Abstract
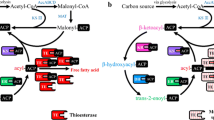
The expression of exogenous genes encoding acetyl-CoA carboxylase (Acc) and pantothenate kinase (CoaA) in Escherichia coli enable highly effective fatty acid production. Acc-only strains grown at 37 °C or 23 °C produced an approximately twofold increase in fatty acid content, and additional expression of CoaA achieved a further twofold accumulation. In the presence of pantothenate, which is the starting material for the CoA biosynthetic pathway, the size of the intracellular CoA pool at 23 °C was comparable to that at 30 °C during cultivation, and more than 500 mg/L of culture containing cellular fatty acids was produced, even at 23 °C. However, the highest yield of cellular fatty acids (1100 mg/L of culture) was produced in cells possessing the gene encoding type I bacterial fatty acid synthase (FasA) along with the acc and coaA, when the transformant was cultivated at 30 °C in M9 minimal salt medium without pantothenate or IPTG. This E. coli transformant contained 141 mg/L of oleic acid attributed to FasA under noninducible conditions. The increased fatty acid content was brought about by a greatly improved specific productivity of 289 mg/g of dry cell weight. Thus, the effectiveness of the foreign acc and coaA in fatty acid production was unambiguously confirmed at culture temperatures of 23 °C to 37 °C. Cofactor engineering in E. coli using the exogenous coaA and acc genes resulted in fatty acid production over 1 g/L of culture and could effectively function at 23 °C.




Similar content being viewed by others
References
Milke L, Marienhagen J (2020) Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis. Appl Microbiol Biotechnol 104:6057–6065. https://doi.org/10.1007/s00253-020-10643-7
Cronan JE Jr, Waldrop GL (2002) Multi-subunit acetyl-CoA carboxylases. Prog Lipid Res 41:407–435. https://doi.org/10.1016/s0163-7827(02)00007-3
Rathnasingh C, Raj SM, Lee Y, Catherine C, Ashok S, Park S (2012) Production of 3-hydroxypropionic acid via malonyl-CoA pathway using recombinant Escherichia coli strains. J Biotechnol 157:633–640. https://doi.org/10.1016/j.jbiotec.2011.06.008
Cheng Z, Jiang J, Wu H, Li Z, Ye Q (2016) Enhanced production of 3-hydroxypropionic acid from glucose via malonyl-CoA pathway by engineered Escherichia coli. BioresourTechnol 200:897–904. https://doi.org/10.1016/j.biortech.2015.10.107
Maharjan S, Park JW, Yoon YJ, Lee HC, Jae Kyung Sohng JK (2010) Metabolic engineering of Streptomyces venezuelae for malonyl-CoA biosynthesis to enhance heterologous production of polyketides. Biotechnol Lett 32:277–282. https://doi.org/10.1007/s10529-009-0152-9
Zha W, Rubin-Pitel SB, Shao Z, Zhao H (2009) Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering. Metab Eng 11:192–198. https://doi.org/10.1016/j.ymben.2009.01.005
Wattanachaisaereekul S, Lantz AE, Nielsen ML, Nielsen J (2008) Production of the polyketide 6-MSA in yeast engineered for increased malonyl-CoA supply. Metab Eng 10:246–254. https://doi.org/10.1016/j.ymben.2008.04.005
Miyahisa I, Kaneko M, Funa N, Kawasaki H, Kojima H, Ohnishi Y, Horinouchi S (2005) Efficient production of (2S)-flavanones by Escherichia coli containing an artificial biosynthetic gene cluster. Appl Microbiol Biotechnol 68:498–504. https://doi.org/10.1007/s00253-005-1916-3
Miyahisa I, Funa N, Ohnishi Y, Martens S, Moriguchi T, Horinouchi S (2006) Combinatorial biosynthesis of flavones and flavonols in Escherichia coli. Appl Microbiol Biotechnol 71:53–58. https://doi.org/10.1007/s00253-005-0116-5
Leonard E, Lim K-H, Saw P-N, Koffas MA (2007) Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli. Appl Environ Microbiol 73:3877–3886. https://doi.org/10.1128/AEM.00200-07
Leonard E, Yan Y, Fowler ZL, Li Z, Lim C-G, Lim K-H, Koffas MA (2008) Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Mol Pharm 5:257–265. https://doi.org/10.1021/mp7001472
Kim BG, Lee H, Ahn J-H (2014) Biosynthesis of pinocembrin from glucose using engineered Escherichia coli. J Microbiol Biotechnol 24:1536–1541. https://doi.org/10.4014/jmb.1406.06011
Davis MS, Solbiati J, Cronan JE Jr (2000) Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem 275:28593–28598. https://doi.org/10.1074/jbc.M004756200
Lu X, Vora H, Khosla C (2008) Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab Eng 10:333–339. https://doi.org/10.1016/j.ymben.2008.08.006
Ruenwai R, Cheevadhanarak S, Laoteng K (2009) Overexpression of acetyl-CoA carboxylase gene of Mucor rouxii enhanced fatty acid content in Hansenula polymorpha. Mol Biotechnol 42:327–332. https://doi.org/10.1007/s12033-009-9155-y
Jeon E, Lee S, Won JI, Han SO, Kim J, Lee J (2011) Development of Escherichia coli MG1655 strains to produce long chain fatty acids by engineering fatty acid synthesis (FAS) metabolism. Enzyme Microb Technol 49:44–51. https://doi.org/10.1016/j.enzmictec.2011.04.001
Meng X, Yang J, Cao Y, Li L, Jiang X, Xu X, Liu W, Xian M, Zhang Y (2011) Increasing fatty acid production in E. coli by simulating the lipid accumulation of oleaginous microorganisms. J Ind Microbiol Biotechnol 38:919–925. https://doi.org/10.1007/s10295-010-0861-z
Xu P, Gu Q, Wang W, Wong L, Bower AG, Collins CH, Koffas MA (2013) Modular optimization of multi-gene pathways for fatty acids production in E. coli. Nat Commun 4:1409. https://doi.org/10.1038/ncomms2425
Cao Y, Liu W, Xu X, Zhang H, Wang J, Xian M (2014) Production of free monounsaturated fatty acids by metabolically engineered Escherichia coli. Biotechnol Biofuels 7:59. https://doi.org/10.1186/1754-6834-7-59
Shin KS, Lee SK (2017) Introduction of an acetyl-CoA carboxylation bypass into Escherichia coli for enhanced free fatty acid production. Bioresour Technol 245:1627–1633. https://doi.org/10.1016/j.biortech.2017.05.169
Chen D, Yuan X, Liang L, Liu K, Ye H, Liu Z, Liu Y, Huang L, He W, Chen Y, Zhang Y, Xue T (2019) Overexpression of acetyl-CoA carboxylase increases fatty acid production in the green alga Chlamydomonas reinhardtii. Biotechnol Lett 41:1133–1145. https://doi.org/10.1007/s10529-019-02715-0
Xu P, Ranganathan S, Fowler ZL, Maranas CD, Koffas MAG (2011) Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab Eng 13:578–587. https://doi.org/10.1016/j.ymben.2011.06.008
Janßen HJ, Steinbüchel A (2014) Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnol Biofuels 7:7. https://doi.org/10.1186/1754-6834-7-7
Kurosawa K, Boccazzi P, de Almeida NM, Sinskey AJ (2010) High-cell-density batch fermentation of Rhodococcus opacus PD630 using a high glucose concentration for triacylglycerol production. J Biotechnol 147:212–218. https://doi.org/10.1016/j.jbiotec.2010.04.003
Gande R, Dover LG, Krumbach K, Besra GS, Sahm H, Oikawa T, Eggeling L (2007) The two carboxylases of Corynebacterium glutamicum essential for fatty acid and mycolic acid synthesis. J Bacteriol 189:5257–5264. https://doi.org/10.1128/JB.00254-07
Satoh S, Ozaki M, Matsumoto S, Nabatame T, Kaku M, Shudo T, Asayama M, Chohnan S (2020) Enhancement of fatty acid biosynthesis by exogenous acetyl-CoA carboxylase and pantothenate kinase in Escherichia coli. Biotechnol Lett 42:2595–2605. https://doi.org/10.1007/s10529-020-02996-w
Vallari DS, Jackowski S, Rock CO (1987) Regulation of pantothenate kinase by coenzyme A and its thioesters. J Biol Chem 262:2468–2471. https://doi.org/10.1016/S0021-9258(18)61527-3
Brand LA, Strauss E (2005) Characterization of a new pantothenate kinase isoform from Hricobacter pylori. J Biol Chem 280:20185–20188. https://doi.org/10.1074/jbc.C500044200
Hong BS, Yun MK, Zhang Y-M, Chohnan S, Rock CO, White SW, Jackowski S, Park H-W, Leonardi R (2006) Prokaryotic type II and type III pantothenate kinases: the same monomer fold creates dimers with distinct catalytic properties. Structure 14:1251–1261. https://doi.org/10.1016/j.str.2006.06.008
Ogata Y, Chohnan S (2015) Prokaryotic typeIII pantothenate kinase enhances coenzyme A biosynthesis in Escherichi coli. J Gen Appl Microbiol 61:266–269. https://doi.org/10.2323/jgam.61.266
Schweizer E, Hofmann J (2004) Microbial type I fatty acid synthase (FAS): major players in a network of cellular FAS systems. Microbiol Mol Biol Rev 68:501–517. https://doi.org/10.1128/MMBR.68.3.501-517.2004
Ariga N, Maruyama K, Kawaguchi A (1984) Comparative studies of fatty acid synthases of corynebacteria. J Gen Appl Microbiol 30:87–95. https://doi.org/10.2323/jgam.30.87
Stuible H-P, Wagner C, Andreou I, Hunter G, Haselmann J, Schweizer E (1996) Identification and functional differentiation of two type I fatty acid synthases in Brevibacterium ammoniagenes. J Bacteriol 178:4787–4793. https://doi.org/10.1128/jb.178.16.4787-4793.1996
Radmacher E, Alderwick LJ, Besra GS, Brown AK, Gibson KJC, Sahm H, Eggeling L (2005) Two functional FAS-I type fatty acid synthases in Corynebacterium glutamicum. Microbiology 151:2421–2427. https://doi.org/10.1099/mic.0.28012-0
Haushalter RW, Groff D, Deutsch S, The L, Chavkin TA, Brunner SF, Katz L, Keasling JD (2015) Development of an orthogonal fatty acid biosynthesis system in E. coli for oleochemical production. Metab Eng 30:1–6. https://doi.org/10.1016/j.ymben.2015.04.003
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Takamura Y, Nomura G (1988) Changes in the intracellular concenrration of acetyl-CoA and malonyl-CoA in relation to the carbon and energy metabolism of Escherichia coli K12. J Gen Microbiol 134:2249–2253. https://doi.org/10.1099/00221287-134-8-2249
Takamura Y, Kitayama Y, Arakawa A, Yamanaka S, Tosaki M, Ogawa Y (1985) Malonyl-CoA: acetyl-CoA cycling. A new micromethod for determination of acyl-CoAs with malonate decarboxylase. Biochim Biophys Acta 834:1–7. https://doi.org/10.1016/0005-2760(85)90170-5
Chohnan S, Takamura Y (1991) A simple micromethod for measurement of CoASH and its use in measuring intracellular levels of CoASH and short chain acyl-CoAs in Escherichia coli K12 cells. Agric Biol Chem 55:87–94. https://doi.org/10.1271/bbb1961.55.87
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Amiri-Jami M, Griffiths MW (2010) Recombinant production of omega-3 fatty acids in Escherichia coli using a gene cluster isolated from Shewanella baltica MAC1. J Appl Microbiol 109:1897–1905. https://doi.org/10.1111/j.1365-2672.2010.04817.x
Amiri-Jami M, Abdelhamid AG, Hazaa M, Kakuda Y, Griffiths MW (2015) Recombinant production of omega-3 fatty acids by probiotic Escherichia coli Nissle 1917. FEMS Microbiol Lett 362:fnv166. https://doi.org/10.1093/femsle/fnv166
Peng YF, Chen WC, Xiao K, Xu L, Wang L, Wan X (2016) DHA production in Escherichia coli by expressing reconstituted key genes of polyketide synthase pathway from marine bacteria. PLoS ONE 11:e0162861. https://doi.org/10.1371/journal.pone.0162861
Marr AG, Ingraham JL (1962) Effect of temperature on the composition of fatty acids in Escherichia coli. J Bacteriol 84:1260–1267. https://doi.org/10.1128/jb.84.6.1260-1267.1962
Sullivan KH, Hegeman GD, Cordes EH (1979) Alteration of the fatty acid composition of Escherichia coli by growth in the presence of normal alcohols. J Bacteriol 138:133–138. https://doi.org/10.1128/jb.138.1.133-138.1979
de Mendoza D, Garwin JL, Croban JE Jr (1982) Overproduction of cis-vaccenic acid and altered temperature control of fatty acid synthesis in a mutant of Escherichia coli. J Bacterial 151:1608–1611. https://doi.org/10.1128/jb.151.3.1608-1611.1982
Jackowski S, Rock CO (1981) Regulation of coenzyme A biosynthesis. J Bacteriol 148:926–932. https://doi.org/10.1128/jb.148.3.926-932.1981
Jackowski S, Rock CO (1984) Metabolism of 4’-phosphopantetheine in Escherichia coli. J Bacteriol 158:115–120. https://doi.org/10.1128/jb.158.1.115-120.1984
Liu T, Vora H, Khosla C (2010) Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab Eng 12:378–386. https://doi.org/10.1016/j.ymben.2010.02.003
Xu L, Wang L, Zhou XR, Chen WC, Singh S, Hu Z, Huang FH, Wan X (2018) Stepwise metabolic engineering of Escherichia coli to produce triacylglycerol rich in medium-chain fatty acids. Biotech Biofuels 11:177. https://doi.org/10.1186/s13068-018-1177-x
Zhang F, Ouellet M, Batth TS, Adams PD, Petzold CJ, Mukhopadhyay A, Keasling JD (2012) Enhancing fatty acid production by the expression of the regulatory transcription factor FadR. Metab Eng 14:653–660. https://doi.org/10.1016/j.ymben.2012.08.009
Okuyama H, Orikasa Y, Nishida T, Watanabe K, Morita N (2007) Bacterial genes responsible for the biosynthesis of eicosapentaenoic acid and docosahexaenoic acids and their heterologous expression. Appl Environ Microbiol 73:665–670. https://doi.org/10.1128/AEM.02270-06
Acknowledgements
We would like to thank Enago (www.enago.jp) for the English language review.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (C) JP19K05761 from The Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
MK performed the experimental work, data collection, and analysis. MI was a major contributor to the experimental work. SS and MO performed the experimental work. DK performed the statistical analysis. SC designed the study and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaku, M., Ishidaira, M., Satoh, S. et al. Fatty Acid Production by Enhanced Malonyl-CoA Supply in Escherichia coli. Curr Microbiol 79, 269 (2022). https://doi.org/10.1007/s00284-022-02969-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02969-4