Abstract
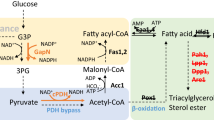
Malonyl-CoA is an essential precursor for fatty acid biosynthesis that is generated from the carboxylation of acetyl-CoA. In this work, a gene coding for acetyl-CoA carboxylase (ACC) was isolated from an oleaginous fungus, Mucor rouxii. According to the amino acid sequence homology and the conserved structural organization of the biotin carboxylase, biotin carboxyl carrier protein, and carboxyl transferase domains, the cloned gene was characterized as a multi-domain ACC1 protein. Interestingly, a 40% increase in the total fatty acid content of the non-oleaginous yeast Hansenula polymorpha was achieved by overexpressing the M. rouxii ACC1. This result demonstrated a significant improvement in the production of fatty acids through genetic modification in this yeast strain.



Similar content being viewed by others
References
van Meer, G., Voelker, D. R., & Feigenson, G. W. (2008). Membrane lipids: Where they are and how they behave. Nature Reviews. Molecular Cell Biology, 9, 112–124. doi:10.1038/nrm2330.
Daum, G., Lees, N. D., Bard, M., & Dickson, R. (1998). Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast (Chichester, England), 14, 1471–1510. doi:10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y.
Ratledge, C., & Wynn, J. P. (2002). The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Advances in Applied Microbiology, 51, 1–51. doi:10.1016/S0065-2164(02)51000-5.
Somashekar, D., Venkateshwaran, G., Sambaiah, K., & Lokesh, B. R. (2003). Effect of culture conditions on lipid and gamma-linolenic acid production by mucoraceous fungi. Process Biochemistry, 38, 1719–1724. doi:10.1016/S0032-9592(02)00258-3.
Neels, J. G., & Olefsky, J. M. (2006). Cell signaling. A new way to burn fat. Science, 312, 1756–1758. doi:10.1126/science.1130476.
Roesler, K., Shintani, D., Savage, L., Boddupalli, S., & Ohlrogge, J. (1997). Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiology, 113, 75–81. doi:10.1104/pp.113.1.75.
Davis, M. S., Solbiati, J., & Cronan, J. E., Jr. (2000). Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. The Journal of Biological Chemistry, 275, 28593–28598. doi:10.1074/jbc.M004756200.
Klaus, D., Ohlrogge, J. B., Neuhaus, H. E., & Dörmann, P. (2004). Increased fatty acid production in potato by engineering of acetyl-CoA carboxylase. Planta, 219, 389–396. doi:10.1007/s00425-004-1236-3.
Bailey, A., Keon, J., Owen, J., & Hargreaves, J. (1995). The ACC1 gene, encoding acetyl CoA carboxylase, is essential for growth in Ustilago maydis. Molecular & General Genetics, 249, 191–201. doi:10.1007/BF00290366.
Cronan, J. E., Jr., & Waldrop, G. L. (2002). Multi-subunit acetyl-CoA carboxylases. Progress in Lipid Research, 41, 407–435. doi:10.1016/S0163-7827(02)00007-3.
Sasaki, Y., & Nagano, Y. (2004). Plant acetyl-CoA carboxylase: Structure, biosynthesis, regulation, and gene manipulation for plant breeding. Bioscience, Biotechnology, and Biochemistry, 68, 1175–1184. doi:10.1271/bbb.68.1175.
Tong, L. (2005). Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cellular and Molecular Life Sciences, 62, 1784–1803. doi:10.1007/s00018-005-5121-4.
Hoja, U., Marthol, S., Hofmann, J., Stegner, S., Schulz, R., Meier, S., et al. (2004). HFA1 encoding an organelle-specific acetyl-CoA carboxylase controls mitochondrial fatty acid synthesis in Saccharomyces cerevisiae. The Journal of Biological Chemistry, 279, 21779–21786. doi:10.1074/jbc.M401071200.
Barber, M. C., Price, N. T., & Travers, M. T. (2005). Structure and regulation of acetyl-CoA carboxylase genes of metazoa. Biochimica et Biophysica Acta, 1733, 1–28.
Wongsumpanchai, W., Anamnart, S., Laoteng, K., & Petsom, A. (2004). Elongation of C16:0 to C18:0 fatty acids in methylotrophic yeast Hansenula polymorpha CBS 1976 and fatty acid auxotrophic mutants. FEMS Microbiology Letters, 237, 213–218.
Laoteng, K., Ruenwai, R., Tanticharoen, M., & Cheevadhanarak, S. (2005). Genetic modification of essential fatty acids biosynthesis in Hansenula polymorpha. FEMS Microbiology Letters, 245, 169–178. doi:10.1016/j.femsle.2005.03.006.
Laoteng, K., Anjard, C., Rachadawong, S., Tanticharoen, M., Maresca, B., & Cheevadhanarak, S. (1999). Mucor rouxii Δ9-desaturase gene is transcriptionally regulated during cell growth and by low temperature. Molecular Cell Biology Research Communications, 1, 36–43. doi:10.1006/mcbr.1999.0107.
Gietl, C., Faber, K. N., van der Klei, I. J., & Veenhuis, M. (1994). Mutational analysis of the N-terminal topogenic signal of watermelon glyoxysomal malate dehydrogenase using the heterologous host Hansenula polymorpha. Proceedings of the National Academy of Sciences of the United States of America, 91, 3151–3155. doi:10.1073/pnas.91.8.3151.
Stearns, T., Ma, H., & Botstein, D. (1990). Manipulating yeast genome using plasmid vectors. Methods in Enzymology, 185, 280–297. doi:10.1016/0076-6879(90)85025-J.
Lepage, G., & Roy, C. C. (1986). Direct transesterification of all classes of lipids in a one-step reaction. Journal of Lipid Research, 27, 114–120.
Marchler-Bauer, A., Anderson, J. B., Derbyshire, M. K., DeWeese-Scott, C., Gonzales, N. R., Gwadz, M., et al. (2007). CDD: A conserved domain database for interactive domain family analysis. Nucleic Acids Research, 35, D237–D240. doi:10.1093/nar/gkl951.
Shorrosh, B. S., Dixon, R. A., & Ohlrogge, J. B. (1994). Molecular cloning, characterization, and elicitation of acetyl-CoA carboxylase from alfalfa. Proceedings of the National Academy of Sciences of the United States of America, 91, 4323–4327. doi:10.1073/pnas.91.10.4323.
Pfeifer, B. A., & Khosla, C. (2001). Biosynthesis of polyketides in heterologous hosts. Microbiology and Molecular Biology Reviews, 65, 106–118. doi:10.1128/MMBR.65.1.106-118.2001.
Al-Feel, W., DeMar, J. C., & Wakil, S. J. (2003). A Saccharomyces cerevisiae mutant strain defective in acetyl-CoA carboxylase arrests at the G2/M phase of the cell cycle. Proceedings of the National Academy of Sciences of the United States of America, 100, 3095–3100. doi:10.1073/pnas.0538069100.
Kamiryo, T., Nishikawa, Y., Mishina, M., Terao, M., & Numa, S. (1979). Involvement of long-chain acyl coenzyme A for lipid synthesis in repression of acetyl-coenzyme A carboxylase in Candida lipolytica. Proceedings of the National Academy of Sciences of the United States of America, 76, 4390–4394. doi:10.1073/pnas.76.9.4390.
Wijeyaratne, S. C., Ohta, K., Chavanich, S., Mahamontri, V., Nilubol, N., & Hayashida, S. (1986). Lipid composition of a thermotolerant yeast, Hansenula polymorpha. Agricultural and Biological Chemistry, 50, 827–832.
Perrière, G., & Gouy, M. (1996). WWW-query: An on-line retrieval system for biological sequence banks. Biochimie, 78, 364–369. doi:10.1016/0300-9084(96)84768-7.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882. doi:10.1093/nar/25.24.4876.
Acknowledgments
This work was funded by a grant (BT-B-02-NG-B5-4905) from National Science and Technology Development Agency, Thailand. Rawisara Ruenwai was supported by the Thailand Graduate Institute of Science and Technology. We thank Prof. Morakot Tanticharoen and Dr. Sansanalak Rachdawong for their helpful comments on this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruenwai, R., Cheevadhanarak, S. & Laoteng, K. Overexpression of Acetyl-CoA Carboxylase Gene of Mucor rouxii Enhanced Fatty Acid Content in Hansenula polymorpha . Mol Biotechnol 42, 327–332 (2009). https://doi.org/10.1007/s12033-009-9155-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-009-9155-y