Abstract
Purpose
Greater scrutiny is being placed on developing a full understanding of potential cardiotoxicity of therapeutic agents, especially on the potential to prolong the QTc interval which can lead to arrhythmias such as torsade de pointes and sudden death. This trial was designed to specifically evaluate the effect, if any, of cetuximab on the QTc interval in patients with advanced solid tumors.
Methods
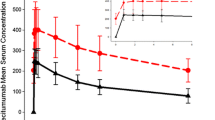
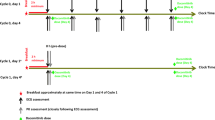
Cetuximab was administered as an initial dose of 400 mg/m2 on day 1 (week 1) followed by a maintenance dose of 250 mg/m2 weekly thereafter. ECG monitoring was performed at screening, baseline (week 1 preceding dosing), and during week 1 to 5 of treatment. Cetuximab concentration-to-QTc relationship was evaluated based on cetuximab serum samples obtained at the time of each ECG measurement to allow for accurate correlation between any observed QT/QTc changes and cetuximab serum concentration.
Results
At the recommended dose (400 mg/m2 on day 1 followed by 250 mg/m2 weekly), cetuximab had no clinically meaningful effect on QTc interval, PR or QRS intervals, or heart rate and there was no apparent concentration-dependent effect of cetuximab on any of these electrocardiogram parameters. Safety observations in patients treated with cetuximab in this study were consistent with the agent’s known safety profile.
Conclusion
These results suggest that cetuximab can be safely administered as a single agent without risk of effect on QTc interval.



Similar content being viewed by others
References
Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM (2010) Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. JNCI 102:14–25
Ederhy S, Izzedine H, Massard C, Dufaitre G, Spano JP, Milano G et al (2011) Cardiac side effects of molecular targeted therapies: towards a better dialogue between oncologists and cardiologists. Crit Rev Oncol/Hematol 80:369–379
Brana I, Taberno J (2010) Cardiotoxicity. Ann Oncol 21(Suppl 7):vii173–vii179
Yeh ETH, Bickford CL (2009) Cardiovascular complications of cancer therapy. J Am Coll Cardiol 53:2231–2247
Mirvis DM, Goldberger AL (2005) Electrocardiography. In: Zipes DP, Libby P, Braunwald E, Bonow RO (eds) Braunwald’s heart disease: a textbook of cardiovascular medicine, 7th edn. Saunders, Philadelphia, pp 107–151
Belardinelli L, Antzelevitch C, Vos MA (2003) Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol Sci 24:619–625
Bazett HC (1920) An analysis of the time relationship of electrocardiograms. Heart 7:353–370
Fridericia LS (1920) Die Systolendauer im Elektrokardiogramm bei normalen menschen und bei herzkranken [letter]. Acta Med Scand 53:489
Hodges M, Salerno Q, Erlien D (1983) Bazett’s QT correction reviewed. Evidence that a linear QT correction for heart rate is better (abstract). J Am Coll Cardiol 1:694
Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D (1992) An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 70:797–801
Strevel EL, Ing DJ, Siu LL (2007) Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 25:3362–3371
Bagnes C, Panchuk PN, Recondo G (2010) Antineoplastic chemotherapy induced QTc prolongation. Curr Drug Safety 5:93–96
Raschi E, Vasina V, Ursino MG, Boriani G, Martoni A, De Ponti F (2010) Anticancer drugs and cardiotoxicity: insights and perspectives in the era of targeted therapy. Pharmacol Ther 125:196–218
Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH (2002) Timing of new black box warnings and withdrawals for prescription medications. JAMA 287:2215–2220
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Steering Committee: ICH Harmonized Tripartite Guideline: The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, E14. Geneva, Switzerland, ICH 2005. Available at: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html
FDA Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs 2006. Available at: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM129357.pdf
National Cancer Institute: Cancer therapy evaluation program, Common terminology for adverse events, version 3.0 DCTD, NCI, NIH, DHHS, 2006. Available from: http://ctep.cancer.gov/forms/CTCAEv3.pdf
Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J (1995) Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1:1311–1318
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Jonker DJ, O’Callaghan CJ, Karapetis CS et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Karapetis CS, Khambata-Ford S, Jonker DJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Van Cutsem E, Kohne C-H, Lang I et al (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29:2011–2019
Bonner JA, Harai PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous cell carcinoma of the head and neck. N Engl J Med 345:567–578
Burtness G, Goldwasser MA, Flood W, Mattar B, Forastiere AA (2005) Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer. An Eastern Cooperative Oncology Group study. J Clin Oncol 23:8646–8654
Vermorkin JB, Mesia R, Rivera F et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127
Guidance for industry, E14 Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, 2005. Available at http://www.fda.gov/cder/Guidance/6922fnl.pdf
Garnett CE, Beasley N, Bhattaram VA et al (2007) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18
Fracasso PM, Howard Burris III, Arquette MA et al (2007) Pharmacodynamic rationale for dosing study of cetuximab: pharmacokinetic and a phase 1 escalating single-dose and weekly fixed-dose. Clin Cancer Res 13:986–993
Pettrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Barni S (2012) Risk of anti-EGFR monoclonal antibody-related hypomagnesemia: systematic review and pooled analysis of randomized studies. Expert Opin Drug Saf 11(Suppl 1):S9–S19
Raschi E, De Ponti F (2012) Cardiovascular toxicity of anticancer-targeted therapy: emerging issues in the era of cardio-oncology. Intern Emerg Med 7:113–131
Albini A, Cesana E, Dontaelli F, Cammarota R, Bucci EO, Baravelli M et al (2011) Cardio-oncology in targeting the HER receptor family: the puzzle of different cardiotoxicities of HER2 inhibitors. Futur Cardiol 7:693–704
Keating GM (2010) Panitumumab: a review of its use in metastatic colorectal cancer. Drugs 70:1059–1078
NCT01412957: A Phase 3, Multicenter, Randomized, Open-label Trial to Evaluate the Survival Benefit of Panitumumab and Best Supportive Care, Compared to Best Supportive Care Alone, in Subjects with Chemorefractory Wild-type KRAS Metastatic Colorectal Cancer. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01412957?id=NCT01412957&rank=1. Accessed 12 July 2012
Al-Khatib SM, Allen La Pointe NM, Kramer JM, Califf RM (2003) What clinicians should know about the QT interval. JAMA 30:2120–2127
Acknowledgments
This study (NCT00698841) was sponsored by Bristol-Myers Squibb. The authors would like to acknowledge all the investigators, and their sites, participating in this study: Richy Agajanian, Vincent A. Armenio, John Deeken, Lourdes J. Feliciano, Troy H. Guthrie Jr, Jayne Gurtler, Donald W. Hill, Charles W. Hollen, Haresh S. Jhangjiani, Lawrence P. Leichman, Andre K.D. Liem, Hima Bindu Lingham, An D. Nguyen, Craig H. Reynolds, and. Brian J. Shimkus. The authors would also like to thank the patients as well as their families and caregivers. Medical writing and editorial support were provided by Jamie H. Zhang, PhD, an employee of Bristol-Myers Squibb.
Conflict of interest
Authors JFD, BS, AL, DH, and JG declared no conflict of interest. Authors EB, LT, and HL are employed by Bristol-Myers Squibb (BMS) and may own stock. Authors OT and SZ were both employed by BMS at time of study conduct and during early stages of manuscript preparation, but have since left that employment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deeken, J.F., Shimkus, B., Liem, A. et al. Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol 71, 1473–1483 (2013). https://doi.org/10.1007/s00280-013-2146-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2146-5