Abstract
Fungal bio-control agents (BCA) can minimize use of agro-chemicals while increasing plant productivity and tolerance to biotic-abiotic stressors. Ideally, BCA should tolerate varying environmental conditions they are introduced into, to successfully dominate and protect plants from stressors. However, BCA are living micro-organisms, their survival and efficacy can be impeded by extreme conditions. The current study aimed at evaluating whether indigenous fungal isolates, viz, Aspergillus flavus, A. terreus, Penicillium sp. AL-38 IRH-2012b, Talaromyces minioluteus, T. purpureogenus, T. sayulitensis, Trichoderma ghanense and T. viride can tolerate different levels of salinity, pH, nutrient and temperature. Certain fungal species are pests with potential of destroying many crops; the pathogenic effects of the aforementioned fungal isolates were further assessed on different crops’ seeds. The results showed that, although being indigenous, Aspergillus, T. sayulitensis and T. ghanense failed to thrive in high salinity and pH. While Penicillium sp. AL-38 IRH-2012b failed to thrive under reduced nutrient level and all fungal isolates failed to grow at 10–20 °C. Furthermore, it was noted species within the same genus could affect crops in both favorable and unfavorable ways. The study demonstrated that the selected indigenous fungal isolates can tolerate different abiotic conditions and have potential to improve seed germination and seedling growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their importance in human diet, leafy vegetables and grains are globally cultivated and consumed as sources of essential nutrients and fiber [1, 2]. These crops are grown either in open fields or intensively managed environmentally controlled settings. However, soil-borne pathogens represent a severe threat to optimal crop production [3, 4]. Consequently, low crop production could impede farmers and communities from contributing to 2030 Sustainable Development Goals (SDGs 1–3) of No Poverty, Zero Hunger, and Good Health and Well-being (United Nations 2020). Moreover, as consumers demand for year-round production of fresh produce with little to no chemical residues, growers face even more difficulties managing pathogens. Currently, researchers are focusing on studying microbes that are both safe for humans and the environment [5, 6].
In South Africa (SA), indigenous fungal isolates have demonstrated tremendous potential as bio-control agents (BCA) against plant pathogens [7, 8]. However, abiotic conditions like temperature have an impact on BCA effectiveness [9]. Aspergillus, Penicillium, Talaromyces and Trichoderma species have been the subject of extensive research in plant pathogen management [10, 11]. Several studies have attributed fungal BCA capacity for bio-control to the production of antimicrobial substances, lytic enzymes, pathogenesis-related proteins, phenolic secondary metabolites, as well as their ability to outcompete other root pathogens and inducing systemic resistance in plants [12, 13].
Soil-borne plant diseases caused by soil-borne pathogen are brought about by ecosystem imbalances such as changes in planting conditions and local environment. As a result, abiotic stress is a crucial element that significantly affects agricultural soil fertility and productivity [14]. Abiotically stressed soils that are acidic with high salinity levels and nutrient-deficiency were shown to benefit very slightly from the use of BCA; regardless of whether bio-agents have bio-control or bio-fertilizer qualities [15]. Therefore, it is imperative to determine abiotic stress tolerance in beneficial soil fungi. This is because fungal BCA that have evolved to thrive in harsh conditions, such as high salinity or relatively high temperatures, may in turn have an impact on their symbiotic hosts’ ability to adapt to a comparable spectrum of situations.
It is generally recognized that two key characteristics must be present or improved when choosing acceptable fungal BCA for use as biocides: (i) high virulence against the intended pests and (ii) environmental stress tolerance [15, 16]. Despite the fact that these BCA have been studied for their potential to parasitize plant pathogens [5, 6], little research focus has been placed on determining how well these isolates can withstand abiotic stress and/or recover from it. Additionally, there are several species of fungi that are pests in and of themselves, destroying unfathomable amounts of crops, forests, stored goods and structures, while also harming both human and animal health [17, 18]. For these reasons, determining the unintended consequences of using indigenous fungi as BCA is necessary before developing and using them.
Considering that field studies would require handling a wide range of complex soil types, an in vitro study was therefore formulated. The objective of the study was to carry out in vitro assessment of tolerance level of selected indigenous fungal isolates to various abiotic stresses i.e., salinity, pH, nutrient status and temperature. The study also looked at fungal isolates’ biosafety on seeds.
Materials and Methods
Study Area
The study was conducted at North-West University, Mahikeng, SA, during winter season (June–July 2022). The climate of the area is classified as subtropical, is characterized by an average temperature of 5 °C in winter and 32 °C in summer and summer rainfall range between 1100 and 1600 mm/year.
Fungal isolates
Fifty soil samples around plant roots, with no specific target plants, were collected using a garden trowel at North-West University, agricultural research farm, Molelwane, Mmabatho (latitude of 25.810 S and longitude of 25.630 E). The soil samples were transported to the laboratory in sterile polythene zip-lock bags and investigated for the presence of fungi. For fungal isolation, soil serial dilution plate method was used [19]. Serial dilutions were made from 10 g in 100 mL soil suspension with sub-subsamples being cultured on Petri dishes containing potato dextrose agar (PDA) media (Biolab, Lawrenceville, Georgia, USA) (39 mg L−1) treated with Streptomycin (0.5 g L−1) (Mast Diagnostics, UK). The PDA media was specifically used to enable isolation of both endophytic and free-living fungi that have direct and indirect soil ecological functions such as antibiosis and mycoparasitism. The Petri dishes were then incubated at 25 ± 2 °C and observed every two days for re-isolation of pure cultures [20]. Individual fungal colonies were each isolated and purified. Seven-day-old pure culture mycelia of respective fungal isolates were collected from the colony edges and microscopically examined. The hyphae and spore color, size and shape were used to identify the different species [21]. The morphological identification was supplemented by molecular identification using sequencing of internal transcribed spacer (ITS) gene region (with ITS1 and ITS2 sub-regions), and their identities were confirmed by taxonomist, Inqaba Laboratory, Tshwane, Gauteng Province, SA.
Soil Tests
The same soil samples used for serial dilutions were air-dried and sieved through a 2-mm sieve. Soil pH, electrical conductivity (EC), soil texture and organic compost (OC) were determined. Soil pH was tested using the Bouyoucos hydrometer technique [22], and EC was measured following Richard [23] procedure. Soil texture was determined using standard glass/calomel electrodes in a 1:2.5 w/v soil–water suspension [24]. Soil OC was tested following a method by Walkley [25].
Tolerance of Indigenous Fungal Isolates To Extreme Environmental Conditions
To investigate the abiotic stress tolerance of the selected indigenous isolates, a concentration gradient culture was established following a modified procedure by Yu et al. [26]. For matching saline-alkali land, NaCl and NaHCO3 were added at various concentrations to the PDA medium. The medium concentrations (w/v, g/mL) of 1, 4, 7 and 10% m/v were added to generate final salt stress. Likewise, alkali stress media (w/v, g/mL) of pH levels of 6.25, 6.50, 0.7 and 7.00 were obtained, with an untreated control of 5.77. To simulate a nutrient-deficient soil, PDA medium was diluted to 25, 50, 75 and 100%. To test for extreme temperatures, cultures were grown under 10, 20, 30 and 40 °C temperatures and growth rates measured. When NaCl−, pH and nutrient deficiency-amended media in the Petri dishes had solidified and cooled, a 5-mm-diameter mycelial disk of the respective test isolate was placed in the middle of the Petri dish and incubated at 25 ± 2 °C. For temperature tests, Petri dishes were incubated at different temperature levels. For each tolerance test, the culture conditions consisted of five replicates per treatment level and the radius of the colony was recorded using a ruler every 48 h for eight days.
Fungal Isolates Biosafety Tests on Crop Seeds
Eight treatments of fungal BCA and untreated control, with five replications, were laid out in a completely randomized design (CRD) in vitro. Seeds of tomato (Solanum lycopersicum ‘Hotstuff’), swiss chard (Beta vulgaris subsp. vulgaris ‘Fordhock giant’), beetroot (B. vulgaris subsp. vulgaris ‘Detroit’), dry bean (Phaseolus vulgaris ‘PAN 148’), pumpkin (Cucurbita moschata L.), maize (Zea mays L.) and sorghum (Sorghum bicolor L.) were purchased at NWK store, Mahikeng, SA and were tested for fungal pathogenicity. Following a modified procedure by Coninck et al. [27], seeds were disinfected with 10% NaOCl solution for 5 min, washed three times for 5 min with sterile distilled water. The seeds were put on sterile paper towel and allowed to air dry for 24 h inside a laminar flow cabinet (FILTA-MATIX) before being coated and tested. To avoid aggregation formation, each fungal isolate was dissolved in water before being added to chitosan mixture and agitated frequently to make a slurry solution. Previously sterilized and dried seeds were submerged in their corresponding slurry solutions for 2–5 s before being allowed to air dry for 48 h at room temperature, i.e., 27 °C. Ten seeds per treatment were tested for germination in Petri dishes using Whatman No. 1 filter paper and 5 mL of sterile distilled water. When roots longer than 5 mm were seen, seeds were deemed to have germinated and inspected for necrosis and rotting. The experiment was repeated twice for validation, data compared and did not differ between the experiments.
Data Analysis
Based on the homoscedascity assumption, i.e., that different fungal species vary in their tolerance to abiotic factors and may also differ in their biosafety on seed germination, the data were subjected to analysis of variance (regression) using SAS version 9.4 package (SAS Institute Inc., Cary, NC, USA) to test significance of the variation. Tukey’s Honestly Significant Difference (HSD) post-hoc test was used for mean comparisons at the probability level of 5%. Fungal dose–response curves were modeled by the regression estimations.
Results
Soil Characteristics
The results on soil physical properties indicated that the soils were silt loam (bulk density: 1 420 kg/m, field capacity: 320 mm/m, wilting point: 120 mm/m and porosity: 46%), slightly acidic with pH ranging from 5.85 to 6.25, EC ranged between 132.12 and 151.80 µs, while OC ranged from 22.50 to 26.10% and local temperatures fluctuated from 18 to 40 °C during collection of soil samples.
Indigenous Fungal Isolates
Fungal identities were confirmed through molecular identification as follows: A. flavus, A. terreus, Penicillium sp. AL-38 IRH-2012b, T. minioluteus, T. purpureogenus, T. sayulitensis, T. ghanense and T. viride (Table 1).
Response of Indigenous Fungal Isolates to Extreme Environments
The growth responses of A. flavus, A. terreus, Penicillium sp. AL-38 IRH-2012b, T. minioluteus, T. purpureogenus, T. sayulitensis, T. ghanense and T. viride differed significantly (P ≤ 0.05, F = 840.36, degrees of freedom = 7) to various levels of salinity, pH, nutrient deficiency and temperature (Fig. 1).
a Effect of different levels of salinity on fungi radial growth (mm) at eight days incubation period. b Effect of different levels of pH on fungi radial growth (mm) at eight days incubation period. c Effect of different levels of nutrient on fungi radial growth (mm) at eight days incubation period (n = 20). d Effect of different levels of temperature on fungi radial growth (mm) at eight days incubation period (n = 20)
Salinity
The isolates’ radii shrank significantly (P ≤ 0.05, F = 888.15, degrees of freedom = 7) with increasing salt concentration, except T. viride and T. purpureogenus that demonstrated superior adaptation (Table 2, supplementary data file). All eight selected fungal isolates thrived at 1%m/v salt concentration, with T. sayulitensis showing the least tolerance to salt stress than the other isolates. The radii of A. flavus significantly decreased as salt concentration increased (Fig. 2a). Nonetheless, all eight isolates could grow at 10% salt concentration (Fig. 2b).
PH
There was a highly significant difference (P ≤ 0.05, F = 687.86, degrees of freedom = 7) among all the eight fungal isolates at all levels (Table 3, supplementary data file). From the Talaromyces genus, T. purpureogenus adapted to changing pH levels (Fig. 3a) compared to T. minioluteus and T. sayulitensis. Even though T. viride showed better adaptation to pH levels (Fig. 3b), T. ghanense failed to grow at 6.75 pH-amended PDA. Penicillium radii growth decreased as pH levels increased but was still able to grow at a slow rate. Both Aspergillus species halted their radii growth at 1% pH level.
Nutrient Deficiency
Change in nutrient status had significant effect (P ≤ 0.05, F = 104.38, degrees of freedom = 7) on seven fungal isolates, except T. minioluteus. Even at 100% nutrient status, T. minioluteus showed the least growth rate (Table 4, supplementary data file). Although their thickness was reduced and was growing sparsely (Fig. 4). The results further showed that the A. flavus tolerated the nutrient stress better than the other isolates.
Temperature
At 10 and 40 °C, none of the fungal isolates examined grew. The optimal growth temperatures varied from 27 to 32 °C and did not differ significantly (P ≤ 0.05) between eight of the local isolates. The current study demonstrated that there was no growth observed on the isolates at temperatures below 10 °C. Additionally, increasing the temperature above 40 °C resulted in the inhibition of mycelial growth (Table 5, supplementary data file). However, it was observed that it kept the fungal spores viable. Isolates’ growth rates increased when the temperature was raised from above 20 °C. The range between 27 and 30 °C was found to be ideal for mycelial development.
Fungal Isolates Effects on Crops’ Seeds
Based on seed germination rate, plumule growth, radicle growth and seedling vigor index, the two Trichoderma species showed the highest seedling growth, followed by Talaromyces, A. terreus and Penicillium sp. AL-38 IRH-2012b, respectively. While A. flavus inhibited seed germination and caused seed rot. Aspergillus terreus promoted seed germination and subsequent improved seedling growth (Fig. 5).
Discussion
Soil abiotic stress is a worldwide problem and has become a major factor limiting agricultural and forestry production [28]. Soil characteristics influence establishment and subsequent growth of micro-organisms in the rhizosphere. Due to their failure to colonize the host rhizosphere in stressful natural conditions, several fungal BCA that have promising potential when tested in vitro often fail to thrive under field conditions. As such, to maintain viability of BCA spores throughout storage and following formulation operations, tolerance to extreme conditions is important. The tolerance of plants to abiotic and biotic stress can be improved by the application of BCA [29]; however, the applied BCA isolates must acclimatize to the abiotic stress well.
Fungal Isolates Tolerance to Salt
Results from the current study showed that the growth of T. viride was not hindered by 10% (1709.40 mM) NaCl. However, as incubation time increased, the promotion effect gradually weakened for the seven fungal isolates, except for T. viride, thus exhibiting remarkable NaCl tolerance. This observation showed that different fungal species from the same genus can have different tolerance capacity for the same salinity level, which could be attributed to different metabolites from individual species. For instance, Yusnawan et al. [30] identified high concentrations of isoprophyl-1-methyl, ledene oxide and acetone in T. virens T.v3. While gurjunene, butanol and himachalene were found in T. asperellum T.v8 [31]. This demonstrated the potential of T. viride to be applied in soils with high salinity levels.
This has been corroborated in previous field studies where T. asperellum RM-28, which tolerates saline (4% NaCl) environments, improved the growth and resistance of sorghum-sudan grass (Sorghum bicolor ssp. drummondii) seedlings [32]. Similarly, Zhang et al. [33] observed that Cucumber (Cucumis sativus ‘Chuanlv 21’) leaves' total chlorophyll content dropped from 1.29 to 1.00 mg/g when NaCl concentrations increased (0, 100 and 200 mM). Application of T. atroviride (HN082102.1), however, caused the content of total chlorophyll to rise by 16.57, 13.93 and 19.68%. All the tested indigenous fungal isolates have potential to be applied for crop improvement and protection in soils with approximately 4% (700 mM) salinity.
Fungal Isolates Tolerance to pH
The pH of the rhizosphere affected the population of soil microbes as well as a number of metabolic activities. Also, the pH of the growth medium affected mycelial development and bio-control potential by regulating the synthesis of extracellular enzymes. The indigenous T. viride was more tolerant to a wide range of pH, followed by T. purpureogenus and then Penicillium sp. AL-38 IRH-2012b. This further demonstrated how different enzymes and other metabolites from different fungal isolates within the same genus play a role in their respective adaptation capabilities. These metabolites are known to aid in adapting to a wide range of pH and promote greater mycelial development and biological activity [34]. Trichoderma viride, T. purpureogenus and Penicillium sp. AL-38 IRH-2012b species’ potential as inoculant bio-agents in soils where changing pH is anticipated is supported by their improved tolerance and survival at increased pH levels.
The current results showed that T. viride changed the pH of the medium to levels close to neutrality, which is an impressive development that sets it apart from the other examined isolates. This was observed when the association between biomass production and pH modification in the pH media was evaluated. Given that the influence of pH stress reduces the generation of mycelium, there is sufficient evidence to support the use of T. viride in crop production under pH soils. This is due to the fact that not only did the isolate prove adaptation to high pH, but altered the pH to the level more suitable for most agricultural crops [35].
Cabral-Miramontes et al. [36] identified that T. harzianum has an adaptation to high pH, like T. viride isolates from the current study, it exhibited the ability to alter the pH of the medium. As a result, the effects of pH do not prevent mycelial development, and biomass increase is beneficial in the creation of the plant–microbe correlation since mycelium serves to house plant roots and give nutrients, resulting in a mutualistic connection. Extreme pH settings do not harm these micro-organisms. They adjust to their surroundings by secreting extracellular metabolites to reduce stress. These molecules work in conjunction with the proper molecular niche development for the advantageous exchange of protons and this is crucial for the adjustment of valences in chemical compounds. As a result, they are taken up by plants and microbes as macro- and micronutrients [37].
Fungal Isolates Tolerance to Nutrient Deficiency
Survival BCA techniques involve competition for the growing site and eventual overtaking it. The capacity to develop quickly is the key advantage in the race for resources like nutrients and space [38]. Considering that there are possibilities of fungal BCA to grow slowly in a nutrient-poor environment, they might not be able to successfully establish in the growth site and outcompete other local plant-pathogenic micro-organisms when placed in such soils. Such BCA with weak growth potential may also not survive in the soil for a long period. Important bio-control techniques involve competition for the growing site and eventual overtaking it [39].
The fungal isolates morphology was examined under a compound microscope, and it was observed that the hyphae in the 100% nutrient level had distinct and turgid hyphal structures. When compared to A. terreus at equivalent test durations and nutrient levels, this isolate developed flaccid hyphae; the growth and development phenotype reverse of Penicillium sp. AL-38 IRH-2012b and T. viride. It could be that the metabolism reacts differently to prevent potential hydroxyl ion damage. Furthermore, it had a sparse structure, indicating that it does not cluster to react at excessive nutrient deficiency [36]. The observed thin and sparsely distributed growth (Fig. 4) could mean that under insufficient nutrient level, the BCA would be less effective and may be even overgrown by other pathogenic competing microbes.
Fungal Isolates Tolerance to Temperature
Fungal BCA tolerance capacity to temperature fluctuations is an important criterion for the organism's survival under field conditions. In this study, all eight selected indigenous isolates showed the best growth at 27–30 °C. Trichoderma isolates were found to show comparatively better growth characteristics in all tested temperatures, i.e., from 10 to 40 °C. In contrast, it was noted that Talaromyces isolates exhibited the lowest growth level. Similarly, Affokpon et al. [38] observed that the sporulation as well as mycelia development were both greater at 28 °C. Following three weeks of the incubation, fungal isolates were able to develop normally and distinguished by strongly stimulated sporulation.
All the isolates failed to develop at temperatures from 10 °C and below, in contrast to Trichoderma species, albeit at significantly slower rates than the other temperatures examined. One of the species' survival mechanisms in extremely cold conditions, such those between 10 and 20 °C, seems to be the induction of chlamydospores. Trichoderma species spores demonstrated heat resistance at temperatures between 45 and 75 °C. Contrarily, three strains of Trichoderma, ID11D, ID4A and ID4B, were found to be able to maintain the viability of spores at 75 °C in this study heat tolerance test by Kucuk and Kivanc [40]. Current findings are comparable with those of Kucuk and Kivanc [40] which suggested that the fungal isolates were primarily adapted to local environmental conditions from when they were collected.
Fungal Isolate Effects on Crops’ Seeds
While A. flavus caused seed rot and reduced seedling growth, A. terreus improved seed germination and seedling growth of tested vegetables. This shows that species from the same genus can inhibit seed germination and seedling growth, while other species promote growth. Aspergillus is a genus of filamentous fungi that are typically opportunistic, prevalent in soil, air and decaying plant matter [41]. Previous research has shown the inhibitory effects of Aflatoxin B1 from A. flavus on seed germination and seedling development in other agricultural plants [42]. Yoo et al. [43] previously reported that four weeks tomato seed treatment with A. terreus isolates, JF02, JF07, JF27 and JF44, seedlings had significantly increased shoot length in comparison with the control group.
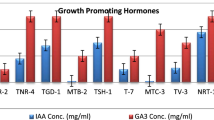
In the present study, it was observed that Penicillium isolate promoted the growth of grain seeds, maize and sorghum, more than those of vegetable seeds. An increased root biomass could promote abiotic stress tolerance and aiding the plants in absorption of water and nutrients in far proximity. The stimulation of seed germination by Penicillium sp. AL-38 IRH-2012b may have resulted from synthesis of plant growth hormones such auxins, gibberellic acids, abscisic acid, ethylene and jasmonate. It has been shown in several investigations that Penicillium species generate gibberellic acid, which is necessary for the mitotic division of seeds [44]. In a study by Mushatq et al. [45], pre-soaking tomato seeds with Penicillium isolates considerably enhanced seed germination and subsequent seedling growth of up to 90% higher than untreated seeds.
The two Trichoderma species showed the fastest seed germination and seedling growth compared to other tested isolates. Furthermore, 100% seed germination was observed on Trichoderma-treated seeds. According to Shahid et al. [46], chickpea (Cicer arietinum L.) germination and vigor were improved when seeds were treated with T. viride; while Lalitha and Arunalakshmi [44] discovered improved seed germination and root length in mustard. The application of T. viride promoted wheat plant height, root length, leaf length, number of leaves and grain production [47]. Trichoderma ghanense improved shoot and root length of rye (Secale cereale L.) seedlings [48]. The inoculation of Trichoderma isolates led to an increase in auxin, a decrease in cytokinin and abscisic acid content in melon (C. melo) and further stimulated melon growth [49].
Compared to untreated control, T. minioluteus improved germination rate of S. lycopersicum significantly by 99%. Talaromyces isolates promoted the formation of lateral roots in vegetable seeds, which would result in an improved nutrient uptake capacity and an increase in the biomass of the roots and shoots. Talaromyces species have been shown to increase crop growth through their abilities to produce siderophores, Indole Acetic Acid (IAA) and dissolving phosphorus [50].
Conclusion
The findings demonstrated that although being indigenous, Aspergillus, T. sayulitensis and T. ghanense did not thrive in conditions of high salinity and pH; Penicillium sp. AL-38 IRH-2012b failed to grow at lower nutrition levels and all fungal isolates failed to grow at temperatures between 10 and 20 °C. The production of chlamydospores by Trichoderma was acknowledged as a means of surviving unfavorable conditions. Additionally, Aspergillus species from the same genus can have both favorable and unfavorable effects on crops. The adaptation of BCA to habitats exposed to harsh and sudden fluctuating climatic conditions is a significant challenge to the transition from conventional to organic agriculture, even more so now due to climate change. The fact that the assessed fungal isolates live a multitrophic existence in nature; one that includes soil saprophytism and plant root endophytism indicates that they have the potential to adapt throughout time and geographical zones. The tolerance characteristics of fungal isolates indicate the need for continuous study of soil-borne fungal isolates with particular focus on the probability of certain species evolving into being plant parasitic.
References
Zihad S, Gupt Y, Uddin SJ, Islam MT, Alam MR, Aziz S et al (2019) Nutritional value, micronutrient and antioxidant capacity of some green leafy vegetables commonly used by southern coastal people of Bangladesh. Heliyon 5:e02768. https://doi.org/10.1016/j.heliyon.2019.e02768
Poutanen KS, Kårlund AO, Gómez-Gallego C, Johansson DP, Scheers NM, Marklinder IM et al (2022) Grains: a major source of sustainable protein for health. Nutr Rev 80:1648–1663. https://doi.org/10.1093/nutrit/nuab084
Nazarov PA, Baleev DN, Ivanova MI, Sokolova LM, Karakozova MV (2020) Infectious plant diseases: etiology, current status, problems and prospects in plant protection. Acta Nat 12:46–59. https://doi.org/10.32607/actanaturae.11026
Phani V, Khan MR, Dutta TK (2021) Plant-parasitic nematodes as a potential threat to protected agriculture: Current status and management options. Crop Prot 144:105573. https://doi.org/10.1016/j.cropro.2021.105573
Messa VR, Torres da Costa AC, Kuhn OJ, Stroze CT (2020) Nematophagous and endomycorrhizal fungi in the control of Meloidogyne incognita in soybean. Rhizosphere 15:100222. https://doi.org/10.1016/j.rhisph.2020.100222
Chaudhary S, Sindhu SS, Dhanker R, Kumari A (2023) Microbes-mediated sulphur cycling in soil: Impact on soil fertility, crop production and environmental sustainability. Microbiol Res 271:127340. https://doi.org/10.1016/j.micres.2023.127340
Coombes C, Hill M, Moore S, Dames J (2016) Entomopathogenic fungi as control agents of Thaumatotibia leucotreta in citrus orchards: field efficacy and persistence. Biocontrol 61:729–739. https://doi.org/10.1007/s10526-016-9756-x
Ngoma L, Esau B, Babalola OO (2013) Isolation and characterization of beneficial indigenous endophytic bacteria for plant growth promoting activity in Molelwane Farm, Mafikeng, South Africa. Afr J Biotechnol 12.
Jaronski ST (2010) Ecological factors in the inundative use of fungal entomopathogens. Biocontrol 55:159–185. https://doi.org/10.1007/s10526-009-9248-3
Frisvad JC (2015) Taxonomy, chemodiversity, and chemoconsistency of Aspergillus, Penicillium, and Talaromyces species. Front Microbiol 5:773. https://doi.org/10.3389/fmicb.2014.00773
Patkowska E, Mielniczuk E, Jamiołkowska A, Skwaryło-Bednarz B, Błażewicz-Woźniak M (2020) The Influence of Trichoderma harzianum Rifai T-22 and other biostimulants on rhizosphere beneficial microorganisms of carrot. Agron 10:1637. https://doi.org/10.3390/agronomy10111637
Silva L, Freire K, Araújo-Magalhães G, Agamez-Montalvo G, Sousa M, Costa-Silva T et al (2018) Penicillium and Talaromyces endophytes from Tillandsia catimbauensis, a bromeliad endemic in the Brazilian tropical dry forest, and their potential for L-asparaginase production. World J Microbiol Biotechnol 34:162. https://doi.org/10.1007/s11274-018-2547-z
Toghueo RMK, Boyom FF (2020) Endophytic Penicillium species and their agricultural, biotechnological, and pharmaceutical applications. 3 Biotech 10:107. https://doi.org/10.1007/s13205-020-2081-1
Yadav S, Modi P, Dave A, Vijapura A, Patel D, Patel M (2020) Effect of abiotic stress on crops. In: Hasanuzzaman M, Filho MCMT, Fujita M, Nogueira TAR (eds) Sustainable crop production. Intech
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M et al (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front Plant Sci 10:1068. https://doi.org/10.3389/fpls.2019.01068
Ibarra-Cortés KH, Guzmán-Franco AW, González-Hernández H, Suarez-Espinosa J, Baverstock J (2013) Selection of a fungal isolate for the control of the pink hibiscus mealybug Maconellicoccus hirsutus. Pest Manag Sci 69:874–882. https://doi.org/10.1002/ps.3452
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL et al (2012) Emerging fungal threats to animal, plant and ecosystem health. Nat 484:186–194. https://doi.org/10.1038/nature10947
Rokas A (2022) Evolution of the human pathogenic lifestyle in fungi. Nat Microbiol 7:607–619. https://doi.org/10.1038/s41564-022-01112-0
Pradhan N, Sukla L (2006) Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol 5
Dada E, Aruwa C (2014) Microorganisms associated with urine contaminated soils around lecture theatres in Federal. Int J Appl Microbiol Biotechnol 2:79–85
Gams W, Bissett J (1998) Trichoderma and Gliocladium. Taylor and Francis, Bristol, pp 3–34
Bouyoucos GJ (1951) A recalibration of the hydrometer method for making mechanical analysis of soils 1. Agron J 43:434–438
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Soil Sci 78:154
McLean E (1983) Soil pH and lime requirement. Methods Soil Anal Part 2 Chem Microbiol Prop 9:199–224
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils - Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–264
Yu Z, Wang Z, Zhang Y, Wang Y, Liu Z (2021) Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil. Microbiol Res 242:126596
Coninck E, Scauflaire J, Gollier M, Liénard C, Foucart G, Manssens G et al (2020) Trichoderma atroviride as a promising biocontrol agent in seed coating for reducing Fusarium damping-off on maize. J Appl Microbiol 29:637–651. https://doi.org/10.1111/jam.14641
Patil M, Yenjerappa S, Amaresh Y, Naik M (2013) Salinity stress tolerance in native Trichoderma isolates. Environ Ecol 31:727–729
Rawat L, Singh Y, Shukla N, Kumar J (2013) Salinity tolerant Trichoderma harzianum reinforces NaCl tolerance and reduces population dynamics of Fusarium oxysporum f.sp. ciceri in chickpea (Cicer arietinum L.) under salt stress conditions. Arch Phytopathol Plant Prot. https://doi.org/10.1080/03235408.2013.769316
Yusnawan E, Taufiq A, Wijanarko A, Susilowati DN, Praptana RH, Chandra-Hioe MV et al (2021) Changes in volatile organic compounds from salt-tolerant Trichoderma and the biochemical response and growth performance in saline-stressed groundnut. Sustain 13:13226. https://doi.org/10.3390/su132313226
Guo R, Wang Z, Huang Y, Fan H, Liu Z (2018) Biocontrol potential of saline- or alkaline-tolerant Trichoderma asperellum mutants against three pathogenic fungi under saline or alkaline stress conditions. Braz J Microbiol 49:236–245. https://doi.org/10.1016/j.bjm.2018.02.008
Anam GB, Reddy MS, Ahn YH (2019) Characterization of Trichoderma asperellum RM-28 for its sodic/saline-alkali tolerance and plant growth promoting activities to alleviate toxicity of red mud. Sci Total Environ 662:462–469. https://doi.org/10.1016/j.scitotenv.2019.01.279
Zhang C, Wang W, Hu Y, Peng Z, Ren S, Xue M et al (2022) A novel salt-tolerant strain Trichoderma atroviride HN082102.1 isolated from marine habitat alleviates salt stress and diminishes cucumber root rot caused by Fusarium oxysporum. BMC Microbiol 22:67. https://doi.org/10.1186/s12866-022-02479-0
Kredics L, Antal Z, Manczinger L, Szekeres A, Kevei F, Nagy E (2003) Influence of environmental parameters on Trichoderma strains with biocontrol potential. Food Technol Biotechnol 41:37–42
Suarau Odutola O (2018) Relevance of soil pH to agriculture. In: Suarau O (ed) Soil pH for nutrient availability and crop performance. IntechOpen, Rijeka
Cabral-Miramontes JP, Olmedo-Monfil V, Lara-Banda M, Zúñiga-Romo ER, Aréchiga-Carvajal ET (2022) Promotion of plant growth in arid zones by selected Trichoderma species strains with adaptation plasticity to alkaline pH. Biol 11:1206. https://doi.org/10.3390/biology11081206
Yan S, Xu Y, Yu XW (2021) From induction to secretion: A complicated route for cellulase production in Trichoderma reesei. Bioresour Bioprocess 8:107. https://doi.org/10.1186/s40643-021-00461-8
Affokpon A, Coyne DL, De Proft M, Coosemans J (2015) In vitro growth characterization and biocontrol potential of naturally occurring nematophagous fungi recovered from root-knot nematode infested vegetable fields in Benin. Int J Pest Manag 61:273–283. https://doi.org/10.1080/09670874.2015.1043971
Contreras-Cornejo HA, Macías-Rodríguez L, Del-Val E, Larsen J (2016) Ecological functions of Trichoderma species and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol Ecol 92:fiw036. https://doi.org/10.1093/femsec/fiw036
Kucuk C, Kivanc M (2003) Isolation of Trichoderma species and determination of their antifungal, biochemical and physiological features. Turk J Biol 27:247–253
Abdel-Azeem A, Abdel Azeem M, Abdul-Hadi S, Darwish A (2019) Aspergillus: Biodiversity, ecological significances, and industrial applications: 3rd ISNPS, Avignon, France. pp 121–79
Avilala J, Subramanyam D, Arthala P, Pradeep M, Golla N (2011) Aflatoxin impacts on germinating seeds. Ann Biol Res 2:180–188
Yoo SJ, Shin DJ, Won HY, Song J, Sang MK (2018) Aspergillus terreus JF27 promotes the growth of tomato plants and induces resistance against Pseudomonas syringae pv. tomato. Mycobiol 46:147–153
Lalitha P, Arunalakshmi K (2012) Effect of Trichoderma viride on germination of mustard and survival of mustard seedlings. IJLBPR 1:137–140
Mushatq S, Nasim G, Khokhar I (2011) Effect of Penicillium Extractsa on germination vigor in subsequent seedling growth of tomato (Solanum lycopersicum L). Arch Phytopathol Plant Prot. https://doi.org/10.1080/03235408.2011.603965
Shahid M, Singh A, Srivastava M, Sachan C, Biswas S (2011) Effect of seed treatment on germination and vigour in chickpea. Trends Biosci 4:205–207
Mahato S, Bhuju S, Shrestha J (2018) Effect of Trichoderma viride as biofertilizer on growth and yield of wheat. Malays J Sustain Agric 2:1–5. https://doi.org/10.26480/mjsa.02.2018.01.05
Bridžiuvienė D, Raudonienė V, Švedienė J, Paškevičius A, Baužienė I, Vaitonis G et al (2022) Impact of soil chemical properties on the growth promotion ability of Trichoderma ghanense, T. tomentosum and their complex on rye in different land-use systems. J Fungi 8:85. https://doi.org/10.3390/jof8010085
Martínez-Medina A, Del Mar AM, Pascual JA, Van Wees SC (2014) Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J Chem Ecol 40:804–815. https://doi.org/10.1007/s10886-014-0478-1
Zhao L, Zhao W, Deng H (2021) Effects of Talaromyces purpureogenus on cucumber growth promotion and its mechanism. J Bacteriol Mycol. https://doi.org/10.26420/jbacteriolmycol.2021.1173
Acknowledgements
The authors acknowledge Inqaba Biotechnical Industries (Pty) Ltd for molecularly identifying fungal species.
Funding
Open access funding provided by North-West University. This work was financed by Potatoes South Africa.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Mukondeleli Ndivhuwo Ramatsitsi] and [Khosi Ramachela]; Methodology: [Mukondeleli Ndivhuwo Ramatsitsi]; Formal analysis and investigation: [Mukondeleli Ndivhuwo Ramatsitsi]; Writing—original draft preparation: [Mukondeleli Ndivhuwo Ramatsitsi]; Writing—review and editing: [Mukondeleli Ndivhuwo Ramatsitsi, Khosi Ramachela, Mbokota Candy Khosa and Chuene Victor Mashamaite]; Funding acquisition: [Mukondeleli Ndivhuwo Ramatsitsi]; Resources: [Mukondeleli Ndivhuwo Ramatsitsi, Khosi Ramachela, Mbokota Candy Khosa and Chuene Victor Mashamaite]; Supervision: [Khosi Ramachela and Mbokota Candy Khosa].
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramatsitsi, M.N., Khosa, M.C., Mashamaite, C.V. et al. In Vitro Assessment of Eight Selected Indigenous Fungal Isolates Tolerance to Various Abiotic Stresses and their Effects on Seed Germination. Curr Microbiol 80, 386 (2023). https://doi.org/10.1007/s00284-023-03507-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03507-6