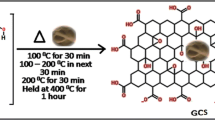
Abstract
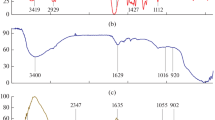
Cr (VI) is a highly toxic pollutant that is known to be carcinogenic and mutagenic, and it must be completely removed from waste water before disposal. A novel graphene-silicate composite (GS-Composite) adsorbent was produced from asphalt and sand. The synthesized GS-Composite was characterized using X-ray diffraction. The adsorption of Cr (VI) ions from aqueous solution was investigated using a batch adsorption technique under various experimental circumstances. Nano-zeolite (NZ) was used as comparative adsorbent and added with various ratio to GS-Composite. The surface charge of both sorbents was determined via DLS measurements. The results declare that GS-composite is strong adsorbent for chromium “III” from aqueous media. The specific surface area of the GS-Composite and NZ was determined to be 88.20 m2/g and 50.46 m2/g, respectively. At pH 1.5, GS-composite was found to be a powerful reducing agent to Cr (VI). The maximum adsorption capacity of GS-Composite for Cr (III) was determined to be 50.00 mg/g, with an R2 value of 0.9959, and the equilibrium sorption data were adequately matched to the Langmuir adsorption model. The findings of this investigation showed that GS-Composite is a powerful and cost-effective as a reducing-adsorbent agent for Cr (VI) and Cr (III), respectively.









Similar content being viewed by others
References
Elshamy OA, El-Fawal EM (2021) Synthesis of NiFe2 O4 @AC/UiO-66(Zr) for enhancement of the photocatalytic performance of alizarin yellow R under visible-light. ChemistrySelect 6:995–1007. https://doi.org/10.1002/slct.202004567
El-Fawal EM, El-Shamy OAA (2019) Photodegradation enhancement of 2-chlorophenol using ZnO–CdS@CS nanocomposite under visible light. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02249-y
El-Tabey AE, Mady AH, El-Shamy OAA, Ragab AA (2020) Sustainable approach: utilizing modified waste styrofoam as an eco-friendly catalyst for dual treatment of wastewater. Polym Bull. https://doi.org/10.1007/s00289-020-03135-1
Choi K, Lee S, Park JO, Park J-A, Cho S-H, Lee SY, Lee JH, Choi J-W (2018) Chromium removal from aqueous solution by a PEI-silica nanocomposite. Sci Rep 8:1–10
Crisostomo CAB, Lima FA, Dias RM, Cardoso VL, De Resende MM (2016) Joint assessment of bioreduction of chromium (VI) and of removals of both total chromium and total organic carbon (TOC) in sequential hybrid bioreactors. Water Air Soil Pollut 227:51
Shi L, Zhang X, Chen Z (2011) Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res 45:886–892
El-Shamy OAA, El-Azabawy RE, El-Azabawy O (2019) Synthesis and characterization of magnetite-alginate nanoparticles for enhancement of nickel and cobalt ion adsorption from wastewater. J Nanomater 2019:1–8
Lin C, Qiao S, Luo W, Liu Y, Liu D, Li X, Liu M (2014) Thermodynamics, kinetics, and regeneration studies for adsorption of Cr (VI) from aqueous solutions using modified cellulose as adsorbent. BioResources 9:6998–7017
Li L-L, Feng X-Q, Han R-P, Zang S-Q, Yang G (2017) Cr (VI) removal via anion exchange on a silver-triazolate MOF. J Hazard Mater 321:622–628
El-Sharaky EA, Mishrif MR, El-Shamy OAA (2018) Synthesis and evaluation of a new trianionic surfactant for the removal of Pb(II) by flotation method. Tenside Surfactants Deterg 55:148–152. https://doi.org/10.3139/113.110545
Khlyustova A, Sirotkin N, Titov V (2019) Plasma-induced precipitation of metal ions in aqueous solutions. J Chem Technol Biotechnol 94:3987–3992
Qiu Y-R, Mao L-J (2013) Removal of heavy metal ions from aqueous solution by ultrafiltration assisted with copolymer of maleic acid and acrylic acid. Desalination 329:78–85
Yusof AM, Malek NANN (2009) Removal of Cr (VI) and As (V) from aqueous solutions by HDTMA-modified zeolite Y. J Hazard Mater 162:1019–1024
Wu Z, Zhao D (2011) Ordered mesoporous materials as adsorbents. Chem Commun 47:3332–3338
Shutov DA, Sungurova AV, Manukyan AS, Rybkin VV (2018) Reduction-oxidation of chromium ions in aqueous solution by treatment with atmospheric-pressure direct-current discharge in argon. High Energy Chem 52:429–432
Ighere JO, Honjoya K, Chawla RC (2014) Using ferrous ion for the reductive degradation of hexavalent chromium. Adv Chem Eng Sci 5:15
Wang J, Ji B, Shu Y, Chen W, Zhu L, Chen F (2018) Cr (VI) removal from aqueous solution using starch and sodium carboxymethyl cellulose-coated Fe and Fe/Ni nanoparticles. Pol J Environ Stud 27(6):2785
Asuquo E, Martin A, Nzerem P (2018) Evaluation of Cd(II) Ion removal from aqueous solution by a low-cost adsorbent prepared from white yam (Dioscorea rotundata) waste using batch sorption. ChemEngineering 2:35. https://doi.org/10.3390/chemengineering2030035
Asif Z, Chen Z (2017) Removal of arsenic from drinking water using rice husk. Appl Water Sci 7:1449–1458
Dubey R, Bajpai J, Bajpai AK (2015) Green synthesis of graphene sand composite (GSC) as novel adsorbent for efficient removal of Cr (VI) ions from aqueous solution. J Water Process Eng 5:83–94
Fontes L, Tostes N, Braga M, Guerra B, Manhas F, Pereira V, Ernesto C, Reynaud G, Pereira-filho ER (2013) Proposition of a simple method for chromium (VI) determination in soils from remote places applying digital images : a case study from Brazilian Antarctic Station. Microchem J 109:165–169. https://doi.org/10.1016/j.microc.2012.03.007
Khairnar RS, Wakde MD, Mahabole MP, Krishna R, Titus E (2014) Development of ethanol sensor using sodium A nano zeolite. Development 3
Chapi S, Devendrappa H (2016) Optical, electrical, thermal and electrochemical studies of spin-coated polyblend-ZnO nanocomposites. J Mater Sci Mater Electron 27:11974–11985. https://doi.org/10.1007/s10854-016-5344-1
Chapi S, Raghu S, Devendrappa H (2016) Enhanced electrochemical, structural, optical, thermal stability and ionic conductivity of (PEO/PVP) polymer blend electrolyte for electrochemical applications. Ionics (Kiel) 22:803–814. https://doi.org/10.1007/s11581-015-1600-2
Abdel-karim AM, Shahen S, Elsisi DM, Hyba AM, El-Shamy OAA (2022) Experimental and theoretical studies of corrosion resistance enhancement of carbon steel in 1 M HCl by quinoxalinosulfonamide hybrid-bearing theophylline moiety. J Bio- Tribo-Corrosion 8:70. https://doi.org/10.1007/s40735-022-00666-0
El Feky AA, Shalaby MN, El-Shamy OAA, Selim SA (2010) Adsorption of some surfactants onto polyvinyl alcohol as hydrophobic polymer surface. J Dispers Sci Technol 31:1091–1099
El-Dib FI, Mohamed DE, El-Shamy OAA, Mishrif MR (2020) Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt J Pet 29:1–7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared the absence of conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Shamy, O.A.A., Nassar, I.M. & Ragab, A.A. Facile synthesis of graphene sand composite from asphalt as an effective adsorbent for chromium ions in aqueous media. Polym. Bull. 80, 9899–9911 (2023). https://doi.org/10.1007/s00289-022-04545-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04545-z