Abstract
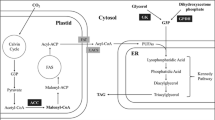
Chlamydomonas reinhardtii is a photosynthetic unicellular model algae with multiple biotechnological advantages, and its fatty acids can be used to produce biofuels. Numerous studies suggest that acetyl-coA carboxylase (ACCa) catalyzes the first committed and rate-limiting step of fatty acid biosynthesis, thereby playing a central role in oil accumulation. Here, we cloned and overexpressed ACCa in C. reinhardtii to directly evaluate its effect on fatty acid synthesis. GC–MS analysis found that the unsaturated FAs contents of the CW15-24 and CW15-85 strains were 55.45% and 56.15%, which were significantly enriched compared to the wild type CW15 (48.39%). Under the optimized conditions, the content of lipid by overexpressed the ACCa gene in the mutant CW15-85 (0.46 g/l) was 1.16-fold greater than control through optimization of N and P sources. Altogether, our data clearly demonstrate that ACCa overexpression in C. reinhardtii can directly increase the synthesis of fatty acids.





Similar content being viewed by others
References
Avidan O, Pick U (2015) Acetyl-coa synthetase is activated as part of the pdh-bypass in the oleaginous green alga chlorella desiccata. J Exp Bot 66:7287–7298
Blank GS, Dale HR, Cornelius WS (2010) Diatom mineralization of silicic acid. viii. metabolic requirements and the timing of protein synthesis. J Phycol 22:382–389
Brennan L, Fernandez AB, Mostaert AS, Owende P (2012) Enhancement of BODIPY505/515 lipid fluorescence method for applications in biofuel-directed microalgae production. J Microbiol Methods 90:37–143
Carrera P, Saskya E, Hankamer B, Oey M (2018) Optimising light conditions increases recombinant protein production in, chlamydomonas reinhardtii, chloroplasts. Algal Res 32:329–340
Cooksey KE, Williams SA, Callis PR (1987) Nile red: a fluorophore useful in assessing the relative lipid content of single cells. Metabol Struct Funct 37:645–647
Davis MS, Solbiati J, Cronan JE (2000) Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem 275:28593–28598
Dunahay TG, Jarvis EE, Dais SS, Roessler PG (1996) Manipulation of microalgal lipid production using genetic engineering. Appl Biochem Biotechnol 57:223
Fernandez-Pastor I, Fernandez-Hernandez A, Perez-Criado S, Rivas F, Martinez A, Garcia-Granados A (2017) Microwave-assisted extraction versus soxhlet extraction to determine triterpene acids in olive skins. J Sep Sci 40:1209–1217
Fowler SD, Brown WJ, Warfel J, Greenspan P (1987) Use of nile red for the rapid in situ quantitation of lipids on thin-layer chromatograms. J Lipid Res 28:1225–1232
Giroud C, Eichenberger W (2017) Lipids of chlamydomonas reinhardtii: incorporation of 14c-acetate, palmitate and oleate into different lipids and evidence for lipid-linked desaturation of fatty acids. Plant Cell Physiol 30:121–128
Hasan H, Abd-Rahim MH, Campbell L, Carter D, Abbas A, Montoya A (2018) Overexpression of acetyl-coa carboxylase in, aspergillus terreus, to increase lovastatin production. New Biotechnol 44:64
Hippler M, Redding K, Rochaix JD (2015) Chlamydomonas genetics, a tool for the study of bioenergetic pathways. BBA-Bioenerg 1367:56–62
Huang F, Huang Q, Huang M, Yi XL, Zheng HB, Qiao DR, Cao Y (2018) Construction of a convenient method for Chlamydomonas reinhardtii glass-bead transformation assay. J Sichuan Univ 45:694–699
Jia B, Xie XF, Wu M, Lin ZJ, Yin JB, Lou SL (2019) Understanding the functions of endogenous dof transcript factor in chlamydomonas reinhardtii. Biotechnol Biofuels 12:67
Kindle KL (1990) High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 87:1228–1232
Klaus D, Ohlrogge JB, Neuhaus HE, Dormann P (2004) Increased fatty acid production in potato by engineering of acetyl-CoA carboxylase. Planta 219:389–396
Kumar EM, Enamala S, Chavali M, Donepudi J, Yadavalli R, Kolapalli B (2018) Production of biofuels from microalgae-a review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew Sustain Energy Rev 94:49–68
Laing L, Utting SD (1980) The influence of salinity on the production of two commercially important unicellular marine algae. Aquaculture 21:79–86
Li Y, Han D, Hu G, Dauvillee D, Sommerfeld M, Ball S (2010) Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metab Eng 12:387–391
Li NN, Zhang Y, Meng HJ, Li ST, Wu SF, Xu ZC (2019) Characterization of fatty acid exporters involved in fatty acid transport for oil accumulation in the green alga chlamydomonas reinhardtii. Biotechnol Biofuels 12:14
Liu L, Chen J, Lim PE, Wei D (2018) Enhanced single cell oil production by mixed culture of chlorella pyrenoidosa and rhodotorula glutinis using cassava bagasse hydrolysate as carbon source. Bioresour Technol 255:140–148
Lumbreras V, Stevens DR, Purton S (2010) Efficient foreign gene expression in chlamydomonas reinhardtii mediated by an endogenous intron. Plant J 14:441–447
Mutsumi T, Karseno M, Toshiomi Y (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226
Nagy V, Vidal-Meireles A, Podmaniczki A, Szentmihályi K, Tóth SZ (2018) The mechanism of photosystem ii inactivation during sulphur deprivation-induced h 2 production in chlamydomonas reinhardtii. Plant J 94:548
Nodooshan KG, Moraga RJ, Shi-Jie GC, Nguyen C, Wang Z, Mohseni S (2018) Environmental and economic optimization of algal biofuel supply chain with multiple technological pathways. Eng Chem Res 57:6910–6925
Page RA, Okada S, Harwood JL (1994) Acetyl-CoA carboxylase exerts strong flux control over lipid synthesis in plants. BBA-Bioenerg 1210:369–372
Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J (1997) Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol 113:75–81
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403:25–34
Salas-Montantes CJ, González-Ortega O, Ochoa-Alfaro AE, Camarena-Rangel R, Paz-Maldonado LMT, Rosales-Mendoza S (2018) Lipid accumulation during nitrogen and sulfur starvation in chlamydomonas reinhardtii overexpressing a transcription factor. J Appl Phycol 30:1721–1733
Sandoval-Vargas JM, Jiménez-Clemente LA, Macedo-Osorio KS, Oliver-Salvador MC, Fernández-Linares LC, Durán-Figueroa NV (2019) Use of the ptxd gene as a portable selectable marker for chloroplast transformation in chlamydomonas reinhardtii. Mol Biotechnol 3:1–8
Shu Q, Qin L, Yuan ZH, Zhu SN, Xu J, Xu ZB (2018) Comparison of dairy wastewater and synthetic medium for biofuels production by microalgae cultivation. Energy Sour Part A 40:751–758
Siaut M, Cuine S, Cagnon C, Fessler B, Nguyen M, Carrier P (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:11–17
Sierra LS, Dixon CK, Wilken LR (2017) Enzymatic cell disruption of the microalgae, chlamydomonas reinhardtii, for lipid and protein extraction. Algal Res 25:149–159
Su Y, Song KH, Zhang PD, Su YQ, Cheng J, Chen X (2017) Progress of microalgae biofuel’s commercialization. Renew Sustain Energy Rev 74:402–411
Traller JC, Cokus SJ, David A (2016) Genome and methylome of the oleaginous diatom cyclotella cryptica reveal genetic flexibility toward a high lipid phenotype. Biotechnol Biofuels 9:258
Voelker T, Kinney AT (2001) Variations in the biosynthesis of seed-storage lipids. Annu Rev Plant Biol 52:335–361
Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U (2009) Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryot Cell 8:1856–1868
Wang Y, Yu J, Wang P, Deng S, Chang J, Ran Z (2017) Response of energy microalgae chlamydomonas reinhardtii, to nitrogen and phosphorus stress. Environ Sci Pollut R 25:1–9
Wang C, Li Y, Lu J, Deng X, Li H, Hu Z (2018) Effect of overexpression of lpaat and gpd1 on lipid synthesis and composition in green microalga chlamydomonas reinhardtii. J Appl Phycol 30:1711–1719
Wu J, Hu Z, Wang C, Li S, Lei A (2008) Efficient expression of green fluorescent protein (gfp) mediated by a chimeric promoter in chlamydomonas reinhardtii. Chin J Oceanol Limnol 26:242–247
Xu D, Gao Z, Li F, Fan X, Zhang X, Ye N (2013) Detection and quantitation of lipid in the microalga Tetraselmis subcordiformis (wille) butcher with BODIPY 505/515 staining. Bioresour Technol 27:386–390
Yang L, Chen J, Qin S, Zeng M, Wang JX (2018) Growth and lipid accumulation by different nutrients in the microalga chlamydomonas reinhardtii. Biotechnol Biofuels 11:40
Zalutskaya Z, Ostroukhova M, Filina V, Ermilova E (2017) Nitric oxide upregulates expression of alternative oxidase 1 in chlamydomonas reinhardtii. J Plant Physiol 219:123–127
Acknowledgements
We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript. This work was supported by the Natural Science Foundation of Fujian Province, China (Grant Number 2017J01622) and the Sugar Crop Research System (Grant Number CARS-170501).
Supporting information
Supplementary Fig. 1—Characterization of the PHKA overexpression construct by PCR detection of Acca and mCherry and restriction analysis. (A) Acca amplification from three independent PHKA clones confirms insertion of Acca coding sequence (lane M, molecular weight marker; lanes 1-3, Acca). (B) Restriction validation of PHKA clones (lane M, molecular weight marker; lane 1, products of PHKA; lanes 2-3, EcoR I digestion; lanes 4-5, EcoR V and EcoR I digestion). (C) mCherry amplification from C. reinhardtii mutant genomic DNA (lane M, molecular weight marker; lanes 1-5, mCherry).
Supplementary Fig. 2−Protein spot hybridization using anti-mCherry and anti-GAPDH antibodies. (A) GAPDH (endogenous control) is detected in wild type (CW15) and ACCa-overexpressing transgenic clones (CW15-24, CW15-85). (B) mCherry is detected only in the ACCa-overexpressing (CW15-24, CW15-85) clones, suggesting successful transfection of the PHKA overexpression vector.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, D., Yuan, X., Liang, L. et al. Overexpression of acetyl-CoA carboxylase increases fatty acid production in the green alga Chlamydomonas reinhardtii. Biotechnol Lett 41, 1133–1145 (2019). https://doi.org/10.1007/s10529-019-02715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02715-0