Abstract
Purpose
Necitumumab is a second-generation, recombinant, human immunoglobulin G1 monoclonal antibody that blocks the ligand binding site of the epidermal growth factor receptor. The primary objective of this phase 2 study, conducted in accordance with International Conference on Harmonisation E14 guidance, was to determine the effect of necitumumab treatment on QT/QTc interval in patients with advanced solid tumors.
Methods
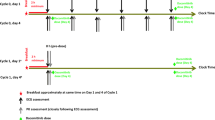
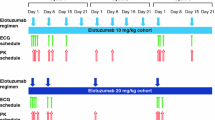
Patients received necitumumab monotherapy at an absolute dose of 800 mg, once per week for each 6-week cycle. Triplicate electrocardiogram readings were taken at pretreatment (baseline) and then weekly at multiple timepoints that were time-matched with blood samples to determine necitumumab concentrations.
Results
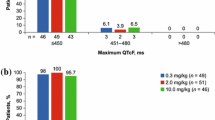
Seventy-five patients received treatment. Overall, the upper bound of the two-sided 90 % confidence interval for mean change from baseline in QTc in cycle 1 did not exceed 10 ms. No patients had a mean QTcF interval >500 ms, and no patients had an increase of >60 ms. Necitumumab concentration–QTc analysis also showed that necitumumab is unlikely to cause QTc prolongation.
Conclusions
The results demonstrate lack of effect of necitumumab on the QTc interval in heavily pretreated patients with advanced solid tumors, suggesting that QT prolongation is not a major safety concern for necitumumab at the recommended therapeutic dose. The safety profile was consistent with the known safety profile of necitumumab.



Similar content being viewed by others
References
Dienstmann R, Felip E (2011) Necitumumab in the treatment of advanced non-small cell lung cancer: translation from preclinical to clinical development. Expert Opin Biol Ther 11:1223–1231
Salomon DS, Brandt R, Ciardiello F, Normanno N (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19:183–232
Ciardiello F, Tortora G (2008) EGFR antagonists in cancer treatment. N Engl J Med 358:1160–1174
Woodburn JR (1999) The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther 82(2–3):241–250
Blick SK, Scott LJ (2007) Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs 67:2585–2607
Patel SB, Gill D, Garrido-Laguna I (2015) Profile of panitumumab as first-line treatment in patients with wild-type KRAS metastatic colorectal cancer. Onco Targets Ther 9:75–86
Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy G, Kazarnowicz A, Park K, Schumann C, Reck M, Depenbrock H, Nanda S, Kruljac-Letunic A, Kurek R, Paz-Ares L, Socinski MA, Investigators SQUIRE (2015) Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 16:763–774
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Steering Committee (2005) ICH Harmonised Tripartite Guideline: The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, E14. http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html. Accessed 11 November 2015
Darpo B, Benson C, Dota C, Ferber G, Garnett C, Green CL, Jarugula V, Johannesen L, Keirns J, Krudys K, Liu J, Ortemann-Renon C, Riley S, Sarapa N, Smith B, Stoltz RR, Zhou M, Stockbridge N (2015) Results from the IQ-CSRC prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin Pharmacol Ther 97:326–335
Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornøe CW, Wang Y, Zhu H, Gobburu JV (2008) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18
France NP, Della Pasqua O (2015) The role of concentration-effect relationships in the assessment of QTc interval prolongation. Br J Clin Pharmacol 79:117–131
Shah RR, Morganroth J, Shah DR (2013) Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf 36:295–316
Shah RR, Morganroth J (2015) Update on cardiovascular safety of tyrosine kinase inhibitors: with a special focus on QT interval, left ventricular dysfunction and overall risk/benefit. Drug Saf 38:693–710
Schrieber SJ, Zhao H, Keegan P, Booth B, Rahman NA (2014) Evaluation of the potential for QT/QTc interval prolongation for therapeutic biotechnology products. J Clin Oncol 32(suppl):5s (abstr 2600)
Jackson T, Kondic J, Seebeck J (2015) QT-prolonging effects of monoclonal antibody drugs in humans: a systematic review of two literature and a public adverse event database. Int J Clin Pharmacol Ther 53:705–711
Deeken JF, Shimkus B, Liem A, Hill D, Gurtler J, Berghorn E, Townes L, Lu H, Trifan O, Zhang S (2013) Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol 71:1473–1483
Kuenen B, Witteveen PO, Ruijter R, Giaccone G, Dontabhaktuni A, Fox F, Katz T, Youssoufian H, Zhu J, Rowinsky EK, Voest EE (2010) A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res 16:1915–1923
Vargas HM, Bass AS, Breidenbach A, Feldman HS, Gintant GA, Harmer AR, Heath B, Hoffmann P, Lagrutta A, Leishman D, McMahon N, Mittelstadt S, Polonchuk L, Pugsley MK, Salata JJ, Valentin JP (2008) Scientific review and recommendations on preclinical cardiovascular safety evaluation of biologics. J Pharmacol Toxicol Methods 58:72–76
Carlson GF, Tou CK, Parikh S, Birmingham BK, Butler K (2011) Evaluation of the effect of dapagliflozin on cardiac repolarization: a thorough QT/QTc study. Diabetes Ther 2:123–132
Tyl B, Kabbaj M, Azzam S, Sologuren A, Valiente R, Reinbolt E, Roupe K, Blanco N, Wheeler W (2012) Lack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: results of a thorough QT study (TQTS) with QT-concentration analysis. J Clin Pharmacol 52:893–903
Greillier L, Tomasini P, Barlesi F (2015) Necitumumab for non-small cell lung cancer. Expert Opin Biol Ther 15:1231–1239
Acknowledgments
This study was sponsored by Eli Lilly and Company. Eli Lilly and Company contracted with inVentiv Health Clinical for medical writing support provided by Stacey E. Shehin, PhD, and editorial support provided by Angela C. Lorio, ELS, and Teri Tucker.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David C. Smith received funding from Eli Lilly and Company. John Powderly received remuneration from Carolina BioOncology Institute, has consultant/advisory roles with Bristol Myers Squibb and Merck, has stock ownership in BioCytics, Lion BioTech, Juno Therapeutics, BlueBird Bio, Kite Pharma, and ZioPharm Oncology, and received funding from Bristol Myers Squibb, Genentech, EMD Serono, AstraZeneca, InCyte, MacroGenics, and Eli Lilly and Company. James J. Lee, Dale R. Shepard, and Patricia LoRusso have no conflicts to disclose. Johan Wallin, Archana Chaudhary, Grace Yi Chao, Wee Teck Ng, Gerrit Grau, and Raffael Kurek are employees of and own stock in Eli Lilly and Company. Malcolm I. Mitchell is a former employee and owns stock in Eli Lilly and Company.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smith, D.C., Powderly, J., Lee, J.J. et al. Evaluation of the effect of necitumumab on the corrected QT interval in patients with advanced solid tumors. Cancer Chemother Pharmacol 78, 271–280 (2016). https://doi.org/10.1007/s00280-016-3074-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3074-y