Abstract
We aimed to summarise effects and use of non-pharmacological and pharmacological treatments for sarcoidosis with musculoskeletal manifestations. We systematically searched the Cochrane Library, Ovid MEDLINE, Embase, CINAHL, AMED, Scopus, clinical.trials.gov, PROSPERO and PEDro for systematic reviews from 2014 to 2022 and for primary studies from date of inception to March 29, 2022, and studies with patients diagnosed with sarcoidosis with musculoskeletal manifestations. Inclusion criteria required that studies reported effects of non-pharmacological and/or pharmacological treatments or number of patients receiving these treatments. Results were reported narratively and in forest plots. Eleven studies were included. No systematic reviews fulfilled our inclusion criteria. None of the included studies had a control group. We found that between 23 and 100% received corticosteroids, 0–100% received NSAIDs, 5–100% received hydroxychloroquine, 12–100% received methotrexate, 0–100% received TNF inhibitors, and 3–4% received azathioprine. Only ten patients in one study had used non-pharmacological treatments, including occupational therapy, chiropractic and acupuncture. There are no controlled studies on treatment effects for patients with sarcoidosis with musculoskeletal manifestations. We found 11 studies reporting use of pharmacological treatments and only one study reporting use of non-pharmacological treatments. Our study identified major research gaps for pharmacological and non-pharmacological treatment in musculoskeletal sarcoidosis and warrant randomised clinical trials for both.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a rare disease of unknown aetiology which can affect several organs [1]. Prevalence and presenting symptoms of sarcoidosis vary significantly by sex, racial group, and country, and in the United States, an age-adjusted annual incidence of 35.5 per 100,000 for blacks was observed as compared with 10.9 per 100,000 for whites [2].
In most cases, the respiratory system is involved [3], other affected organ systems are skin, eyes, and systemic symptoms, such as fever, night sweats, fatigue, and malaise. Within the musculoskeletal system, ankles, knees and wrists are the most commonly involved joints, with oligo-articular involvement more prominent in acute arthritis [4].
Löfgren’s syndrome is a typical musculoskeletal manifestation with acute onset of Erythema nodosum with periarticular ankle inflammation together with hilar lymphadenopathy and is associated with a benign disease course [5]. Myopathy can result in pain and muscle weakness, whilst chronic arthropathy and osseous manifestations are rather uncommon [1].
The clinical presentation of sarcoidosis can be quite variable, as can the prognosis, ranging from mild and self-limited to severe disease that leads to significant organ impairment and increased mortality [6]. Chronic progressive disease may require long-term treatment with corticosteroids, cytotoxics and other agents which have a potential for considerable adverse events [3].
Glucocorticosteroids are considered first line treatment in sarcoidosis [1, 7]. Disease-modifying antirheumatic drugs, such as methotrexate, azathiorine, hydroxychloroquine, or a tumour necrosis factor inhibitor (TNFi or TNF inhibitors), have been used successfully and in combination with corticosteroids [8]. In a recent review, TNFi was given in 12% of patients with sarcoidosis in rheumatology practices in the United States [9].
The disease requires cooperation between specialists, but the role of non-pharmacological management has not been a topic of interest in the literature for patients with musculoskeletal manifestations as compared to rehabilitation supported in pulmonary sarcoidosis [10].
The aim of this study was to systematically review the literature on sarcoidosis with musculoskeletal manifestations for evidence of non-pharmacological and pharmacological treatment effects. Results will inform clinicians on evidence-based pharmacological and non-pharmacological treatment decisions in patients with musculoskeletal manifestations of sarcoidosis. The review will also identify research gaps. If no effect studies were found, we included studies with reported use of specified treatments. This review was not registered in a prospective register of systematic reviews.
Materials and methods
Inclusion criteria
We included patients with sarcoidosis with joint and bone manifestations, including Löfgren’s syndrome. We searched primarily for systematic reviews. If no systematic reviews were found, we searched for primary studies with or without a control group. Included designs were: Randomised controlled trials (including cluster-randomised, quasi-randomised, stepped wedge), regression discontinuity design, controlled clinical trials, longitudinal studies, case series, and cohort studies. Excluded designs were qualitative studies, case–control studies, cross-sectional studies and case studies, and publications in abstract format. Our PICO (patients-interventions-comparisons-outcomes) for the effect question was:
P: Patients with sarcoidosis with joint and bone manifestations, including Löfgren’s syndrome.
I: Non-pharmacological and pharmacological interventions.
C: Standard care, placebo, other active non-pharmacological or pharmacological intervention.
O: All outcomes.
If we did not find effect studies, we had the same ‘P’ and ‘I’ but aimed to describe the use of non-pharmacological and pharmacological interventions.
Literature search
First, we performed a search for systematic reviews. The search for systematic reviews (in March 2022) was conducted in Cochrane Library, MEDLINE, Embase, Scopus and CINAHL, using a basic search strategy including keywords and MeSH-terms for patient group only. Thereafter, we searched for primary studies in Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations and Daily < 1946 to March 28, 2022 > , Embase < 1974 to 2022 March 28 > , CINAHL and Scopus, using a more extensive search strategy. The full search strategies for MEDLINE and Embase are presented in Appendix 1.
We did not apply any language restrictions in the literature search. We included only studies in English. A librarian (AMK) performed the literature searches.
Two authors (GS and TU) independently screened titles/ abstracts and full texts according to the inclusion criteria. One author (GS) extracted data, and the other author (TU) checked the data extractions. For appraisal of methodological quality, we used the Joanna Briggs Case Series Critical Appraisal Tool [11]. The tool has 10 domains on which to assess risk of bias for each study (Appendix 3). The tool does not have a sum score, so it is not feasible to assess the within-study risk of bias. For a similar reason, we did not assess the level of risk of bias per medication/ medication group. One author (GS) performed the appraisal, and the second author (TU) checked the appraisal. We planned to perform meta-analyses of effect studies. If no such studies were found, we considered to perform meta-analyses of the proportion of patients that used different types of non-pharmacological or pharmacological treatments in the studies. A prerequisite for performing meta-analyses is that studies are sufficiently homogeneous with respect to patients, medications, outcomes, and study designs. Studies with low methodological quality were not judged to be eligible for meta-analysis. We also supply a narrative description of the results of each included study.
Statistical analysis
We constructed forest plots with the R package “meta” and the command “metaprop”.[12]. We present plots on the proportion of patients in each study who used the different kinds of medications. We present one plot for each specified medication (corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), hydroxychloroquine, methotrexate, TNF inhibitors, and azathioprine).
Results
We identified five reviews [1, 13,14,15,16], none of which were fulfilling our inclusion criteria. The review by Shariatmaghani et al. [1] was not a systematic review. Adler [13] studied effects of TNF inhibitors on sarcoidosis, but there were no patients with musculoskeletal manifestations. Another (non-systematic) review by Baughman [14] proposed a treatment scheme but did not report treatment effects. A fourth review by Bechman [15] was not a systematic review and did not report treatment effects. Finally, the article by Drent [16] was not a systematic review.
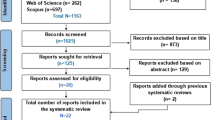
The combined search for systematic reviews and primary studies found 1425 unique records after removal of duplicates. The flow chart (Fig. 1) shows the inclusion process. We obtained 41 articles in full text and included 11 articles [4, 17,18,19,20,21,22,23,24,25,26], which are listed in Table 1. One article [53] was in Spanish and was therefore excluded. The 30 excluded articles [5, 9, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] are listed in Appendix 2.
PRISMA_2020_flow_diagram. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71. For more information, visit:http://www.prisma-statement.org/
Characteristics of included studies Table 1 shows characteristics of the included studies. They were published between 1984 and 2020. Three studies were from France, three were from USA, two from India, and the remainder from Norway, Spain, and Iceland. No studies had a control group, and none reported effects of treatment. The total number of patients was 329. The number of patients in individual studies varied between 5 and 117. The mean per cent males were 54.1 (the mean of the means in each study), and ranged from 22 to 100. Mean age in the studies was 41.5 (standard deviation: 7.4) years. The aims of the studies were mostly to describe occurrence of different diagnoses and symptoms and/or describe treatments given to patients. One study (Garg [20]) tried to improve on the classification to Löfgren’s syndrome or Ponchet’s disease. Banse [17] studied the effects of TNF inhibitors. Glennås [21] explored seasonal variations of disease onset.
Methodological quality appraisal. Only two of the studies [21, 23] were assessed as fulfilling all the quality items in the quality appraisal, whilst six studies had serious shortcomings. The most common shortcomings were lack of consecutive inclusion, unclear reporting of outcomes or follow-up, and unclear reporting of the presenting site(s)/clinic(s) demographic information. Appendix 3 is a description of how we assessed the included studies on methodological quality.
Description of the studies
Arthritis in Sarcoidosis Group 2018 recruited patients from 11 centres in India. The medical records from these centres were reviewed to locate patients with sarcoidosis who had arthritis from 2005 onward. Patients were categorised into acute sarcoid arthritis (≤ 6 months) or chronic sarcoid arthritis (> 6 months). The authors compared acute and chronic sarcoid arthritis groups for differences in demographic profile. Of 117 patients, 88 had acute and 29 had chronic sarcoid arthritis. Forty-five patients in the acute group had Löfgren’s syndrome, and one patient in the chronic group had Heerfordt’s syndrome. Treatments given: All patients had received non-steroidal anti‐inflammatory drugs. For extra‐articular manifestations, 35 received corticosteroids, 17 received weekly methotrexate, 12 were on hydroxychloroquine and three on azathioprine. Prognosis: About four‐fifths of the 49 patients with acute sarcoid arthritis followed up for a median of about 2 years had attained complete remission with non‐steroidal anti‐inflammatory drugs, with corticosteroids and other DMARDs used for extra‐articular features. Acute sarcoid arthritis is mostly self‐limiting. Conclusions: The authors concluded that ankles, knees and wrists are the most commonly involved joints, with oligo-articular involvement more prominent in acute arthritis as opposed to similar oligo‐ and poly-articular involvement in chronic arthritis. Both acute and chronic articular sarcoidosis have greater propensity for hilar involvement, ILD and erythema nodosum. However, uveitis and peripheral adenopathy are more common in chronic sarcoid arthritis.
Banse 2013 evaluated the efficacy and safety of three TNF inhibitors to treat joint manifestations of sarcoidosis. Included patients were refractory to conventional therapy (NSAIDs, corticosteroids, and/or disease-modifying antirheumatic drugs (DMARDs). Amongst the ten patients, five had arthralgias without swelling and five had arthritis, including two mono-, one oligo-, and two polyarthritis. Before initiation of TNF inhibitors, according to disease activity (DAS28-CRP) scores, six patients had low articular disease activity (DAS28 < 3.2), two had moderate (DAS28 3.2–5.1), and two had high disease activity (DAS28 > 5.1). Treatments given: All patients received TNF inhibitors, and the results were reported after 3, 6, and 12 months. The total duration of TNF inhibitor exposure was 17.6 patient-years, which was started a median of 3 (0.33–17) years after sarcoidosis diagnosis. Infliximab, adalimumab and etanercept was first- and second-/third-line choices, respectively. Conclusions: The authors concluded that no significant impact of a TNF inhibitor on articular manifestations (numbers of painful and swollen joints, DAS28 with ESR or CRP, global VAS score), extra-articular involvement (pulmonary, ocular, cardiac, muscular), or biological indicators of inflammatory syndrome were observed. However, TNF inhibitor use obtained significant, albeit moderate, corticosteroid sparing effects. Moreover, their safety seems good, with no severe adverse events occurring under treatment.
Cacciatore 2020 included 39 patients with sarcoid arthropathy in a retrospective study. They contrasted 19 patients with acute disease (Löfgren’s syndrome) with 20 chronic patients. Treatments given: Amongst 20 patients with chronic sarcoidosis, treatment was used in 17 cases, and they used either NSAIDs alone (n = 5), steroids alone (n = 5), hydroxychloroquine (n = 2), methotrexate (n = 3), or TNF inhibitors (n = 2). Conclusions: Sarcoid arthropathies have different clinical phenotypes in acute and chronic forms and various treatment regimens, such as hydroxychloroquine and methotrexate, could be used in chronic forms.
Fayad 2006 performed a retrospective study from hospital charts over the period 1985–2001 in two academic French rheumatology centres. Five patients with muscle sarcoidosis were included. Treatments given: All patients were given prednisolone and two patients used hydroxychloroquine. Conclusion: Symptomatic muscle involvement may be an initial feature of chronic, and usually the systemic form of, sarcoidosis. It responds to corticosteroid therapy, but relapse seems to be frequent.
Garg 2009 examined patients with acute inflammatory ankle arthritis to establish whether they had Löfgren’s syndrome (acute presentation of sarcoidosis) or Ponchet’s disease (reactive arthritis due to tuberculosis infection). They also presented an algorithm for differential diagnosis of such patients. Of 18 patients, 10 were classified with Löfgren’s syndrome, and all of them had a negative Mantoux test (computerised tomography). The remaining patients were classified with Ponchet’s disease, all of them had a positive Mantoux test. Treatments received: The patients also received drug treatment (glucocorticoids and glucocorticoid-sparing drug methotrexate and hydroxychloroquine in patients with Löfgren’s syndrome and anti-tuberculosis drugs in Ponchet’s disease. The numbers treated with each drug were not reported.). Prognosis: All patients with Löfgren’s syndrome responded rapidly to the drugs and became symptom-free over a period of 8–12 weeks. The authors reported that all the patients with Ponchet’s disease responded satisfactorily with complete clinical as well as radiological response. Conclusions: The algorithm was successful at distinguishing between Löfgren’s syndrome and Ponchet’s disease.
Glennås 1995 followed 186 patients presenting with acute arthritis and with suspicion for reactive arthritis for 2 years and sought to classify according to diagnosis. The number of new cases of sarcoid arthritis (SA) per year in Oslo was 2.9/100 000 persons between 18 and 60 years of age. The authors found a clustered onset of SA in the spring (February-June, p = 0.01). All 17 cases of SA had complete remission of arthritis at the 104-week follow-up. Treatments received: Eight of eleven patients received corticosteroids, and 13 of 17 received NSAIDs. Conclusions: The authors concluded that the outcome of acute sarcoid arthritis appeared favourable.
Loupasakis 2015 described a case series with sarcoid arthritis in eleven firefighters in New York who worked at the World Trade Center (WTC) site on September 11, 2001. Nine of 60 firefighters who developed sarcoidosis after this date presented with poly-articular arthritis. Two others diagnosed pre-9/11/2001 developed sarcoid arthritis post-WTC-exposure. All eleven were never cigarette smokers and all performed rescue/recovery at the WTC-site within three days of the attacks. All had biopsy-proven pulmonary sarcoidosis. Treatments received: All required additional disease-modifying anti-rheumatic drugs (DMARDs) for adequate control (stepwise progression from hydroxychloroquine to methotrexate to TNF inhibitors) of their joint manifestations. Conclusions: The authors concluded that chronic inflammatory polyarthritis appears to be an important manifestation of sarcoidosis in firefighters with sarcoidosis and WTC-exposure. Their arthritis is chronic, and unlike arthritis in non-WTC-exposed sarcoid patients, inadequately responsive to conventional oral DMARDs, often requiring TNF inhibitors.
Mana 2003 studied recurrence of sarcoidosis following complete remission in 17 patients from Barcelona, Spain. Sixteen of the patients were women, and they experienced a total of 24 recurrences. The mean follow-up time was 143 ± 80 months. Löfgren’s syndrome was present in 16 patients at onset and in all patients at recurrence. Treatments given: Five patients received corticosteroids. Conclusions: The authors concluded that acute sarcoidosis, and particularly Löfgren’s syndrome, may recur many years after complete remission, and, in general, still has a good outcome.
Miller 2019 retrospectively followed the musculoskeletal and pulmonary outcomes of 24 patients with osseous sarcoidosis. They collected 1-year follow-up and last follow-up outcomes after diagnosis. The authors constructed a composite outcome consisting of (1) osseous sarcoidosis symptoms, (2) musculoskeletal imaging of affected bone, (3) chest imaging, and (4) pulmonary function testing. Treatments given: Three patients were already being treated with DMARDs or glucocorticoids for other sarcoid manifestations at the time of the osseous diagnosis. Miller et al. noted that current DMARD or glucocorticoid use at baseline was associated with a lower proportion of patients with positive worsening composite outcome (22% vs. 60%, p = 0.10). Conclusions: Most patients had a favourable outcome according to symptoms, musculoskeletal/chest imaging, and PFTs, even though only a minority were treated with glucocorticoids or DMARDs.
Perruquet 1984 conducted a retrospective review of all records of patients with a diagnosis of sarcoidosis who were seen at one centre between 1972 and 1982. Thirty-two (21%) of 150 patients with sarcoidosis had articular symptoms. Treatments given: Patients were given salicylates, NSAIDs, prednisone, and colchicine. Conclusion: Joint symptoms were generally transient. Acute sarcoid arthritis has a favourable prognosis.
Petursdottir 2007 used data from the Icelandic Sarcoidosis Study, which contains all tissue-verified cases of sarcoidosis in Iceland since 1981. Their aim was to elucidate the prevalence, clinical manifestations and long-term prognosis of sarcoid arthritis in a nationwide cohort. Forty-seven of 234 patients reported skeletal symptoms. Treatments given: The authors reported that patients were taking NSAIDs, DMARDs and glucocorticosteroids. Of 39 participants, 10 had used physiotherapy or occupational therapy. Three patients had used chiropractic treatment, and one had used acupuncture. Conclusion: Around a fifth of all sarcoidosis patients develop joint symptoms, most frequently in the ankle. The prognosis is mostly favourable, but a subgroup of female patients may develop chronic polyarthritis.
Because the studies were heterogeneous and the methodological quality was so low, we decided to not perform meta-analyses, but present the results as forest plots.
Treatments received in each study.
Pharmacological treatments
Figure 2 is a forest plot of the proportion of patients receiving corticosteroids and NSAIDs in each study. The upper panel shows that between 23 and 100 per cent received corticosteroids in the studies. The lower panel shows that between 0 and 100% received NSAIDs. Hence, the figures show substantial variation amongst studies.
Figure 3 shows the use of two DMARDs (hydroxychloroquine and methotrexate) in the same way. It is estimated that between 5 and 100% received hydroxychloroquine in the studies and that between 12 and 100% received methotrexate.
Figure 4 shows the use of TNF inhibitors (upper panel) and of azathioprine (lower panel). It is estimated that between 0 and 100% received TNF inhibitors, and that between 3 and 4% received azathioprine. There were, however, only two studies with a total of four patients that used azathioprine.
Other pharmacological treatments
Petursdottir 2007 [26] reported that six participants had used DMARDs following diagnosis, whilst five had used these medications at the time of follow-up. The specific DMARDs used were not reported. Eleven participants in the study by Perruquet 1984 [25] had used salicylates, and two had used colchicine.
Non-pharmacological treatments
Only the study by Petursdottir [26] reported use of various non-pharmacological treatments in altogether 10 patients.
Discussion
We did not find any systematic review about non-pharmacological and pharmacological treatments in sarcoidosis with musculoskeletal manifestations. We, therefore, searched for primary studies, without finding a single study which had the aim to estimate treatment effects. Banse [17], Garg [20], and Miller [24] reported before-and-after data for patients using medications, but none of these studies were controlled. Hence, we cannot conclude about treatment effects for patients with musculoskeletal sarcoidosis. This is an important knowledge gap.
All of the ten included studies reported the number of patients using one medication or more. But because of large heterogeneity and poor methodological quality, we did not conduct meta-analyses for proportions of medications used. The forest plots showed large differences between studies in proportion of medication use and should be interpreted with caution. However, the plots give a visual representation of the pharmacotherapies provided in the included studies.
Thus, when clinicians decide on drugs for sarcoidosis, they have no specific evidence to guide them for what works and does not for this disease. Treatment alternatives may, thus, often be chosen with drugs that are known for their anti-inflammatory effects in other arthritic diseases, e.g. rheumatoid arthritis. Drugs against arthritis will therefore be used in this rare disease which may present with Löfgren’s syndrome as one form of reactive arthritis. It is surprising that no RCT for musculoskeletal manifestations of sarcoidosis has been performed. A reason for this could be that acute manifestations of musculoskeletal sarcoidosis are often successfully treated with medium doses of corticosteroids. If chronic manifestations develop, they may affect the musculoskeletal system in various areas and treatment will then be individualised. Another reason may be that frequent lung manifestations in sarcoidosis leave less focus on the chronic musculoskeletal disease burden.
In this systematic review, we included 10 studies and report proportions on the use of the following drug groups in the included studies: corticosteroids, NSAIDs, DMARDs (methotrexate and hydroxychloroquine), TNF inhibitors, and the immunosuppressive agent azathioprine. Because many patients were also participants in research studies with specific medication examined, we cannot be confident about the proportion of use of these drugs outside a research context.
In this systematic review, we were especially interested in evidence for non-pharmacological treatment in musculoskeletal sarcoidosis. Physical training or pulmonary rehabilitation (PR) is an important element of the comprehensive care of people with chronic diseases, including musculoskeletal disorders. The lack of studies with systematic assessment of rehabilitative measures in musculoskeletal sarcoidosis is another important knowledge gap.
To collect information on the benefits of physical activity and training in sarcoidosis, Strookappe et al. [55] performed a comprehensive literature review. As might be expected, all selected publications involved the pulmonary organ and only one was an RCT [56]. One reason for this lack of published evidence may be that most patients with Löfgren’s syndrome have a good prognosis and are initially not in need of physical therapy. Nonetheless, the absence of effect studies on non-pharmacological therapy of musculoskeletal sarcoidosis is striking.
This is, to our best knowledge, the first systematic review on pharmacological and non-pharmacological treatment of musculoskeletal sarcoidosis. A further strength is the application of a systematic literature search and quality assessment of the included studies. A major limitation of this review is that the studies had a high heterogeneity in included patients, and did not always differentiate clearly between musculoskeletal and pulmonary outcomes. Second, the methodological quality of the included studies was poor. Finally, our review was not registered in a prospective register of systematic reviews.
In conclusion, findings in our review do not allow guidance on the effect of pharmacological and non-pharmacological treatment of musculoskeletal manifestations in sarcoidosis. Future studies should use randomised controlled designs and include homogeneous patient samples.
References
Shariatmaghani S, Salari R, Sahebari M, Tabrizi PS, Salari M (2019) Musculoskeletal manifestations of sarcoidosis: a review article. Curr Rheumatol Rev 15:83–89
Arkema EV, Grunewald J, Kullberg S, Eklund A, Askling J (2016) Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J 48:1690–1699
Iannuzzi MC, Fontana JR (2011) Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA 305:391–399
Arthritis in Sarcoidosis G, Agarwal V, Agrawal V, Aggarwal A, Aggarwal P, Chowdhury AC, et al (2018) Arthritis in sarcoidosis: a multicentric study from India. Int J Rheum Dis 21:1728–1733
Rubio-Rivas M, Franco J, Corbella X (2020) Sarcoidosis presenting with and without Lofgren’s syndrome: Clinical, radiological and behavioral differences observed in a group of 691patients. Joint Bone Spine 87:141–147
Jeon MH, Kang T, Yoo SH, Swan HS, Kim HJ, Ahn HS (2020) The incidence, comorbidity and mortality of sarcoidosis in Korea, 2008–2015: a nationwide population-based study. Sarcoidosis Vasc Diffuse Lung Dis 37:24–26
Beegle SH, Barba K, Gobunsuy R, Judson MA (2013) Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Dev Ther 7:325–338
Baughman RP, Wells A (2019) Advanced sarcoidosis. Curr Opin Pulm Med 25:497–504
Hammam N, Evans M, Morgan E, Reimold A, Anastasiou C, Kay JL, et al. (2020) Treatment of Sarcoidosis in U.S. Rheumatology Practices: Data from ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry. Arthritis Care & Research n/a.
Wallaert B, Kyheng M, Labreuche J, Stelianides S, Wemeau L, Grosbois JM (2020) Long-term effects of pulmonary rehabilitation on daily life physical activity of patients with stage IV sarcoidosis: a randomized controlled trial. Respir Med Res 77:1–7
Moola S. Systematic reviews of etiology and risk in Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute; 2017.
Balduzzi S, Rucker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 22:153–160
Adler BL, Wang CJ, Bui TL, Schilperoort HM, Armstrong AW (2019) Anti-tumor necrosis factor agents in sarcoidosis: a systematic review of efficacy and safety. Semin Arthritis Rheum 48:1093–1104
Baughman RP, Papanikolaou I (2017) Current concepts regarding calcium metabolism and bone health in sarcoidosis. Curr Opin Pulm Med 23:476–481
Bechman K, Christidis D, Walsh S, Birring SS, Galloway J (2018) A review of the musculoskeletal manifestations of sarcoidosis. Rheumatology (Oxford) 57:777–783
Drent M, Cremers JP, Jansen TL (2014) Pulmonology meets rheumatology in sarcoidosis: a review on the therapeutic approach. Curr Opin Rheumatol 26:276–284
Banse C, Bisson-Vaivre A, Kozyreff-Meurice M, Vittecoq O, Goeb V (2013) No impact of tumor necrosis-factor antagonists on the joint manifestations of sarcoidosis. Int J Gen Med 6:605–611
Cacciatore C, Belnou P, Thietart S, Desthieux C, Versini M, Abisror N et al (2020) Acute and chronic sarcoid arthropathies: characteristics and treatments from a retrospective nationwide french study. Front Med (Lausanne) 7:565420
Fayad F, Liote F, Berenbaum F, Orcel P, Bardin T (2006) Muscle involvement in sarcoidosis: a retrospective and followup studies. J Rheumatol 33:98–103
Garg S, Malaviya AN, Kapoor S, Rawat R, Agarwal D, Sharma A (2011) Acute inflammatory ankle arthritis in northern India–Lofgren’s syndrome or Poncet’s disease? J Assoc Physicians India 59:87–90
Glennas A, Kvien TK, Melby K, Refvem OK, Andrup O, Karstensen B et al (1995) Acute sarcoid arthritis: occurrence, seasonal onset, clinical features and outcome. Br J Rheumatol 34:45–50
Loupasakis K, Berman J, Jaber N, Zeig-Owens R, Webber MP, Glaser MS et al (2015) Refractory sarcoid arthritis in World Trade Center-exposed New York City firefighters: a case series. J Clin Rheumatol 21:19–23
Mana J, Montero A, Vidal M, Marcoval J, Pujol R (2003) Recurrent sarcoidosis: a study of 17 patients with 24 episodes of recurrence. Sarcoidosis Vasc Diffuse Lung Dis 20:212–221
Miller ER, Fanta CH, McSparron JI, Pan B, Coblyn JS, Sparks JA (2019) Musculoskeletal and pulmonary outcomes of sarcoidosis after initial presentation of osseous involvement. Sarcoidosis Vasc Diffuse Lung Dis 36:60–73
Perruquet JL, Harrington TM, Davis DE, Viozzi FJ (1984) Sarcoid arthritis in a North American Caucasian population. J Rheumatol 11:521–525
Petursdottir D, Haraldsdottir SO, Gislason T, Gudbjornsson B (2007) Clinical manifestation, prevalence and prognosis of sarcoid arthropathy. A nationwide study: the Icelandic Sarcoidosis Study. Sarcoidosis Vasc Diffuse Lung Dis 24:113–120
Androdias G, Maillet D, Marignier R, Pinede L, Confavreux C, Broussolle C et al (2011) Mycophenolate mofetil may be effective in CNS sarcoidosis but not in sarcoid myopathy. Neurology 76:1168–1172
Banse C, Bisson-Vaivre A, Kozyreff-Meurice M, Dominique S, Vittecoq O, Goeb V. Impact of TNF antagonists on the joint manifestations of sarcoidosis. Annals of the Rheumatic Diseases Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR,2013.
Barnard J, Newman LS (2001) Sarcoidosis: immunology, rheumatic involvement, and therapeutics. Curr Opin Rheumatol 13:84–91
Bello S, Bonali C, Serafino L, Rotondo C, Terlizzi N, Lapadula G (2014) Intra-articular therapy with tumor necrosis factor-α antagonists: an update. Reumatismo 65:257–263
Ben Hassine I, Rein C, Comarmond C, Glanowski C, Saidenberg-Kermanac’h N, Meunier B et al (2019) Osseous sarcoidosis: a multicenter retrospective case-control study of 48 patients. Joint Bone Spine 86:789–793
Crommelin HA, Vorselaars AD, van Moorsel CH, Korenromp IH, Deneer VH, Grutters JC (2015) Is there evidence for anti-TNF drugs in joint involvement in sarcoidosis? Immunotherapy 7:601
Glanowski C, Mestiri R, Bialé L, Carmoi T, Lechevalier D, Leroux G et al (2018) THU0612 Bone sarcoidosis: a retrospective multicenter study of 27 cases. Ann Rheum Dis 77:505
Hassine I, Rein C, Comarmond C, Chapelon-Abric C, Saidenberg N, Meunier B, et al. Bone sarcoidosis: a french case control study. 2018 ACR/ARP Annual Meeting, Oct 2018; Chicago, United States: Arthritis Rheumatol., 70 (suppl 10). Abstract Number: 2259. ⟨hal-01998033⟩; 2018. p. 2495.
Hobbs K (2005) Chronic sarcoid arthritis treated with intraarticular etanercept. Arthritis Rheum 52:987–988
Huang A, Harty LC, Ryan CM, Veale DJ (2011) Clinical images: infliximab therapy of polyarticular small joint sarcoid arthritis. Arthritis Rheum 63:2030
James DG, Neville E, Carstairs LS (1976) Bone and joint sarcoidosis. Semin Arthritis Rheum 6:53–81
Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI (1986) Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and complications. Ann N Y Acad Sci 465:702–712
Khanna D, Liebling MR, Louie JS (2003) Etanercept ameliorates sarcoidosis arthritis and skin disease. J Rheumatol 30:1864–1867
Madronal Garcia I. Joint manifestations in a cohort of patients with sarcoidosis in a third level hospital. Annual European Congress of Rheumatology, EULAR 2020 Germany2020. p. 1814.
Mana J, Rubio-Rivas M, Villalba N, Marcoval J, Iriarte A, Molina-Molina M et al (2017) Multidisciplinary approach and long-term follow-up in a series of 640 consecutive patients with sarcoidosis: cohort study of a 40-year clinical experience at a tertiary referral center in Barcelona. Spain Med (Baltimore) 96:e7595
Martín-Varillas JL, Bilbao LS, González-Mazón I, Ramón RF, Prieto-Peña D, Martinez-Lopez D et al (2019) FRI0604 Systemic sarcoidosis Study of 381 patients from a tertiary university hospital in the north of Spain. Ann Rheumatic Dis 78:997
Mathur A, Kremer JM (1993) Immunopathology, musculoskeletal features, and treatment of sarcoidosis. Curr Opin Rheumatol 5:90–94
Medici T, Mannhart R, Tanner E (1977) Effect of steroid therapy on acute sarcoidosis (Loefgren’s syndrome). Am Rev Respir Dis 115:141
Neville E, Walker AN, James DG (1983) Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 52:525–533
Oshagbemi OA, Driessen JHM, Pieffers A, Wouters EFM, Geusens P, Vestergaard P et al (2017) Use of systemic glucocorticoids and the risk of major osteoporotic fractures in patients with sarcoidosis. Osteoporos Int 28:2859–2866
Patil S, Arakane M, Jenigiri S, Field E, Singh N (2018) Musculoskeletal sarcoidosis: a 15-year experience from a tertiary care center in the us. Arthr Rheumatol 70:2496–2497 (PubMed PMID: 626435339)
Retamozo S (2019) ACR/ARP annual meeting abstract supplement. Arthritis Rheumatol 2019:1–5362
Rubio-Rivas M, Corbella X, Mana J (2019) Elderly sarcoidosis: a comparative study from a 42-year single-centre experience. Respir Med 152:1–6
Russell E, O'Connor C, Hussain H. Long term follow-up of infliximab efficacy in pulmonary and extrapulmonary sarcoidosis refractory to conventional therapy. Arthritis and Rheumatism Conference: Annual Scientific Meeting of the American College of Rheumatology and Association of Rheumatology Health Professionals 20112011.
Schmajuk G, editor Patterns of Medication Use for Patients with Sarcoidosis: Data from the ACR ’ s RISE Registry. 2019 ACR/ARP Annual Meeting November 8–13, 2019; 2019; Atlanta.
Somayeh S, Roshanak S, Maryam S, Payman Shalchian T, Masoumeh S (2019) Musculoskeletal manifestations of sarcoidosis: a review article. Curr Rheumatol Rev 15:83–89
Tejera Segura B, Holgado S, Mateo L, Pego-Reigosa JM, Carnicero Iglesias M, Olivé A (2014) Löfgren syndrome: a study of 80 cases. Med Clin (Barc) 143:166–169
Zhou Y, Lower EE, Li H, Farhey Y, Baughman RP (2017) Clinical characteristics of patients with bone sarcoidosis. Semin Arthritis Rheum 47:143–148
Strookappe B, Saketkoo LA, Elfferich M, Holland A, De Vries J, Knevel T et al (2016) Physical activity and training in sarcoidosis: review and experience-based recommendations. Expert Rev Respir Med 10:1057–1068
Karadalli MN, Bosnak-Guclu M, Camcioglu B, Kokturk N, Turktas H (2016) Effects of inspiratory muscle training in subjects with sarcoidosis: a randomized controlled clinical trial. Respir Care 61:483–494
Funding
Open access funding provided by Diakonhjemmet Hospital. No specific funding was provided for this work.
Author information
Authors and Affiliations
Contributions
All authors provided substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. GS drafted the work and TU and MK revised it critically for important intellectual content. All authors gave final approval of the version to be published. Finally, all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smedslund, G., Kotar, A.M. & Uhlig, T. Sarcoidosis with musculoskeletal manifestations: systematic review of non-pharmacological and pharmacological treatments. Rheumatol Int 42, 2109–2124 (2022). https://doi.org/10.1007/s00296-022-05171-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05171-8