G, Ahmad F, Meena M, Rehman RU, Prasad R, Sharma RK, Singh R, Seth CS (2024) Comprehensive journey from past to present to future about seed priming with hydrogen peroxide and hydrogen sulfide concerning drought, temperature, UV and ozone stresses-a review. Plant Soil. https://doi.org/10.1007/s11104-024-06499-9
Corpas FJ (2017) Reactive nitrogen species (RNS) in plants under physiological and adverse environmental conditions: current view. In: Cánovas F, Lüttge U, Matyssek R (eds) Progress in botany. Springer, Cham, pp 97–119. https://doi.org/10.1007/124_2016_3
Corpas FJ, Palma JM (2023) Functions of NO and H2S signal molecules against plant abiotic stress. In: Couée I (ed) Plant abiotic stress signaling. Springer, New York, pp 97–109. https://doi.org/10.1007/978-1-0716-3044-0_5
Corpas FJ, González-Gordo S, Muñoz-Vargas MA, Rodríguez-Ruiz M, Palma JM (2021) The modus operandi of hydrogen sulfide (H2S)-dependent protein persulfidation in higher plants. Antioxidants 10(11):1686. https://doi.org/10.3390/antiox10111686
Da-Silva CJ, Mollica DCF, Vicente MH, Peres LEP, Modolo LV (2018) NO, hydrogen sulfide does not come first during tomato response to high salinity. Nitric Oxide 76:164–173. https://doi.org/10.1016/j.niox.2017.09.008
de Bont L, Mu X, Wei B, Han Y (2022) Abiotic stress-triggered oxidative challenges: Where does H2S act? JGG 49(8):748–755. https://doi.org/10.1016/j.jgg.2022.02.019
Demiralay M, Sağlam A, Yetişsin F, Kadioğlu A (2022) Investigation of the roles of hydrogen peroxide and NADPH oxidase in the regulation of polyamine metabolism in maize plants under drought stress conditions. J Agric Sci 28(4):613–625. https://doi.org/10.15832/ankutbd.861008
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Faraji J, Sepehri A (2020) Exogenous nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J Soil Sci Plant Nutr 20(2):703–714. https://doi.org/10.1007/s42729-019-00158-0
Farouk S, Al-Ghamdi AAM (2021) Sodium nitroprusside application enhances drought tolerance in marjoram herb by promoting chlorophyll biosynthesis and enhancing osmotic adjustment capacity. Arab J Geosci 14:430. https://doi.org/10.1007/s12517-021-06846-5
Fielding JL, Hall JL (1978) A biolchemical and cytochemical study of peroxidase activity in roots of Pisum sativum; I. a comparison of dab-peroxidase and guaiacol-peroxidase with particular emphasis on the properties of cell wall activity. J Exp Bot 29:969–981. https://doi.org/10.1093/jxb/29.4.969
Gautam H, Fatma M, Sehar Z, Mir IR, Khan NA (2022) Hydrogen sulfide, ethylene, and nitric oxide regulate redox homeostasis and protect photosynthetic metabolism under high temperature stress in rice plants. Antioxidants 11(8):1478. https://doi.org/10.3390/antiox11081478
Gow A, Doctor A, Mannick J, Gaston B (2007) S-Nitrosothiol measurements in bio-logical systems. Chromatogr B Analyt Technol Biomed Life Sci 851:140–151. https://doi.org/10.1016/j.jchromb.2007.01.052
Guo YY, Li HJ, Zhao CF, Xue JQ, Zhang RH (2020) Exogenous melatonin improves drought tolerance in maize seedlings by regulating photosynthesis and the ascorbate–glutathione cycle. Russ J Plant Physl 67(5):809–821. https://doi.org/10.1134/S1021443720050064
Gupta P, Seth CS (2020) Interactive role of exogenous 24 epibrassinolide and endogenous NO in Brassica juncea L. under salinity stress: evidence for NR-dependent NO biosynthesis. Nitric Oxide 97:33–47. https://doi.org/10.1016/j.niox.2020.01.014
Gupta P, Seth CS (2023) 24-epibrassinolide regulates functional components of nitric oxide signalling and antioxidant defense pathways to alleviate salinity stress in Brassica juncea L. cv. Varuna. J Plant Growth Regul 42(7):4207–4222. https://doi.org/10.1016/j.niox.2020.01.014
Hanif S, Saleem MF, Sarwar M, Irshad M, Shakoor A, Wahid MA, Khan HZ (2021) Biochemically triggered heat and drought stress tolerance in rice by proline application. J Plant Growth Regul 40:305–312. https://doi.org/10.1007/s00344-020-10095-3
Hasanuzzaman M, Nahar K, Rahman A, Inafuku M, Oku H, Fujita M (2018) Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol Mol Biol Plants 24(6):993–1004. https://doi.org/10.1007/s12298-018-0531-6
Hasanuzzaman M, Bhuyan MB, Parvin K, Bhuiyan TF, Anee TI, Nahar K, Fujita M (2020) Regulation of ROS metabolism in plants under environmental stress: a review of recent experimental evidence. Int J Mol Sci 21(22):8695. https://doi.org/10.3390/ijms21228695
Heinonen M, Gürbüz G, Ertbjerg P (2021) Oxidation of proteins. In: Rodriguez-Amaya DB, Amaya-Farfan J (eds) Chemical changes during processing and storage of foods. Academic Press, Elsevier, Oxford, UK, pp 85–123. https://doi.org/10.1016/B978-0-12-817380-0.00003-8
Hosseini SA, Réthoré E, Pluchon S, Ali N, Billiot B, Yvin JC (2019) Calcium application enhances drought stress tolerance in sugar beet and promotes plant biomass and beetroot sucrose concentration. Int J Mol Sci 20(15):3777. https://doi.org/10.3390/ijms20153777
Hosseinifard M, Stefaniak S, Ghorbani Javid M, Soltani E, Wojtyla Ł, Garnczarska M (2022) Contribution of exogenous proline to abiotic stresses tolerance in plants: a review. Int J Mol Sci 23(9):5186. https://doi.org/10.3390/ijms23095186
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in ascorbate deficient arabidopsis mutant. J Exp Bot 56:3041–3049. https://doi.org/10.1093/jxb/eri301
Ishida A, Ookubo K, Ono K (1987) Formation of hydrogen peroxide by NAD(P)H oxidation with isolated cell wall-associated peroxidase from cultured liverwort cells, Marchantia polymorpha L. Plant Cell Physiol 28:723–726. https://doi.org/10.1093/oxfordjournals.pcp.a077349
Islam SMF, Karim Z (2019) World’s demand for food and water: the consequences of climate change. In: Farahani MHDA, Vatanpour V, Taheri AH (eds) Desalination-challenges and opportunities. Intech Open Limited, London, pp 1–27
Islam MN, Rauf A, Fahad FI, Emran TB, Mitra S, Olatunde A, Shariati MA, Rebezov M, Rengasamy KRR, Mubarak MS (2022) Superoxide dismutase: an updated review on its health benefits and industrial applications. Crit Rev Food Sci Nutr 62(26):7282–7300. https://doi.org/10.1080/10408398.2021.1913400
Jafari M, Shahsavar AR (2022) Sodium nitroprusside: its beneficial role in drought stress tolerance of “Mexican lime”(Citrus aurantifolia (Christ.) Swingle) under in vitro conditions. In Vitro Cell Dev Biol Plant 58(1):155–168. https://doi.org/10.1007/s11627-021-10218-9
Jafari M, Shahsavar AR, Talebi M, Hesami M (2022) Exogenous melatonin protects lime plants from drought stress-induced damage by maintaining cell membrane structure, detoxifying ROS and regulating antioxidant systems. Horticulturae 8(3):257. https://doi.org/10.3390/horticulturae8030257
Jahan S, Tamta S, Shankhdhar SC, Shankhdhar D (2023) Salicylic acid potential to reversing drought induced oxidative stress in Bacopa monnieri (L.) through enhancement of bioactive compound (Bacoside-A) and antioxidants including physio-biochemical attributes. S Afr J Bot 161:617–626. https://doi.org/10.1016/j.sajb.2023.08.050
Jing XQ, Li WQ, Zhou MR, Shi PT, Zhang R, Shalmani A, Chen KM (2021) Rice carbohydrate-binding malectin-like protein, OsCBM1, contributes to drought-stress tolerance by participating in NADPH oxidase-mediated ROS production. Rice 14:1–21. https://doi.org/10.1186/s12284-021-00541-5
Kataria S, Jain M, Tripathi DK, Singh VP (2020) Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming-induced salt tolerance in soybean. Physiol Plant 168(2):422–436. https://doi.org/10.1111/ppl.13031
Kaur G, Singh HP, Batish DR, Mahajan P, Kohli RK, Rishi V (2015) Exogenous nitric oxide (NO) interferes with lead (Pb)-induced toxicity by detoxifying reactive oxygen species in hydroponically grown wheat (Triticum aestivum) roots. PLoS ONE 10:e0138713. https://doi.org/10.1371/journal.pone.0138713
Kaya C, Ashraf M, Alyemeni MN, Corpas FJ, Ahmad P (2020) Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J Hazard Mater 399:123020. https://doi.org/10.1016/j.jhazmat.2020.123020
Khan MN, Mobin M, Abbas ZK, Siddiqui MH (2017) Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 68:91–102. https://doi.org/10.1016/j.niox.2017.01.001
Khan MA, Khan AL, Imran QM, Asaf S, Lee SU, Yun BW, Lee IJ (2019) Exogenous application of nitric oxide donors regulates short-term flooding stress in soybean. PeerJ 7:e7741. https://doi.org/10.7717/peerj.7741
Kharbech O, Massoud MB, Sakouhi L, Djebali W, Mur LAJ, Chaoui A (2020a) Exogenous application of hydrogen sulfide reduces chromium toxicity in maize seedlings by suppressing NADPH oxidase activities and methylglyoxal accumulation. Plant Physiol Biochem 154:646–656. https://doi.org/10.1016/j.plaphy.2020.06.002
Kharbech O, Sakouhi L, Massoud MB, Mur LAJ, Corpas FJ, Djebali W, Chaoui A (2020b) Nitric oxide and hydrogen sulfide protect plasma membrane integrity and mitigate chromium-induced methylglyoxal toxicity in maize seedlings. Plant Physiol Biochem 157:244–255. https://doi.org/10.1016/j.plaphy.2020.10.017
Kour J, Khanna K, Singh AD, Dhiman S, Devi K, Sharma N, Madaan I, Kapoor N, Sirhindi G, Bhardwaj R (2023) Nitric oxide as a modulator of oxidative stress and antioxidative metabolism in plants. In: Khan MIR, Iqbal N, Poór P, Ferrante A, Singh VP, Tripathi DK, Fotopoulos V (eds) Nitric oxide in developing plant stress resilience. Academic Press, London, pp 91–124. https://doi.org/10.1016/B978-0-323-91209-9.00011-7
Larouk C, Gabon F, Kehel Z, Djekoun A, Nachit M, Amri A (2021) Chlorophyll fluorescence and drought tolerance in a mapping population of durum wheat. Contemp Agric 70(3–4):123–134. https://doi.org/10.2478/contagri-2021-0018
Lau SE, Hamdan MF, Pua TL, Saidi NB, Tan BC (2021) Plant nitric oxide signaling under drought stress. Plants 10(2):360. https://doi.org/10.3390/plants10020360
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for de-termination of oxidatively modified proteins. Methods Enzymol 233:346–357
Li ZG, Min X, Zhou ZH (2016) Hydrogen sulfide: a signal molecule in plant cross-adaptation. Front Plant Sci 7:1621. https://doi.org/10.3389/fpls.2016.01621
Li LH, Yi HL, Liu XP, Qi HX (2021) Sulfur dioxide enhance drought tolerance of wheat seedlings through H2S signaling. Ecotoxicol Environ Saf 207:111248. https://doi.org/10.1016/j.ecoenv.2020.111248
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 603(2):591–592. https://doi.org/10.1042/bst0110591
Majeed S, Nawaz F, Naeem M, Ashraf MY, Ejaz S, Ahmad KS, Tauseed S, Farid G, Khalid I, Mehmood K (2020) Nitric oxide regulates water status and associated enzymatic pathways to inhibit nutrients imbalance in maize (Zea mays L.) under drought stress. Plant Physiol Biochem 155:147–160. https://doi.org/10.1016/j.plaphy.2020.07.005
Mansoor S, Ali Wani O, Lone JK, Manhas S, Kour N, Alam P, Ahmad P, Ahmad P (2022) Reactive oxygen species in plants: from source to sink. Antioxidants 11(2):225. https://doi.org/10.3390/antiox11020225
Marcolongo JP, Venancio MF, Rocha WR, Doctorovich F, Olabe JA (2019) NO/H2S “crosstalk” reactions the role of thionitrites (SNO−) and perthionitrites (SSNO−). Inorg Chem 58(22):14981–14997. https://doi.org/10.1021/acs.inorgchem.9b01978
Mariyam S, Bhardwaj R, Khan NA, Sahi SV, Seth CS (2023) Review on nitric oxide at the forefront of rapid systemic signaling in mitigation of salinity stress in plants: crosstalk with calcium and hydrogen peroxide. Plant Sci. https://doi.org/10.1016/j.plantsci.2023.111835
Maslennikova DR, Lastochkina OV, Shakirova FM (2022) Exogenous sodium nitroprusside improves salt stress tolerance of wheat (Triticum aestivum L.) via regulating the components of ascorbate-glutathione cycle, chlorophyll content and stabilization of cell membranes state. Russ J Plant Physl 69(6):1–12. https://doi.org/10.1134/S102144372206019X
Muñoz-Vargas MA, González-Gordo S, Palma JM, Corpas FJ (2022) H2S in horticultural plants: endogenous detection by an electrochemical sensor, emission by a gas detector, and its correlation with L-cysteine desulfhydrase (LCD) activity. Int J Mol Sci 23(10):5648. https://doi.org/10.3390/ijms23105648
Nabi RBS, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun BG, Yun BW (2019) Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exp Bot 161:120–133. https://doi.org/10.1016/j.envexpbot.2019.02.003
Nasibi F, Kalantari KM, Khodashenas M (2009) The effect of sodium nitroprusside (SNP) pretreatment on some biochemical parameters in tomato seedlings (Lycopersicum esculentum) under drought stress. TJANRS 16(2):121–133
Ozfidan-Konakci C, Yildiztugay E, Elbasan F, Kucukoduk M, Turkan I (2020) Hydrogen sulfide (H2S) and nitric oxide (NO) alleviate cobalt toxicity in wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic redox and antioxidant capacity. J Hazard Mater 388:122061. https://doi.org/10.1016/j.jhazmat.2020.122061
Pandey V, Shukla A (2015) Acclimation and tolerance strategies of rice under drought stress. Rice Sci 22(4):147–161. https://doi.org/10.1016/j.rsci.2015.04.001
Prajapati P, Gupta P, Kharwar RN, Seth CS (2023) Nitric oxide mediated regulation of ascorbate-glutathione pathway alleviates mitotic aberrations and DNA damage in Allium cepa L. under salinity stress. Int J Phytoremediation 25(4):403–414. https://doi.org/10.1080/15226514.2022.2086215
Rajput VD, Harish Singh RK, Verma KK, Sharma L, Quiroz-Figueroa FR, Meena M, Gour VS, Minkina T, Sushkova S, Mandzhieva S (2021) Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 10(4):267. https://doi.org/10.3390/biology10040267
Razi K, Muneer S (2021) Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit Rev Biotechnol 41(5):669–691. https://doi.org/10.1080/07388551.2021.1874280
Rezayian M, Ebrahimzadeh H, Niknam V (2020) Nitric oxide stimulates antioxidant system and osmotic adjustment in soybean under drought stress. J Soil Sci Plant Nutr 20:1122–1132. https://doi.org/10.1007/s42729-020-00198-x
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10(2):277. https://doi.org/10.3390/antiox10020277
Saja-Garbarz D, Ostrowska A, Kaczanowska K, Janowiak F (2021) Accumulation of silicon and changes in water balance under drought stress in Brassica napus var napus L. Plants 10(2):280. https://doi.org/10.3390/plants10020280
Samanta S, Seth CS, Roychoudhury A (2023) The molecular paradigm of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with different phytohormone signaling pathways during drought stress in plants. Plant Physiol Biochem 206:108259. https://doi.org/10.1016/j.plaphy.2023.108259
Samimi M, Mirchi A, Moriasi D, Ahn S, Alian S, Taghvaeian S, Sheng Z (2020) Modeling arid/semi-arid irrigated agricultural watersheds with SWAT: applications, challenges, and solution strategies. J Hydrol 590:125418. https://doi.org/10.1016/j.jhydrol.2020.125418
Saville B (1958) A scheme for the colorimetric determination of microgram amounts of thiols. Analyst 83:670–672. https://doi.org/10.1039/AN9588300670
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-Wajid HH, Battaglia ML (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10:259. https://doi.org/10.3390/plants10020259
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51(3):121–124. https://doi.org/10.1046/j.1365-3040.2001.00778.x
Sharma A, Soares C, Sousa B, Martins M, Kumar V, Shahzad B, Sidhu GPS, Bali AS, Asgher M, Bhardwaj R, Thukral AK, Fidalgo F, Zheng B (2020) Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: a review on molecular and biochemical aspects. Physiol Plant 168(2):318–344. https://doi.org/10.1111/ppl.13004
Shen J, Su Y, Zhou C, Zhang F, Zhou H, Liu X, Wu D, Yin X, Xie Y, Yuan X (2019) A putative rice L-cysteine desulfhydrase encodes a true L-cysteine synthase that regulates plant cadmium tolerance. Plant Growth Regul 89:217–226. https://doi.org/10.1007/s10725-019-00528-9
Shi X, Qiu H (2020) Post-translational S-nitrosylation of proteins in regulating cardiac oxidative stress. Antioxidants 9(11):1051
Siddiqui MH, Khan MN, Mukherjee S, Alamri S, Basahi RA, Al-Amri AA, Al-Munqedhi BMA, Ali HM, Almohisen IA (2021) Hydrogen sulfide (H2S) and potassium (K+) synergistically induce drought stress tolerance through regulation of H+-ATPase activity, sugar metabolism, and antioxidative defense in tomato seedlings. Plant Cell Rep 40(8):1543–1564. https://doi.org/10.1007/s00299-021-02731-3
Signorelli S, Casaretto E, Sainz M, Díaz P, Monza J, Borsani O (2013) Antioxidant and photosystem II responses contribute to explain the drought–heat contrasting tolerance of two forage legumes. Plant Physiol Biochem 70:195–203. https://doi.org/10.1016/j.plaphy.2013.05.028
Singh S, Kumar V, Kapoor D, Kumar S, Singh S, Dhanjal DS, Singh J (2020) Revealing on hydrogen sulfide and nitric oxide signals co-ordination for plant growth under stress conditions. Physiol Plant 168(2):301–317. https://doi.org/10.1111/ppl.13002
Singh G, Patel A, Tiwari S, Gupta D, Prasad SM (2022) Signaling molecules hydrogen sulfide (H2S) and nitric oxide (NO): role in microalgae under adverse environmental conditions. Acta Physiol Plant 44(7):68. https://doi.org/10.1007/s11738-022-03404-8
Sohag AAM, Tahjib-Ul-Arif M, Polash MAS, Belal Chowdhury M, Afrin S, Burritt DJ, Afzal Hossain M (2020) Exogenous glutathione-mediated drought stress tolerance in rice (Oryza sativa L.) is associated with lower oxidative damage and favorable ionic homeostasis. Iran J Sci and Technol A 44:955–971. https://doi.org/10.1007/s40995-020-00917-0
Strain HH, Svec WA (1966) Extraction separation estimation and isolation of the chlorophylls. In: Vernon LP, Seely GR (eds) The chlorophylls. Academic Press, New York, pp 21–66
Sun YY, Li ZG (2022) Key role of reactive oxygen species-scavenging system in nitric oxide and hydrogen sulfide crosstalk-evoked thermotolerance in maize seedlings. Front Plant Sci 13:967968. https://doi.org/10.3389/fpls.2022.967968
Sun C, Zhang Y, Liu L, Liu X, Li B, Jin C, Lin X (2021) Molecular functions of nitric oxide and its potential applications in horticultural crops. Hortic Res 8:71. https://doi.org/10.1038/s41438-021-00500-7
Takata T, Araki S, Tsuchiya Y, Watanabe Y (2020) Oxidative stress orchestrates MAPK and nitric-oxide synthase signal. Int J Mol Sci 21(22):8750. https://doi.org/10.3390/ijms21228750
Tichá T, Luhová L, Petřivalský M (2016) Functions and metabolism of S-nitrosothiols and S-nitrosylation of proteins in plants: the role of GSNOR. In: Lamattina L, García-Mata C (eds) Gasotransmitters in plants: signaling and communication in plants. Springer, Cham, pp 175–200. https://doi.org/10.1007/978-3-319-40713-5_9
Treffon P, Vierling E (2022) Focus on nitric oxide homeostasis: direct and indirect enzymatic regulation of protein denitrosation reactions in plants. Antioxidants 11(7):1411. https://doi.org/10.3390/antiox11071411
Valderrama R, Begara-Morales JC, Chaki M, Mata-Pérez C, Padilla MN, Barroso JB (2019) Hydrogen peroxide (H2O2)-and nitric oxide (NO)-derived posttranslational modifications. In: Gupta D, Palma J, Corpas F (eds) Nitric oxide and hydrogen peroxide signaling in higher plants. Springer, Cham, pp 37–67
Van Rossum MWPC, Alberda M, Van Der Plas LHW (1997) Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci 130:207–216. https://doi.org/10.1016/S0168-9452(97)00215-X
Verma G, Srivastava D, Tiwari P, Chakrabarty D (2019) ROS modulation in crop plants under drought stress. In: Hasanuzzaman M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. John Wiley and Sons Ltd, pp 311–336. https://doi.org/10.1002/9781119468677.ch13
Wahab A, Abdi G, Saleem MH, Ali B, Ullah S, Shah W, Marc RA (2022) Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: a comprehensive review. Plants 11(13):1620. https://doi.org/10.3390/plants11131620
Wang P, Fang H, Gao R, Liao W (2021) Protein persulfidation in plants: function and mechanism. Antioxidants 10(10):1631. https://doi.org/10.3390/antiox10101631
Wang C, Wei L, Zhang J, Hu D, Gao R, Liu Y, Liao W (2022) Nitric oxide enhances salt tolerance in tomato seedlings by regulating endogenous S-nitrosylation levels. J Plant Growth Regul. https://doi.org/10.1007/s00344-021-10546-5
Warrad M, Hassan YM, Mohamed MS, Hagagy N, Al-Maghrabi OA, Selim S, AbdElgawad H (2020) A bioactive fraction from Streptomyces sp. enhances maize tolerance against drought stress. JMB 30(8):1156. https://doi.org/10.4014/jmb.2003.03034
Weatherley PE, Barrs C (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428. https://doi.org/10.1071/BI9620413
Wei L, Zhang M, Wei S, Zhang J, Wang C, Liao W (2020) Roles of nitric oxide in heavy metal stress in plants: cross-talk with phytohormones and protein S-nitrosylation. Environ Pollut 259:113943. https://doi.org/10.1016/j.envpol.2020.113943
Wei L, Zhang J, Wei S, Hu D, Liu Y, Feng L, Liao W (2022a) Nitric oxide enhanced salt stress tolerance in tomato seedlings, involving phytohormone equilibrium and photosynthesis. Int J Mol Sci 23(9):4539. https://doi.org/10.3390/ijms23094539
Wei L, Zhang J, Wei S, Wang C, Deng Y, Hu D, Liu H, Gong W, Pan Y, Liao W (2022b) Nitric oxide alleviates salt stress through protein S-nitrosylation and transcriptional regulation in tomato seedlings. Planta 256:101. https://doi.org/10.1007/s00425-022-04015-w
Wu S, Hu C, Tan Q, Xu S, Sun X (2017) Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front Plant Sci 8:1085. https://doi.org/10.3389/fpls.2017.01085
Yang X, Lu M, Wang Y, Wang Y, Liu Z, Chen S (2021) Response mechanism of plants to drought stress. Horticulturae 7(3):50. https://doi.org/10.3390/horticulturae7030050
Yasir TA, Ahmad M, Wasaya A, Ateeq M, Kanwal S, Wahid A, Aziz M (2023) Hydrogen sulfide (H2S) signaling in plants responding to abiotic stresses. In: Aftab T, Corpas FJ (eds) Gasotransmitters signaling in plants under challenging environment, vol 5. Springer, Cham, pp 241–262
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955–963. https://doi.org/10.1071/FP03091
Yuan S, Patel RP, Kevil CG (2015) Working with nitric oxide and hydrogen sulfide in biological systems. Am J Physiol Lung Cell Mol Physiol 308(5):L403–L415. https://doi.org/10.1152/ajplung.00327.2014
Zhang J, Liao W (2022) Roles of S-nitrosylation in abiotic stress tolerance in plants. In: Singh VP, Singh S, Tripathi DK, Romero-Puertas MC, Sandalio LM (eds) Nitric oxide in plant biology. Academic Press, London, pp 453–475
Zhu CQ, Zhang JH, Sun LM, Zhu LF, Abliz B, Hu WJ, Jin QY (2018) Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast Al contents in rice. Front Plant Sci 9:294. https://doi.org/10.3389/fpls.2018.00294
Zou YN, Wu QS, Kuča K (2021) Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol 23:50–57. https://doi.org/10.1111/plb.13161
Acknowledgements
The author expresses gratitude to the University of Harran (Turkey) for providing partial support for this study.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
The experimental setup was designed and implemented by C.K and FU, who also conducted data analysis C.K. and C.S.S contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors clearly state that they have no conflicting financial interests or personal relationships that may influence their work.
Additional information
Communicated by Muthu Thiruvengadam.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaya, C., Uğurlar, F. & Seth, C.S. Sodium nitroprusside modulates oxidative and nitrosative processes in Lycopersicum esculentum L. under drought stress. Plant Cell Rep 43, 152 (2024). https://doi.org/10.1007/s00299-024-03238-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00299-024-03238-3